Back to Journals » Research and Reports in Urology » Volume 15
Neonatal Cystitis Makes Adult Female Rat Urinary Bladders More Sensitive to Low Concentration Microbial Antigens
Authors Archer AC, DeBerry JJ, DeWitte C, Ness TJ
Received 11 October 2023
Accepted for publication 7 December 2023
Published 11 December 2023 Volume 2023:15 Pages 531—539
DOI https://doi.org/10.2147/RRU.S444167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Panagiotis J Vlachostergios
Ashley C Archer,1 Jennifer J DeBerry,2 Cary DeWitte,2 Timothy J Ness2
1University of Alabama at Birmingham School of Medicine, Birmingham, AL, USA; 2Department of Anesthesiology and Perioperative Medicine, University of Alabama at Birmingham, Birmingham, AL, USA
Correspondence: Timothy J Ness, Department of Anesthesiology and Perioperative Medicine, University of Alabama at Birmingham, PBMR-208, 901 19th St. S, Birmingham, AL, 35205, USA, Tel +1-205-975-9643, Email [email protected]
Purpose: Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic pain disorder. Patients with IC/BPS often experience “flares” of symptom exacerbation throughout their lifetime, initiated by triggers, such as urinary tract infections. This study sought to determine whether neonatal bladder inflammation (NBI) alters the sensitivity of adult rat bladders to microbial antigens.
Methods: Female NBI rats received intravesical zymosan treatments on postnatal days P14-P16 while anesthetized; Neonatal Control Treatment (NCT) rats were anesthetized. In adults, bladder and spinal cord Toll-like receptor type 2 and 4 (TLR2, TLR4) contents were determined using ELISAs. Other rats were injected intravesically with lipopolysaccharide (LPS; mimics an E. coli infection; 25, 50, 100, or 200 μg/mL) or Zymosan (mimics yeast infection; 0.01, 0.1, 1, and 10 mg/mL) solutions on the following day. Visceromotor responses (VMRs; abdominal contractions) to graded urinary bladder distention (UBD, 10– 60 mm Hg, 20s) were quantified as abdominal electromyograms (EMGs).
Results: Bladder TLR2 and TLR4 protein levels increased in NBI rats. These rats displayed statistically significant, dose-dependent, robustly augmented VMRs following all but the lowest doses of LPS and Zymosan tested, when compared with their adult treatment control groups. The NCT groups showed minimal responses to LPS in adults and minimally increased EMG measurements following the highest dose of Zymosan.
Conclusion: The microbial antigens LPS and Zymosan augmented nociceptive VMRs to UBD in rats that experienced NBI but had little effect on NCT rats at the doses tested. The greater content of bladder TLR2 and TLR4 proteins in the NBI group was consistent with increased responsiveness to their agonists, Zymosan and LPS, respectively. Given that patients with IC/BPS have a higher incidence of childhood urinary tract infections, this increased responsiveness to microbial antigens may explain the flares in symptoms following “subclinical” tract infections.
Plain Language Summary: In a rat model of interstitial cystitis, bladder inflammation during childhood resulted in increased bladder sensitivity (ie, pain) to low-level infections in adults.
