Back to Journals » Research and Reports in Urology » Volume 15
Metastatic Castration-Resistant Prostate Cancer: Insights on Current Therapy and Promising Experimental Drugs
Authors Ferretti S, Mercinelli C , Marandino L, Litterio G, Marchioni M, Schips L
Received 19 March 2023
Accepted for publication 15 June 2023
Published 26 June 2023 Volume 2023:15 Pages 243—259
DOI https://doi.org/10.2147/RRU.S385257
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Simone Ferretti,1,* Chiara Mercinelli,2,* Laura Marandino,2 Giulio Litterio,1 Michele Marchioni,1 Luigi Schips1
1Department of Medical, Oral and Biotechnological Sciences, G. d’Annunzio University of Chieti, Urology Unit, Chieti, Italy; 2Division of Experimental Oncology, Urological Research Institute, IRCCS San Raffaele Scientific Institute, Milan, Italy
*These authors contributed equally to this work
Correspondence: Michele Marchioni, Department of Medical, Oral and Biotechnological Sciences, G. d’Annunzio University of Chieti, Urology Unit, Chieti, Italy, Tel +393296544866, Fax +390871357756, Email [email protected]
Abstract: The therapeutic landscape of metastatic hormone sensitive and metastatic castration-resistant prostate cancer (mCRPC) is rapidly changing. We reviewed the current treatment options for mCRPC, with insights on new available therapeutic strategies. Chemotherapy with docetaxel or cabazitaxel (for patients progressing on docetaxel), as well as treatment with androgen receptor axis targeted therapies, and Radium-223 are well-established treatment options for patients with mCRPC. The advent of theragnostic in prostate cancer established Lutetium-177 (177Lu)–PSMA-617 as a new standard of care for PSMA-positive mCRPC previously treated with ARAT and taxane-based chemotherapy. Olaparib, a poly-ADP-ribose polymerase (PARP) inhibitor, is approved for selected patients with mCRPC progressed on ARATs and in combination with abiraterone acetate as first-line treatment for mCRPC. Immunotherapy showed limited efficacy in unselected patients with mCRPC and novel immunotherapy strategies need to be explored. The search for biomarkers is a growing field of interest in mCRPC, and predictive biomarkers are needed to support the choice of treatment and the development of tailored strategies.
Keywords: metastatic castration resistant prostate cancer, chemotherapy, androgen-receptors axis targeted therapies, PARP inhibitors, theragnostic, predictive biomarkers
Introduction
Prostate cancer (PCa) is the first malignant tumor in male population by incidence and second by death, with 27% of new diagnoses of all incident cancer cases in men every year and 11% of all cancer-related deaths.1 Localized PCa has survival rates >90% at 5 years, which decrease to 31% in advanced and metastatic settings.2
Androgen deprivation therapy (ADT) is a backbone therapy for metastatic hormone-sensitive prostate cancer (mHSPC). In years, many other compounds were introduced in the treatment of mHSPC in combination with ADT: chemotherapy (docetaxel), androgen receptor axis targeted therapies (ARATs) such as abiraterone acetate, enzalutamide, apalutamide, and darolutamide.3
However, most of the patients develop metastatic castration-resistant prostate cancer (mCRPC), a condition that carries a worse prognosis. CRPC is defined as biochemical progression while on ADT, defined by three consecutive rises in PSA at least one week apart, or as radiological progression with castration serum testosterone levels, below 50 ng/dL.4
In the last years, the landscape of mCRPC treatment has deeply changed, with the introduction of several new compounds. The choice of the best option as first-line therapy [Table 1] and what to use in further lines [Table 2] depends on several factors, such as the treatment previously received, clinical conditions of the patient, metastatic sites and other tumour characteristics. For this reason, the correct sequence treatment is not well defined.
 |
Table 1 Characteristics of Main Studies First- and Second-Line Treatment of mCRPC |
 |
Table 2 Characteristics of Main Studies of Other-Lines of mCRPC |
We aimed to perform a review of the literature on mCRPC treatment landscape, with insights into currently available drugs and innovative treatment options.
