Back to Journals » International Medical Case Reports Journal » Volume 17
Missed Diagnosis of Perforation and Intraperitoneal Migration of an Intrauterine Device and Its Management in a Resource-Limited Setting: A Case Report
Authors Gebremichael A , Teka H , Abadi KK , Siferih M , Moges M, Arusi M, Shiferaw A
Received 16 October 2023
Accepted for publication 19 January 2024
Published 26 January 2024 Volume 2024:17 Pages 71—76
DOI https://doi.org/10.2147/IMCRJ.S441386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Akebom Gebremichael,1 Hale Teka,2 Kidus Kebede Abadi,3 Melkamu Siferih,4 Menberu Moges,5 Muhudin Arusi,1 Abel Shiferaw6
1Department of Obstetrics and Gynecology, St. Peter’s Specialized Hospital, Addis Ababa, Ethiopia; 2Department of Obstetrics and Gynecology, College of Health Science, Mekelle University, Mekelle, Tigray, Ethiopia; 3Department of Obstetrics and Gynecology, Riwyet Maternal and Child Health Center, Tigray, Ethiopia; 4Department of Obstetrics and Gynecology, Debre Markos University, Debre Markos, Ethiopia; 5Department of Obstetrics and Gynecology, Tayo Hospital, Baidoa, Somalia; 6Department of Obstetrics and Gynecology, Dessie Comprehensive Specialized Hospital, Dessie, Ethiopia
Correspondence: Akebom Gebremichael, Email [email protected]
Background: The intrauterine device is a popular and highly effective form of long-acting reversible contraception. Although generally safe, complications could happen. One of the most serious complications of intrauterine device use is uterine perforation. Risk factors for perforation include, but are not limited to, postpartum period, breastfeeding, levels of experience, and excessive force exerted during insertion. This case is significant because it demonstrates risk factors for uterine perforation, how to handle missing strings, and care in places with little resources.
Case Presentation: We discuss the case of a 27-year-old black Ethiopian woman who presented with chronic pelvic pain and had a perforated intrauterine device discovered in the cul-de-sac. The device had been inserted at six weeks postpartum. The client was unable to feel the strings three months after insertion, and a wrong diagnosis of expulsion was made. After one year of insertion, the intrauterine device was located on a plain abdominal radiograph and removed via laparotomy without complications.
Conclusion: Although uterine perforation is a rare complication of intrauterine device insertion, special attention should be paid to women with risk factors. In the absence of a witnessed expulsion, assessments and investigations should be carried out before declaring a device expelled. In patients with chronic pelvic pain complaints in the presence of an intrauterine device, perforation and migration outside the uterine cavity should be considered. Abdominal X-rays and laparotomies can be used to find and manage extrauterine migrating devices in environments with limited resources.
Keywords: intrauterine device, missed uterine perforation, intraperitoneal migration
Background
Intrauterine devices (copper and hormonal) are generally well tolerated, safe, and efficient. As a result, it is becoming popular as a birth control strategy. Despite the device’s extremely high rate of general safety, problems and negative effects can occur both during insertion and at various points afterward.1–3 Complications are generally rare and include expulsion (3–6%), PID (0.5–1%), contraceptive failure (0.1–0.6%), and perforation (0.01%).4–8 Migration of IUD to the bladder, intestine, peritoneum, appendix, omentum, and mesentery can occur as a result of uterine perforation, which is one of the most significant complications of IUDs despite its rarity.9–12 Early postpartum, breastfeeding, uterine flexion, inexperienced provider, and excessive pressure during insertion are some risk factors that could increase the probability of perforation.13–15 Our case involves missed perforation and translocation of an IUD into the peritoneum of a postpartum lactating mother.
Case Presentation
A 27-year-old black (“Habesha”) lactating woman with gravida 2, para 1, and abortion 1 was referred to our hospital from one of the local health centres with the diagnosis of chronic pelvic pain. Over the previous year, she had multiple visits to different health centers, was repeatedly given medications for acute PID, and was also prescribed different anti-pain medications. Her last delivery was one year ago; it was via cesarean section and uncomplicated. A CU-IUD was provided six weeks after her delivery while she was still breast-feeding. The procedure was followed by abdominal cramps and mild bleeding, which lasted several days.