Keywords: lipopolysaccharide, zymosan, interstitial cystitis, visceromotor responses, TLR2, TLR4, urinary tract infection
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic pain syndrome of unknown etiology.1 The clinical diagnostic criteria for this condition vary, with symptoms that may include severe urgency, frequency of urination, and pain involving the lower abdomen and perineal regions.1 The exact pathophysiology of IC/BPS is yet to be determined; however, the manifestation of this condition is likely a combination of inflammatory and neurophysiological pain factors (visceral and somatic)2 which are modified by multiple other factors, including stress.3,4 Patients with IC/BPS often experience episodes of severe symptom exacerbations (flares) throughout their lifetime.5 Various factors have been identified as triggers of flares, including stress, sexual activity, caffeine, and alcohol, among others.5,6 Notably, urinary tract infection (UTI) has also been identified in IC/BPS patients as a common trigger for bladder symptoms4–8 although, frequent episodes have not been confirmed by urine cultures. The current guideline for the diagnosis of an acute bladder infection is ≥105 CFU/mL in the patient’s urine culture9 with some reports using less stringent values (eg, reference10). Pyuria/leukocyte esterase urine dip measures are commonly employed in clinics as surrogate UTI measures.11 However, the reliability of these measures has been questioned.11 Owing to the heightened inflammatory and functional pain response of patients with IC/BPS, bacteriuria with concentrations below clinical guidelines has been noted to be associated with acute flares in subpopulations of this clinical population.12 Low concentrations of fungal/yeast microbes are also frequently found in the urinary microbiomes of IC/BPS subjects.13–15
Currently, there is no identifiable cure or reliable treatment for this condition, reflecting a lack of knowledge regarding the pathophysiology of IC/BPS.1 Further research on this disease is essential for the development of treatment guidelines and therapies. Epidemiological data suggest that the incidence of UTIs during childhood is higher in the IC/BPS clinical population.16 For this reason, an animal model of IC/BPS, in which rats experience neonatal bladder inflammation (NBI rats), has particular clinical significance because these rat bladders are hypersensitive when tested as adults. Treated on postnatal days P14-P16 with intravesically administered Zymosan, a yeast cell wall component and Toll-Like Receptor 2 (TLR2) agonist, these NBI rats have been demonstrated to display many of the phenotypic features of IC/BPS, particularly following a second insult as adults such as bladder re-inflammation, acute stress, or peri-segmental somatic inflammation.17 These phenotypic features include increased nociceptive responses to UBD, increased frequency, lowered volume and pressure thresholds for micturition, intravesical sensitivity to potassium solutions, bladder vascular fragility, and increased pelvic muscular tone.17 The P14-P16 time period in rats is a critical period of development (bladder inflammation at P7-9) or P21-P23 does not lead to the same alterations in sensation as adults.17,18 The P14-16 time point correlates with the end of the human neonatal period (hence, the name NBI) but overlaps with the early infancy period according to some developmental measures.19 It is also the period during which the inhibitory modulation of spinal cord processing develops.19 The primary bladder nociceptive endpoints utilized in most published studies related to NBI are visceromotor responses (VMRs; abdominal contractions) to urinary bladder distension (UBD) [eg reference20] although alterations in spinal dorsal horn neuronal responses and peripheral tissue inflammatory responses have also been observed.21 It has been proposed that this NBI model can be used to study the mechanisms and treatment of IC/BPS.17
Based on the observation that some individuals with IC/BPS have symptoms associated with bacteriuria below the clinical criterion for a UTI (subclinical infections), the following hypothesis that was tested in the present study was posited: NBI results in increased bladder nociceptive sensitivity to low concentrations of microbial antigens in adults. Specifically, the present study examined the sensitivity of the bladder to the augmenting effects of two known bladder irritants of microbial origin, lipopolysaccharide (LPS; a proxy of E. coli infection and TLR4 agonist) and zymosan (a proxy of yeast infection and TLR2 agonist), by intravesically administering low-concentration antigen solutions to NBI rats and their controls, and then characterizing VMRs to UBD. In addition, the tissue concentrations of TLR2 and TLR4 in NBI rats were compared with those in controls.
Materials and Methods
General
This study was performed using female Sprague-Dawley rats. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and so followed guidelines for animal care as defined by the United States Department of Agriculture. Timed pregnant dams were obtained from Invigo Laboratories (Sprattville, AL, USA) and the date of birth was recorded. Separate groups of female pups underwent neonatal treatments and were raised using standard husbandry methods, with weaning from dams at 3–4 weeks of age. All rats were raised to 12–15 weeks of age before undergoing additional adult pretreatments followed by testing or tissue harvesting.
Neonatal Treatments
On postnatal days P14-P16, all rats underwent one of two treatments. In the neonatal bladder inflammation group (NBI rats), pups were anesthetized with 2–5% isoflurane in oxygen, injected with ampicillin (50–100 mg/kg s.c.), their urethral orifice swabbed with an iodine-povidone solution, and a 24 gauge angiocatheter passed transurethrally into their bladder. A 1% Zymosan solution (0.1 mL) was injected into the bladder and allowed to dwell for 30 min. The pups were kept warm on a heating blanket and were returned to their mothers. Neonatal Control Treatments (NCT rats) consisted of a similar anesthetic for 30 min, iodine-povidone swabbing, ampicillin treatment, and identical recovery protocols.