Chemotherapy
Taxanes – docetaxel and cabazitaxel – are the most active chemotherapy drugs currently used for the treatment of mCRPC.
Docetaxel
Docetaxel is a semisynthetic taxane with a high cytotoxic effect that inhibits microtubular depolymerization, leading to cell arrest in the G(2)M phase of the cell cycle, promoting a cascade of events that ultimately leads to apoptotic cell death.23
Two historical trials support the efficacy of docetaxel in mCRPC. SWOG 99–16 is a randomized Phase III trial that included 674 patients randomized to receive docetaxel and estramustine (D/E arm) or mitoxantrone and prednisone (M/P arm).5 The results showed a higher median overall survival (mOS) in patients treated with docetaxel and estramustine compared to those treated with mitoxantrone and prednisone (17.5 vs 15.6 months; HR for death 0.80).5 The median progression-free survival (mPFS) was longer in the D/E arm compared to the M/P arm (6.3 vs 3.2 months), with a higher rate of PSA decline of at least 50% vs 27%.5
TAX 327 is a randomized phase III trial that included 1006 men with mCRPC and compared docetaxel (given either every three weeks or weekly) plus daily prednisone to mitoxantrone plus prednisone.6 This study confirmed the results of the previous SWOG 99-16, with mOS of 18.9 months for patients treated with docetaxel administered every three weeks, compared to 16.5 months for patients treated with mitoxantrone.6
Cabazitaxel
Cabazitaxel is a microtubule inhibitor belonging to the taxanes family. In vitro studies showed that all taxanes have a certain cross resistance, but cabazitaxel showed the lowest rate of cross-resistance compared to paclitaxel and docetaxel.24 This important property justifies the use of cabazitaxel for the treatment of mCRPC previously treated with a docetaxel-containing treatment regimen.4
De Bono et al demonstrated the superiority of cabazitaxel plus prednisone compared to mitoxantrone plus prednisone in patients with mCRPC with evidence of disease progression during or after treatment with docetaxel (TROPIC trial).11 A total of 755 patients were randomly assigned to receive either 12 mg/mq mitoxantrone or 25 mg/mq cabazitaxel every 3 weeks, plus 10 mg oral prednisone daily.11 Median OS was 15.1 months in the cabazitaxel group and 12.7 months in the mitoxantrone group. Median PFS was 2.8 months and 1.4 months, respectively. Most common clinically significant adverse events were neutropenia and diarrhea, which are more frequent in cabazitaxel group.11
Another milestone in defining the correct therapeutic sequence of the mCRPC is the CARD trial, which evaluated the efficacy of cabazitaxel compared to ARATs (abiraterone acetate or enzalutamide) in patients with mCRPC who were previously treated with docetaxel and had progression within 12 months while receiving the alternative inhibitor (abiraterone or enzalutamide). A total of 255 patients underwent randomization. mOS was longer with cabazitaxel compared to ARATs (13.6 months vs 11.0 months). mPFS too was longer with cabazitaxel (4.4 vs 2.7 months with ARATs).12 Despite the results of the CARD study, the best treatment option in patients who have progressed in more than 12 months to an ARATs used as second line after docetaxel is still being discussed. However, based on the available evidence, even in this category of patients the preferred choice often remains cabazitaxel.