Three months after insertion, she went to the health center because she could not feel the strings. At that time, a presumptive diagnosis of spontaneous expulsion was made. No further attempts were made to confirm its location. She was also not provided with another family planning during that visit. Upon arrival at our hospital, she complained of dull and aching pain in the lower quadrant of eight-month duration. Otherwise, she has no other complaints. Her vital signs were within the normal range; her BMI (20.5) and CBC parameters were within normal limits. A bimanual examination was performed to reveal a tender 3 cm by 3 cm cul-de-sac mass with no clear distinction from the uterus, and a speculum examination was nonrevealing. Pelvic ultrasound showed an undefined posterior cul-de-sac mass. Futher investigation modalities such as laparoscopy and CT were discussed with the patient, but she could not afford them.
Subsequently, suspecting an intraperitoneal IUD from history and the finding of pelvic mass on ultrasound, the radiologist performed an abdominal erect X-ray with a uterine probe inside in the AP and lateral planes, which both showed that the T-shaped CU-IUD is near and posterior (in the lateral plane) to the uterus (Figure 1).
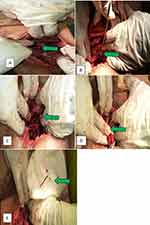
Finally, a laparotomy was decided and the abdominal cavity was accessed by making a 5-cm incision on a previous transverse skin incision. A careful palpation of the pelvic cavity was performed to determine the specific location, and a string was palpated over the posterior wall of the uterus. On close examination, the IUD was found to be enclosed in a mass formed by the left ovary and the left fallopian tube, which adhered to the posterior wall of the uterus at the junction of the body and the cervix.
Considering that part of the string was protruding from the mass, the bowel, vessels, and other pelvic structures were examined for perforation, and there was no perforation. Subsequently, after adhesion was removed, the entire IUD (stem, arms, and string) was retrieved from the mass (Figure 2). The uterus was examined and was in a normal anatomic position. Minor surface bleeding from the posterior wall of the uterus and the adnexa was arrested with pressure. After ascertaining hemostasis, the operation was completed. As part of family planning, she was counseled, provided Implanon-NXT, and discharged without complications two days after surgery. Two weeks following the surgery, she stated that her pain had significantly decreased.
Discussion
IUDs are a highly successful method of birth control, with failure rates ranging from 0.2% to 0.8% for typical users. Although IUD-related problems are uncommon, they are becoming more noticeable as IUD use increases.14–16
Uterine perforation, which commonly occurs during insertion and rarely due to gradual erosion of the myometrium, is the most serious yet rare consequence of an IUD.10,17–19 IUD perforation can mimic myriads of causes of acute and chronic pelvic pain. The most important distinguishing future is the feeling of acute pain and bleeding after insertion that were both evident in this patient. We assume that in this case the perforation, which most likely happened at the time of insertion and then later migrated to the peritoneum.
According to the APEXIUD multisite cohort study, the postpartum period and lactation were considered risk factors for perforation. This can be explained by the fact that women would have more rapid uterine involution and frequent contractions due to hypoestrogenism and oxytocin release, which together increase the risk of perforation.12,20,21 Perforation can also be related to lack of competence and the absence of appropriate materials for insertion. Even though myometrial abnormalities are expected to enhance the risk of uterine perforation, research has shown that having a cesarean section is not a risk factor.22
In approximately 90% of cases, the perforation is not recognized at the time of IUD insertion. Sometimes, perforation is suspected between the time of insertion and follow-up due to persistent symptoms, mainly mild lower abdominal pain.17,23
Of the perforations that occur, most do not cause long-term damage. However, a significant harm associated with perforation may be loss of the contraceptive effect of the IUD, resulting in an unintended pregnancy or trauma to internal organs.22
Therefore, to avoid the grave complications of IUD perforation and the associated risks, family planning providers should be well experienced, use plastic sounds, know the correct position of the uterus, use appropriate force during insertion, use a pull-back mechanism rather than a push-out release mechanism for the device, and take extra care after delivery, especially if the woman is breastfeeding.17,22,24,25
Imaging such as ultrasonography and X-rays can be diagnostic in the management of migrated IUD.26,27 In this patient, before surgery, a CT scan would be preferred to locate the IUD and highlight any potential complications, such as bowel obstruction and abscess formation.7,28 After a pregnancy has been ruled out, the best and least expensive method to locate a lost IUD in a situation with limited resources is basic abdominal pelvic radiography.29
Even in individuals who are asymptomatic, the WHO advises surgical removal of the migrated IUD as soon as possible. Most of the time, minimally invasive procedures such as laparoscopy, hysteroscopy, or colonoscopy surgery are advised; however, invasive procedures with a skilled surgeon may be required if the lost IUD is embedded in organs or close to blood vessels.2,30,31 In this case, laparotomy was performed right away since laparoscopy was expensive.