Adult Bladder Pretreatments
As adults (12–15 weeks of age), rats were anesthetized with 2–5% isoflurane in oxygen and injected with ampicillin (50–100 mg/kg s.c.). Their urethral orifice was swabbed with an iodine-povidone solution, and a 22-gauge angiocatheter was passed transurethrally into the bladder. Solutions of different concentrations of LPS (0, 25, 50, 100 or 200 µg/mL) or Zymosan (0.01, 0.1, 1, or 10 mg/mL) were injected into the bladder in a volume of 500 μL and allowed to dwell for 30 min. The rats were kept warm on a heating blanket, allowed to recover, and returned to their home cages. The control adult treatment consisted of a similar anesthetic for 30 min, iodine-povidone swabbing, ampicillin treatment, and identical recovery protocols. LPS was derived from the O55:B5 E. Coli strain (Sigma-Aldrich, St. Louis, MO), which has been well characterized and utilized by multiple laboratories to induce bladder inflammation in rodents.22 The Zymosan utilized was Zymosan A (Sigma Aldrich, St. Louis, MO), derived from Saccharomyces cerevisiae, has been used extensively in our laboratory.23
Visceromotor Response (VMR) Measures
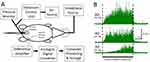
The day after adult pretreatment, the rats were anesthetized with 2–5% isoflurane in oxygen and 1.2 g/kg urethane s.c. A 22-gauge angiocatheter was placed transurethrally into the bladder and held in place by a tight suture around the distal urethral orifice. Silver wire electrodes were placed in the external oblique musculature immediately superior to the inguinal ligament. Isoflurane was then lowered until flexion reflexes were present (typically, 0.25% isoflurane). The UBDs (20 s, 10–60 mm Hg) were produced using compressed air and a distension control device. Intravesical pressure was monitored using an in-line pressure transducer. The (EMG) activity of the external oblique musculature was measured as previously described.17 Briefly, standard differential amplification of the abdominal electrodes was digitized (Spike 2, Cambridge Electronic Design, Inc., Cambridge UK) at a sampling rate of 10 KHz and saved on a computer. Digital rectification allowed for the calculation of the mean EMG activity (in mV) during any defined time period. For each dataset, the same amplifier and filter settings were used for all the rats. A demonstration of typical EMG responses, coupled with a graphical description of the experimental apparatus, is shown in Figure 1.
Enzyme-Linked ImmunoSorbent Assay (ELISA) of TLR2 and TLR4 Content
Adult (12–15 wks) rats that had experienced NBI or NCTs were anesthetized and euthanized, allowing harvesting of the urinary bladder and hydraulic extraction of the spinal cord. The bladder and separate segments of the thoracolumbar (T13-L2) and lumbosacral (L6-S2) spinal cord tissues were weighed, homogenized, and processed according to the manufacturer’s instructions (TLR2: LS Bio, Shirley, MA; kit LS-F56396 - TLR4: Cosmo Bio USA, Carlsbad, CA; kit CSB-E15822r-1) and then analyzed for TLR2 and TLR4 levels.
Statistical Analyses
Data are presented as the mean ± S.E.M. ELISA data were compared using a one way ANOVA. The vigor of VMRs was quantified as the mean rectified EMG activity during the 20s period of UBD minus the mean rectified EMG activity during the immediate pre-stimulus time period. Measures of Visceromotor Response were treated as discrete data points and analyzed using the repeated measures ANOVA. In addition, when utilizing multiple different intensities of UBD, a stimulus-response function was generated which was then associated with a single Area-Under-the-Curve (AUC) statistic which has been demonstrated to be normally distributed [see reference17 for more extensive description]. For different pretreatments, these AUC statistics were normalized to the respective adult pretreatment control measure. The statistical package used for analysis was Systat 12 (SPSS, Inc. San Jose, CA USA). Comparisons with p <0.05 were considered significant.