Platinum Chemotherapy
Platinum-based chemotherapy – cisplatin or carboplatin – has shown limited activity in molecular unselected prostate cancer.25 Taxane-platinum combinations have been demonstrated to be active in patients with mCRPC in multiple Phase 1 and 2 studies.26,27 The association of cabazitaxel with carboplatin may be an option of treatment for patients with specific features as aggressive variants mCRPC (ie, visceral metastases, low PSA and bulky disease, high lactate dehydrogenase [LDH], high carcinoembryonic antigen [CEA], lytic bone metastases, neuroendocrine histological type, or unfavorable genomics as defects in at least 2 of PTEN, TP53, and RB1).28 In a Phase I–II trial, the combination of cabazitaxel-carboplatin demonstrates a longer PFS of 2.8 months compared to cabazitaxel alone.29 In another study, 20 patients with mCRPC were treated with intravenous docetaxel, intravenous carboplatin and oral estramustine. The median OS was 11 months and prostate-specific antigen progression-free survival was 6.5 months.30
Androgen-Receptors Axis Targeted Therapies (ARATs)
The mechanism of development and growth of CRPC is linked to increasing levels of intra-tumoral androgen levels for an up-regulation of androgen biosynthesis enzymes.31 Also, an overexpression of androgen receptors (AR) and the appearance of mutated forms leads to an extension of the tumor.31 ARATs, as abiraterone acetate and enzalutamide, are a class of compounds approved for the treatments of mCRPC, with different mechanisms of action.
Abiraterone Acetate
Abiraterone acetate is an oral irreversible inhibitor of the 17α-hydroxylase/C17,20 lyase (CYP17A1), a steroidal enzyme involved in androgen biosynthesis. Blockade of CYP17A1 inhibits the conversion of 17-hydroxypregnenolone to dehydroepiandrosterone (DHEA), leading to reduction of serum levels of testosterone and other androgens.32 This blockade also results in an accumulation of upstream mineralocorticoids like 11-deoxycorticosterone leading to secondary hyperaldosteronism; for this reason, abiraterone needs to be co-administered with corticosteroids.32
Abiraterone acetate was first evaluated in patients affected by mCRPC progression after docetaxel in the COU-AA-301 phase III trial.13 A total of 1195 eligible patients were randomly assigned (ratio 2:1) to receive abiraterone acetate (1000 mg once daily) plus prednisone (5 mg twice daily) or placebo plus prednisone. Abiraterone showed statistically significant superiority over placebo in terms of mOS (15.8 vs 11.2 months, HR 0.74) and median radiologic progression-free survival (mrPFS; 5.6 vs 3.6 months, HR 0.66).13
A similar study was conducted by the same authors, evaluating abiraterone as a first-line treatment. COU-AA-302 is a randomized phase III trial that included 1088 patients with chemotherapy-naive mCRPC. Patients were randomized to receive either abiraterone acetate plus prednisone or placebo plus prednisone. At a median follow-up of more of 4 years, abiraterone acetate plus prednisone (AAP) significantly prolonged OS compared to placebo group (mOS 34.7 vs 30.3 months; HR 0.81).7
Enzalutamide
Enzalutamide is an oral androgen receptor signaling inhibitor with a high affinity for androgen receptor (AR). It acts at three distinct levels of the signaling pathway, preventing binding of testosterone-AR, nuclear translocation of activated receptors and association with DNA.33,34
As for abiraterone, enzalutamide was first evaluated in patients who progressed after chemotherapy. In the AFFIRM clinical trial, 1199 mCRPCs were randomized with a 2:1 ratio to receive enzalutamide or placebo.14 mOS was 18.4 months in the enzalutamide group vs 13.6 months in placebo group, with a decreased risk of death of about 37% (HR 0.63).14 Moreover, in a post-hoc analysis of the AFFIRM trial, enzalutamide demonstrated its superiority in patients with visceral (liver and/or lung) metastases in terms of mPFS, mOS and time to PSA progression.35
In the PREVAIL phase III trial, enzalutamide significantly improved clinical outcomes versus placebo in patients with chemotherapy-naïve mCRPC.8,36 At a final 5-yr survival analysis, enzalutamide reduced the hazard of death by 17% (HR 0.83), with a mOS of 36 months vs 31 months in the placebo group.8,9
Radionuclide Treatments
Radium-223
Bone metastases are common sites of disease for patients with mCRPC, resulting in a dysregulation in the bone resorption cycle, with abnormal bone formation.37