Conclusion
Uterine perforation with an IUD is a serious problem, even if it is an infrequent occurrence. By taking all necessary precautions to insert devices securely and getting them properly diagnosed and managed, perforation and subsequent risks could be minimized.
In the event that the strings are missed, attempts must be made to rule out pregnancy first, after which the strings must be identified by probing the endocervical cavity and sounding the uterus to assess the device in the endometrial cavity.
A physician should take an abdominal X-ray to locate the device if unable to do so using sound.32 In patients with complaints of chronic pelvic pain in the presence of an intrauterine device, perforation and migration outside the uterine cavity should be considered.
The counseling, care, and follow-up of patients with multiple risk factors of perforation, including this, should be carried out by experienced staff. When less invasive procedures such as laparoscopy and advanced imaging methods such as CT are not available, plain abdominal x-rays and open surgeries may be considered alternatives. Last but not least, this case illustrates a series of mistakes made in IUD care that should serve as a warning to practitioners.
Abbreviations
BMI, Body Mass Index; CU-IUD, Copper coated Intra Uterine Device; IUS, Intrauterine System; PID, Pelvic Inflammatory Disease; WHO, World Health Organization.
Ethics Approval and Consent to Participate
Not applicable. Our institution does not require an approval for publication of case reports.
Consent for Publication
Written informed consent for publication of their clinical details and images was obtained from the patient. A copy of the written consent is available for review by the Editor-in-chief of this journal.
Acknowledgments
The author would like to thank the Department of Obstetrics and Gynecology at Saint Peters Specialized Hospital, Addis Ababa, Ethiopia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any funding from public, commercial, or other funding agencies.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Katherine D. Intrauterine contraception: management of side effects and complications. Post TW, editor. UpToDate. Waltham, MA: UpToDate Inc; 2018. http://www.uptodate.com.
2. Braaten KP, Benson CB, Maurer R, Goldberg AB. Malpositioned intrauterine contraceptive devices: risk factors, outcomes, and future pregnancies. Obstetrics Gynecol. 2011;118(5):1014–1020. doi:10.1097/AOG.0b013e3182316308
3. Sufrin CB, Postlethwaite D, Armstrong MA, Merchant M, Wendt JM, Steinauer JE. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstetrics Gynecol. 2012;120(6):1314–1321. doi:10.1097/AOG.0b013e318273364c
4. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Positive testing for Neisseria gonorrhoeae and Chlamydia trachomatis and the risk of pelvic inflammatory disease in IUD users. J Women’s Health. 2015;24(5):354–359. doi:10.1089/jwh.2015.5190
5. Trussell J. Contraceptive failure in the United States. Contraception. 2004;70(2):89–96. doi:10.1016/j.contraception.2004.03.009
6. Heinemann K, Reed S, Moehner S, Do Minh T. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91(4):274–279. doi:10.1016/j.contraception.2015.01.007
7. Sinha M, Gupta R, Tiwari A. Minimally invasive surgical approach to retrieve migrated intrauterine contraceptive device. Int J Reprod Contraception Obstetrics Gynecol. 2013;2(2):147–152. doi:10.5455/2320-1770.ijrcog20130607
8. Bahamondes L, Fernandes A, Bahamondes MV, Juliato CT, Ali M, Monteiro I. Pregnancy outcomes associated with extended use of the 52-mg 20 μg/day levonorgestrel-releasing intrauterine system beyond 60 months: a chart review of 776 women in Brazil. Contraception. 2018;97(3):205–209. doi:10.1016/j.contraception.2017.10.007
9. Nouioui MA, Taktak T, Mokadem S, Mediouni H, Khiari R, Ghozzi S. A mislocated intrauterine device migrating to the urinary bladder: an uncommon complication leading to stone formation. Case Reports Urol. 2020;2020:1–4. doi:10.1155/2020/2091915
10. Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. I. Classification, complications, mechanism, incidence, and missing string. Obstetrical Gynecological Survey. 1981;36(7):335. doi:10.1097/00006254-198107000-00001
11. Caliskan E, Öztürk N, Dilbaz BÖ, Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reprod Health Care. 2003;8(3):150–155. doi:10.1080/ejc.8.3.150.155
12. Reed SD, Zhou X, Ichikawa L, et al. Intrauterine device-related uterine perforation incidence and risk (APEX-IUD): a large multisite cohort study. Lancet. 2022;399(10341):2103–2112. doi:10.1016/S0140-6736(22)00015-0
13. Gündüz R, Ağaçayak E, Dönmez DA, Findik FM, Evsen MS, Gül T. Evaluation of patients with uterine perforation after intrauterine device placement and determination of risk factors: a Retrospective Case-Control Study. East J Med. 2022;27(2):264–271. doi:10.5505/ejm.2022.88155
14. Torve M, Hansen K. Preventing Unintended Pregnancies: a Review of Long-Acting Reversible Contraception. South Dakota Med. 2023;76(3):132–136.