Results
NBI Results in Increased Bladder Content of TLR2 and TLR4
Rats that received NBI treatments had statistically greater whole-bladder protein content of TLR2 and TLR4 than rats that received NCT (Table 1). The spinal cord contents at both the thoracolumbar (T12-L2) and lumbosacral (L6-S2) levels were not statistically different between the NBI and NCT groups (Table 1).
 |
Table 1 Effect of Neonatal Bladder Inflammation on TLR2 and TLR4 |
NBI Leads to Increased Vigor of Visceromotor Responses (VMRs) Following LPS and Zymosan as Adults
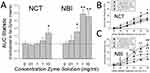
As in previous studies,24 graded urinary bladder distension evoked robust graded VMRs in both adult NBI (Figures 2C and 3C) and NCT (Figures 2B and 3B) rats. AUC statistics related to individual stimulus-response functions for each rat were generated, as this statistic has been demonstrated to be normally distributed across study populations17 and allows for the comparison of different treatment groups (Figures 2A and 3A). NBI rats pretreated with either LPS (Figure 2) or Zymosan (Figure 3) demonstrated dose-dependent effects when compared to NBI rats that received adult control pretreatments (No LPS, No Zymosan). In contrast, the VMRs of NCT rats were not significantly altered by adult pretreatments, except for the highest dose of zymosan, which was statistically greater than the VMRs of NBI rats that received adult control pretreatments. These within-group differences were readily apparent when the AUCs, normalized to the adult control pretreatments, were compared side by side (Figures 2A and 3A). Although the VMRs in NBI rats that received adult control pretreatments tended to be less vigorous than those in NCT rats that received adult control pretreatments (p=0.068), a statistically significant interaction effect between the intensity of UBD and neonatal treatment was observed (repeated-measures ANOVA, F = 2.498; p=0.033). Consequently, we felt that it was indicated to treat the NCT and NBI groups as separate statistical entities.
Discussion
The most important finding of this study was that a bout of cystitis during the rat equivalent of childhood was associated with changes in adult rats, such that low doses of microbial antigens within the bladder were sufficient to induce augmented nociceptive responses to bladder distension, resulting in the increased expression of TLR2 and TLR4 receptors in adult rat bladders. This augmentation of responses correlates with the enhanced responses of humans diagnosed with IC/BPS who report flares in bladder symptoms in association with bacteriuria levels that are below the clinical criteria for a UTI.12 Murine models of IC/BPS have also reported a similar sensitized responsiveness to TLR4 activation25 and require TLR4 to augment autoimmune cystitis-induced pain-like responses.26
The role of fungal or yeast infections as contributing factors to subsequent hypersensitivity is supported by the findings of the MAPP Research Network, which identified that fungal DNA was more frequently present during flares in the urine of subjects with IC/BPS than in control subjects14 and was associated with greater bladder symptom severity.13 There may also be role for obtaining historical information related to neonatal bladder infections as well as laboratory evaluations of TRL2 and TLR4 content within the bladder as part of the diagnostic process related to IC/BPS. Such historical and laboratory phenotyping may be useful in predicting responsiveness to subsequent antimicrobial therapies. The mechanisms underlying this increased sensitivity can be attributed to multiple factors. The easiest explanation for this increased responsiveness to TLR2 and TLR4 agonists is that NBI (induced by zymosan) led to increased expression of receptors upon which these agonists interact, as suggested by the ELISA data. Ongoing studies in our laboratory are attempting to identify specific bladder structures that show increased TLR2 and TLR4 expression following NBI.
Notably, TLR2s and TLR4s have been identified in the bladder urothelium27–29 and TLR4 has also been identified in bladder smooth muscle, systemic white blood cells, and primary afferent neurons in the bladder.28,29 The urothelium has the greatest exposure to intravesical antigens and is therefore a logical site of action. Urothelial cells have been demonstrated to communicate with the underlying primary afferent neurons2 and so a mechanism for altering sensory functions is present. UTIs caused by E. coli have been demonstrated to result in increase the excitability of primary afferent neurons.30 Other mechanisms may also be involved in hypersensitivity and are not exclusive to a urothelial mechanism. These include potential reductions in the glycosaminoglycan (GAG) layer lining the bladder, which has been proposed as the primary mechanism leading to pain in patients with IC/BPS.31 With a reduction in the GAG barrier, intravesical antigens have greater potential to reach TLRs elsewhere in the bladder. NBI rats have been demonstrated to be more