Radium-223 is a α-emitter radionuclide that selectively binds areas of increased metabolic activity and emits high-energy alpha particles with minimal penetration of surrounding tissue, including bone marrow, which minimizes myelosuppression37 These radiation induces a break of DNA-double strand, which is identified as principal mechanism of action of Radium-223.37
ALSYMPCA is a Phase III, randomized, double-blind clinical trial that randomized 921 patients affected by mCRPC, with at least two symptomatic bone metastases and no evidence of visceral metastases, to receive Radium-223 or placebo. Radium-223 was administered as six intravenous infusions every 4 weeks.15 Included patients must have received docetaxel as previous line of treatment, or they had to be ineligible or refusing chemotherapy. mOS with Radium-223 was 14.9 months compared with 11.3 months in the placebo arm (HR 0.70).15 Moreover, Radium-223 significantly prolonged time to first symptomatic skeletal event. The overall incidence of adverse events was consistently lower in the radium-223 group than in the placebo group for adverse events of all grades, grade 3 or 4 adverse events, and serious adverse events.15
The Phase 3 ERA 223 trial randomized patients with chemotherapy-naïve CRPC and asymptomatic or mildly symptomatic bone metastases to receive abiraterone with or without Radium-223.38 The study was unblinded prematurely after more fractures and deaths detected in the Radium-223 group than in the placebo arm, and it did not meet the primary endpoint of symptomatic skeletal event-free survival in the intention-to-treat population. Therefore, the use of the combination of radium-223 and abiraterone is not permitted. The European Medicines Agency’s Pharmacovigilance Risk Assessment Committee has restricted the use of Radium-223 to patients who have received at least two lines of systemic treatment for CRPC (abiraterone/enzalutamide and docetaxel) or who are ineligible for these treatments.39
Lu-PSMA-617
In recent years, there has been a growing interest in theragnostic techniques, such as the use of Prostate Specific Membrane Antigen (PSMA)-Targeted Radioligands for PCa. Theragnostic is a treatment strategy that combines therapeutics with diagnostics. It associates both a diagnostic test that identifies patients most likely to be helped or harmed by a new medication and targeted drug therapy based on the test results. Furthermore, theragnostic aims to monitor the response to the treatment, to increase drug efficacy and safety.40
PSMA is highly expressed in PCa cells, making PSMA a reliable tissue biomarker for PCa functional imaging. Several PSMA ligands for PET imaging are now being explored worldwide.
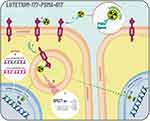
Lutetium-177 (177Lu)–PSMA-617 is a radioligand that delivers beta-particle radiation selectively to PSMA-positive cells and the surrounding microenvironment (Figure 1).21
ANZUP-1603 (TheraP trial) is a randomized Phase 2 trial that compared the activity and safety of 177Lu-PSMA-617 vs cabazitaxel in mCRPC.41 This trial demonstrated an increase in the percentage of patients with PSA reduction of more than 50% compared to baseline with 177Lu-PSMA-617, with a superiority also in terms of mPFS, Objective Response Rate (ORR), safety and patient reported outcomes (PROs).
A subsequent randomized phase III trial, the VISION trial, enrolled a total of 831 patients affected by mCRPC previously treated with at least one ARATs and one or two taxanes, and with PSMA-positive status as determined with the use of centrally read gallium-68 (68Ga)–labeled PSMA-11 (68Ga-PSMA-11) PET–CT imaging.21 These patients were randomly assigned to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for four to six cycles) or standard of care, excepted for chemotherapy, immunotherapy or radium-223.21 mOS and mrPFS were statistically longer in patients treated with 177Lu-PSMA-617 compared to control arm (mOS: 15.3 vs 11.3 months; mrPFS, 8.7 vs 3.4 months).21 Adverse events were documented with higher incidence in 177Lu-PSMA-617 group, but with not significantly worsening of quality of life.21
Based on these studies, 177Lu-PSMA-617 is currently considered as the treatment of choice in patients affected by mCRPC with progression after at least one line with taxanes and one line with ARATs.