15. Contraception LA. ACOG Practice Bulletin No. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:18.
16. Myo MG, Nguyen BT. Intrauterine Device Complications and Their Management. Curr Obstetrics Gynecol Rep. 2023;27:1–8.
17. Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003;67(1):53–56. doi:10.1016/S0010-7824(02)00417-1
18. Mülayim B, Mülayim S, Yigit Celik N. A lost intrauterine device. Guess where we found it and how it happened? Eur J Contracept Reprod Health Care. 2006;11(1):47–49. doi:10.1080/13625180500456791
19. Searle ES. The intrauterine device and the intrauterine system. Best Practice Res Clin Obstetrics Gynaecol. 2014;28(6):807–824. doi:10.1016/j.bpobgyn.2014.05.004
20. Heartwell SF, Schlesselman SA. Risk of uterine perforation among users of intrauterine devices. Obstetrics Gynecol. 1983;61(1):31–36.
21. Andersson K, Ryde-Blomqvist E, Lindell K, Odlind V, Milsom I. Perforations with intrauterine devices: report from a Swedish survey. Contraception. 1998;57(4):251–255. doi:10.1016/S0010-7824(98)00029-8
22. Rowlands S, Oloto E, Horwell DH. Intrauterine devices and risk of uterine perforation: current perspectives. Open Access j Contraception. 2016;16:19–32. doi:10.2147/OAJC.S85546
23. VanGrootheestKSachsBHarrison-WoolrychMCaduff-JanosaPvan PuijenbroekEUterine perforation with levonorgestrel-releasing intrauterine deviceDrug Saf201134838821142273.
24. Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minimally Invasive Gynecol. 2014;21(4):596–601. doi:10.1016/j.jmig.2013.12.123
25. Goldstuck ND, Wildemeersch D. Role of uterine forces in intrauterine device embedment, perforation, and expulsion. Int J Women’s Health. 2014;7:735–744. doi:10.2147/IJWH.S63167
26. Abramovici H, Sorokin Y, Bornstein J, Auslander R. A partial uterine perforation (type 2) by a copper‐T IUD: sonographic diagnosis and management. J Ultrasound Med. 1985;4(7):381–3. 17. doi:10.7863/jum.1985.4.7.381
27. Braaten KP, Goldberg AB. Malpositioned IUDs: when you should intervene (and when you should not). OBG Management. 2012;24(8):38–46.
28. Bilian X. Chinese experience with intrauterine devices. Contraception. 2007;75(6):S31–4. doi:10.1016/j.contraception.2006.12.007
29. Bozkurt M, Yumru AE, Coskun EI, Öndes B. Laparoscopic management of a translocated intrauterine device embedded in the gastric serosa. JPMA. 2011;61(10):1020.
30. World Health Organization. Mechanism of Action, Safety and Efficacy of Intrauterine Devices: Report of a WHO Scientific Group [Meeting Held in Geneva from 1 to 4 December 1986]. World Health Organization; 1987.
31. Sun CC, Chang CC, Yu MH. Far-migrated intra-abdominal intrauterine device with abdominal pain. Taiwanese J Obstetrics Gynecol. 2008;47(2):244–246. doi:10.1016/S1028-4559(08)60095-9
32. Millen A, Austin F, Bernstein GS. Analysis of 100 cases of missing IUD strings. Contraception. 1978;18(5):485–495. doi:10.1016/0010-7824(78)90033-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.