responsive to intravesical administration of potassium solutions,17 a manipulation used by clinicians as an indirect measure of GAG intactness.31 Peripheral nervous system changes may also contribute to hypersensitivity, as there is increased CGRP content in the bladders of NBI rats compared to controls, as well as increased neurogenic inflammation in response to intravesical TRPA1 agonist.20 Central nervous system changes may also explain some of the augmentation of VMRs, as spinal opioidergic and corticotrophinergic mechanisms have been demonstrated to be altered in NBI rats.32,33 Immunological mechanisms can also be at play within bladder tissues,34,35 since immune cells express TLR2 and TLR4 and specific populations of B-cells have been noted in subjects with IC/BPS.34
A concern was that in the present dataset, there was a near-total lack of effects of LPS in NCT rats. Some level of augmentation by LPS was expected given the existing literature that reported the effects of intravesical LPS.34–41 However, these studies had endpoints different from those of the present study and, in most cases, used different rat strains. A broader search for studies using intravesical LPS as a bladder irritant revealed that the concentrations of LPS required to generate a robust effect in models similar to those used in this study were much higher than the concentrations used in the present study. In addition, a robust response often requires pretreatment with agents such as intravesical protamine, presumably stripping the GAG layer of the urothelium of the bladder.42–52 Our own previously reported experience with intravesical LPS in rats failed to observe a statistically significant effect of pretreatment in control rats17 but was robustly present in NBI rats. The responses to intravesical Zymosan in the NCT rats were consistent with those in normal control rats in a previous study.18 Other potential reasons for non-robust responses to LPS in control rats include the potential effect of the strain of E. coli which was used to generate the LPS tested. We chose the O55:B5 strain because of the published robust responses in murine subjects22 but there is ample research that supports the statement that different variations of LPS produce different effects on sensation and inflammation.53,54 Notably, this strain had physiological effects in NBI rats in the present study.
Limitations of this study include the exclusive use of female participants. The decision to exclusively use female rats was based on the perceived ease of cannulation of the urethra of females in comparison to that of male rats, as we did not wish to introduce potential nonspecific injuries. There is an additional observation that IC/BPS disproportionately affects females as opposed to males.1 The use of exclusively female subjects raises the potential for the effects of gonadal hormones and their cyclical effects to occur as part of the estrous cycle. We did not control for the effects of the estrous cycle because previous studies examining inflammation and bladder sensation did not observe an effect of gonadal hormone cycling on the vigor of VMRs to UBD.55 The same was not true for other nociceptive endpoints, such as pseudaffective cardiovascular responses to UBD, which were not measured in this study. Therefore, our experimental conclusions are limited to the use of visceromotor and neurochemical measures.
Conclusion
LPS and Zymosan augmented nociceptive responses to UBD in rats which had experienced NBI and had minimal effects on NCT rats at the doses tested. Higher concentrations of bladder TLR2 and TLR4 proteins in the NBI group correlated with an increased nociceptive response to these antigens. Given that patients with IC/BPS have a higher incidence of childhood UTIs16 and the common incidence of childhood urinary tract infections56,57 this responsiveness to low dose LPS and Zymosan may explain why some patients with IC/BPS become symptomatic following what are defined as “subclinical” UTIs (<50,000 CFU). This, in turn, suggests and supports the controversial clinical practice of long-term antimicrobial treatment to alleviate the symptoms of IC/BPS58,59 and suggests new therapies that may be related to TLR2 and TLR4 mechanisms.60,61
Acknowledgments
Supported by NIH DK51413.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Clemens JQ, Mullins C, Ackerman AL, et al. Urologic chronic pelvic pain syndrome: insights from the MAPP Research Network. Nat Rev Urol. 2019;16(3):187–200. doi:10.1038/s41585-018-0135-5
2. Birder L. Pathophysiology of interstitial cystitis. Inter J Urol. 2019;2019(S1):12–15. doi:10.1111/iju.13985
3. Rahnama’i MS, Javan A, Vyas N, et al. Bladder pain syndrome and interstitial cystitis beyond horizon: reports from the Global Interstitial Cystitis/Bladder Pain Society (GIBS) meeting 2019 Mumbai-India. Anesth Pain Med. 2020;10:e101848.
4. Khullar V, Chermansky C, Tarcan T, et al. How can we improve the diagnosis and management of bladder pain syndrome? Part 1: ICI-RS 2018. Neurourol Neurodyn. 2019;38:S66–S70.
5. Sutcliffe S, Bradley CS, Clemens JQ, et al. Urologic chronic pelvic pain syndrome flares and their impact: qualitative analysis in the MAPP Network. Int Urogynecol J. 2015;26(7):1047–1060. doi:10.1007/s00192-015-2652-6
6. Sutcliffe S, Jemielita T, Lai HH, et al. A case-crossover study of urologic chronic pelvic pain syndrome flare triggers in the MAPP Research Network. J Urol. 2018;199(5):1245–1251. doi:10.1016/j.juro.2017.12.050
7. Warren JW. Interstitial cystitis as an infectious disease. Urol Clin North Am. 1994;21(1):3–39. doi:10.1016/S0094-0143(21)00589-9
8. Warren JW, Brown V, Jacobs S, Horne L, Langenberg P, Greenberg P. Urinary tract infection and inflammation at onset of interstitial cystitis/painful bladder syndrome. Urol. 2008;71(6):1085–1090. doi:10.1016/j.urology.2007.12.091
9. Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Med Micro. 2004;38:1150–1158.
10. Chu CM, Lowder JL. Diagnosis and treatment of urinary tract infections across age groups. Am J Obstet Gynecol. 2018;219(1):40–51. doi:10.1016/j.ajog.2017.12.231
11. Kupelian AS, Horsley H, Khasiriya R, et al. Discrediting microscopic pyuria and leucocyte esterase as diagnostic surrogates for infection in patients with lower urinary tract symptoms: results from a clinical and laboratory evaluation. BJU Int. 2013;112(2):231–238. doi:10.1111/j.1464-410X.2012.11694.x
12. Stanford E, McMurphy C. There is a low incidence of recurrent bacteriuria in painful bladder syndrome/interstitial cystitis patients followed longitudinally. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(5):551–554. doi:10.1007/s00192-006-0184-9
13. Nickel JC, Stephens A, Landis JR, et al. Urinary fungi associated with urinary symptom severity among women with interstitial cystitis/bladder pain syndrome (IC/BPS). World J Urol. 2020;38(2):433–446. doi:10.1007/s00345-019-02764-0
14. Nickel JC, Stephens A, Landis JR, et al. Assessment of the lower urinary tract microbiota during symptom flare in women with urologic chronic pelvic pain syndrome: a MAPP Network Study. J Urol. 2016;195(2):356–362. doi:10.1016/j.juro.2015.09.075
15. Nickel JC, Stephens A, Landis JR, et al. A culture-independent analysis of the microbiota of female interstitial cystitis/bladder pain syndrome participants in the MAPP Research Network. J Clin Med. 2019;8(3):415. doi:10.3390/jcm8030415
16. Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73(2):258–262. doi:10.1016/j.urology.2008.09.014
17. Ness T, DeWitte C, DeBerry J, et al. A model in female rats with phenotypic features similar to interstitial cystitis/bladder pain syndrome. Front Pain Res. 2021;7:791045. doi:10.3389/fpain.2021.791045
18. Randich A, Uzzell TW, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7(7):469–479. doi:10.1016/j.jpain.2006.01.450
19. Schwaller F, Fitzgerald M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur J Neurosci. 2014;39(3):344–352. doi:10.1111/ejn.12414
20. DeBerry J, Randich A, Shaffer A, Robbins M, Ness TJ. Neonatal bladder inflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain. 2010;11(3):247–255. doi:10.1016/j.jpain.2009.07.010
21. Ness TJ, Randich A. Neonatal bladder inflammation alters activity of adult rat spinal visceral nociceptive neurons. Neurosci Lett. 2010;472(3):210–214. doi:10.1016/j.neulet.2010.02.007
22. Saban MR, Hellmich H, Nguyen N-B, et al. Time-course of LPS-induced gene expression in a mouse model of genitourinary inflammation. Physiol Genomics. 2001;5(3):147–160. doi:10.1152/physiolgenomics.2001.5.3.147
23. Randich A, Uzzell TW, Cannon RS, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006;6(1):2. doi:10.1186/1471-2490-6-2
24. Castroman P, Ness TJ. Vigor of visceromotor responses to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neurosci Letts. 2001;306(1–2):97–100. doi:10.1016/S0304-3940(01)01886-9
25. Kogan P, Xu S, Wang Y, et al. Subnoxious intravesical lipopolysaccharide triggers bladder inflammation and symptom onset in a transgenic autoimmune cystitis model: a MAPP Network Animal Study. Sci Rep. 2018;8(1):6573. doi:10.1038/s41598-018-24833-x
26. Cui X, Jing X, Lutgendorf SK, et al. Cystitis-induced bladder pain is Toll-like receptor 4 dependent in a transgenic autoimmune cystitis murine model: a MAPP Research Network animal study. Am J Physiol. 2019;317:F90–F98.
27. Moghadam SO, Zowroozi MR. Toll-like receptors: the role in bladder cancer development, progression and immunotherapy. Scan J Immunol. 2019;90(6):e12818. doi:10.1111/sji.12818
28. Niemczyk G, Fus L, Czarzasta K, et al. Expression of Toll-like receptors in the animal model of bladder outlet obstruction. Biomed Res Int. 2020;2020:6632359. doi:10.1155/2020/6632359
29. Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun. 2004;72(6):3179–3186. doi:10.1128/IAI.72.6.3179-3186.2004
30. Montalbetti N, Dalghi MG, Bastacky SI, et al. Bladder infection with uropathogenic E. Coli increases the excitability of afferent neurons. Am J Physiol. 2022;322:F1–F13.
31. Parsons CL. The potassium sensitivity test: a new gold standard for diagnosing and understanding the pathophysiology of interstitial cystitis. J Urol. 2009;182(2):432–434. doi:10.1016/j.juro.2009.04.089
32. Ness TJ, DeWitte C, Randich A. Neonatal cystitis leads to alterations in spinal corticotropin releasing factor receptor-type 2 content and function in adult rats following bladder re-inflammation. Brain Res. 2022;1788:147927. doi:10.1016/j.brainres.2022.147927
33. Shaffer AD, Ness TJ, Robbins MT, Randich A. Early in life bladder inflammation alters opioid peptide content in the spinal cord and bladder of adult female rats. J Urol. 2013;189(1):352–358. doi:10.1016/j.juro.2012.08.190
34. Tabansky I, Moldwin RM, Liu M, et al. A shared B-cell clonotype inpatients with Interstitial Cystitis/Bladder Pain Syndrome presenting with Hunner lesions. Continence Rep. 2022;4:100015. doi:10.1016/j.contre.2022.100015
35. Yoshizumi M, Tazawa N, Watanabe C, Mizoguchi H. TRPV4 activation prevents lipopolysaccharide-induced painful bladder hypersensitivity in rats by regulating immune pathways. Front Immunol. 2022;2022:1080302. doi:10.3389/fimmu.2022.1080302
36. Wang X, Fan L, Yin H, et al. Protective effect of Aster tataricus extract on NLRP3-mediated pyroptosis of bladder urothelial cells. J Cell Mol Med. 2020;24(22):13336–13345. doi:10.1111/jcmm.15952
37. Podmolikova L, Mukanyangezi MF, Dahlqvist AJ, Naluai AT, Ny L, Giglio D. Radiation of the urinary bladder attenuates the development of lipopolysaccharide-induced cystitis. Int Immunopharmacol. 2020;83:106334. doi:10.1016/j.intimp.2020.106334
38. Lee KW, Kim WB, Lee SW, et al. Alterations of macrophage migration inhibitory factor expression in the nervous system of the rat cystitis model. Urol Int. 2017;98(2):228–235. doi:10.1159/000456077
39. Korgali E, Dundar G, Coskun KA, et al. Effect of strontium chloride on experimental bladder inflammation in rat. Int Scholar Res Not. 2014;2014:369292.
40. Lecci A, Birder LA, Meini S, et al. Pharmacological evaluation of the role of cyclooxygenase isoenzymes on the micturition reflex following experimental cystitis in rats. Br J Phamacol. 2000;130(2):331–338. doi:10.1038/sj.bjp.0703309
41. Luber-Narod J, Austin-Ritchie T, Hollins C, et al. Role of substance P in several models of bladder inflammation. Urol Res. 1997;25(6):395–399. doi:10.1007/BF01268854
42. Shin JH, Ryu C-M, Ju H, et al. Synergistic effects of N-acetylcysteine and mesenchymal stem cell in a lipopolysaccharide-induced interstitial cystitis rat model. Cells. 2020;9(1):86. doi:10.3390/cells9010086
43. Ryu C-M, Shin JH, Yu HY, et al. N-acetylcysteine prevents bladder tissue fibrosis in a lipopolysaccharide-induced cystitis rat model. Sci Reps. 2019;9(1):8134. doi:10.1038/s41598-019-44631-3
44. Li J, Luo H, Dong X, et al. Therapeutic effect of urine-derived stem cells for protamine/lipopolysaccharide-induced interstitial cystitis in a rat model. Stem Cell Res Ther. 2017;8(1):107. doi:10.1186/s13287-017-0547-9
45. Choi B-H, Jin L-H, Kim K-H, et al. Mast cell activation and response to tolterodine in the rat urinary bladder in a chronic model of intravesical protamine sulfate and bacterial endotoxin-induced cystitis. Mol Med Rep. 2014;10(2):670–676. doi:10.3892/mmr.2014.2262
46. Sinanoglu O, Ekici ID, Ekici S. Comparison of intravesical application of chondroitin sulfate and colchicine in rat protamine/lipopolysaccharide induced cystitis model. Urol J. 2014;11(1):1296–1300.
47. Lv J, Huang Y, Zhu S, et al. MCP-1-induced histamine release from mast cells is associated with development of interstitial cystitis/bladder pain syndrome in rat models. Mediators Inflamm. 2012;2012:358184. doi:10.1155/2012/358184
48. Shao Y, Lu G-L, Shen Z-J, He H-C. Reduction of intercellular adhesion molecule 1 may play a role in anti-inflammatory effect of hyaluronic acid in a rat model of severe non-bacterial cystitis. World J Urol. 2013;31(3):535–540. doi:10.1007/s00345-012-0839-8
49. Stein PC, Pham H, Ito T, Parsons CL. Bladder injury model induced in rats by exposure to protamine sulfate followed by bacterial endotoxin. J Urol. 1996;155(3):1133–1138. doi:10.1016/S0022-5347(01)66406-1
50. Meyer-Siegler KL, Ordorica RC, Vera PL. Macrophage migration inhibitory factor is upregulated in an endotoxin-induced model of bladder inflammation in rats. J Interferon Cytokine Res. 2004;24(1):55–63. doi:10.1089/107999004772719918
51. Lecci A, Tramontana M, Giuliani S, Criscuoli M, Maggi CA. Effect of Tachykinin NK 2 receptor blockade on detrusor hyperreflexia induced by bacterial toxin in RATS. J Urol. 1998;160(1):206–209. doi:10.1016/S0022-5347(01)63091-X
52. Diaz-Salmeron R, Cailleau C, Denis S, Ponchel G, Bouchemal K. Hyaluronan nanoplatelets exert an intrinsic anti-inflammatory activity in a rat model of bladder painful syndrome/interstitial cystitis. J Control Release. 2023;356:434–447. doi:10.1016/j.jconrel.2023.03.014
53. Rosen JM, Klumpp DJ. Mechanisms of pain from urinary tract infection. Int J Urol. 2014;21(S1):26–32. doi:10.1111/iju.12309
54. Horvath DJ, Patel AS, Mohamed A, et al. Association of O-antigen serotype with the magnitude of initial systemic cytokine responses and persistence in the urinary tract. J Bacteriol. 2016;198(6):964–972. doi:10.1128/JB.00664-15
55. Ball CL, Ness TJ, Randich A. Opioid blockade and inflammation reveal estrous cycle effects on visceromotor reflexes evoked by bladder distension. J Urol. 2010;184(4):1529–1535. doi:10.1016/j.juro.2010.05.090
56. Arshad M, Seed PC. Urinary tract infections in the infant. Clin Perinatol. 2015;42(1):17–28. doi:10.1016/j.clp.2014.10.003
57. Harb A, Yassine V, Ghssein G, Salami A, Fakih H. Prevalence and clinical significance of urinary tract infection among neonates presenting with unexplained hyperbilirubinemia inLebanaon: a retrospective study. Infect Chemother. 2023;55(2):194–203. doi:10.3947/ic.2022.0117
58. Swamy S, Barcella W, De Iorio M, et al. Recalcitrant chronic bladder pain and recurrent cystitis but negative urinalysis: what should we do? Int Urogyn J. 2018;29(7):1035–1043. doi:10.1007/s00192-018-3569-7
59. Swamy S, Kupelian AS, Khasriya RR, et al. Cross-over data supporting long-term antibiotic treatment in patients with painful lower urinary tract symptoms, pyuria and negative urinalysis. Int Urogynecol J. 2019;30(3):409–414. doi:10.1007/s00192-018-3846-5
60. Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. doi:10.3389/fimmu.2014.00461
61. Oliveira-Nascimento L, Massari P, Wetzler LM. The role of TLR2 in infection and immunity. Front Immunol. 2012;3:79. doi:10.3389/fimmu.2012.00079
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.