Targeted Therapies
Poly-ADP-Ribose Polymerases (PARP) Inhibitors
Germline mutations in Homologous Recombination DNA repair (HRR) genes have been observed in approximately 10–15% of patients with metastatic PCa, while somatic mutations occur in 20–25% of these patients, with BRCA2 and ATM being the most frequently mutated.42 HRR genes are involved in transcriptional regulation and repair of double-strand DNA damage breaks (DSBs) in the DNA molecule.
Poly-ADP-ribose polymerases (PARPs) belong to a protein family that repair single-strand DNA breaks.43,44 PARP inhibitors (PARPi) are a new class of compounds developed for the treatment of different diseases with HRR deficiency, such as ovarian, breast and prostate cancer.45 The inhibition of PARP1 alone is not lethal for the cell, but when these pathways of repair are inefficient, the inhibition of PARP causes cell death (Figure 2).44 The first trial that led to the approval of a PARPi for PCa is PROfound, a trial that studied the role of olaparib in men with mCRPC who had disease progression during or after therapy with ARATs.16,46 Patients were divided into two cohorts. Cohort A included 245 patients with at least one alteration in BRCA1, BRCA2, or ATM, and cohort B included 142 patients with at least one alteration in any other prespecified genes.16 Patients in each cohort were randomly assigned to receive either olaparib or the physician’s choice of enzalutamide or abiraterone. In cohort A, the primary endpoint of imaging-based PFS was significantly longer in the olaparib group than in the control group (median, 7.4 months vs 3.6 months; HR for progression or death, 0.34; 95% confidence interval, 0.25 to 0.47; P < 0.001). Olaparib also had a benefit in terms of imaging-based progression-free survival in the overall population (cohorts A and B). Median OS in cohort A was 18.5 months with olaparib and 15.1 months with control therapy. In cohort B, the median OS was 14.1 months with olaparib and 11.5 months with control therapy.16 Based on the greater advantage seen in cohort A, European guidelines recommend the use of olaparib after new hormonal agents only for patients with mCRPC and alteration in BRCA1 or BRCA2,39 while the drug is approved for patients with pathogenic mutation (germline and/or somatic) in a homologous recombination repair gene (BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, or RAD54L) in the United States.28
Recent data suggest a combined antitumor effect when the PARP inhibitors are combined with next-generation hormonal agents such as abiraterone to treat mCRPC.
In the PROpel phase III trial, patients were randomized to receive olaparib plus abiraterone and prednisone/prednisolone or placebo plus abiraterone and prednisone/prednisolone in the first-line mCRPC setting, regardless of HRR status.47 The combination therapy significantly prolonged imaging-based progression-free survival (ibPFS) compared to abiraterone alone (median 24.8 vs 16.6 months, hazard ratio, 0.66; 95% confidence interval [CI], 0.54 to 0.81; P < 0.001).47 At the data cut-off, OS data were immature. Recently, the combination of olaparib and abiraterone has received approval in the European Union for the treatment of first-line mCRPC.
A trial conducted in the same setting, the MAGNITUDE phase III trial, evaluated the combination of abiraterone and prednisone/prednisolone plus niraparib or placebo as first-line therapy in patients affected by mCRPC with and without HRR gene alterations.48 Patients were divided into two cohorts based on the presence or absence of HRR mutations and then randomized to receive combination therapy or abiraterone alone. First results of MAGNITUDE were presented at the 2022 Genito-Urinary American Society of Clinical Oncology (GU ASCO) annual meeting and showed that niraparib + abiraterone acetate/prednisone significantly improved rPFS in the BRCA1/2 subgroup, reducing the risk of progression or death by 47% (16.6 vs 10.9 months) and in all HRR biomarker positive patients by and 26% (16.5 vs 13.7 months; HR 0.74, 95% CI 0.57–0.97). Contrary to the PROpel study, there was no evidence of benefit with the addition of the PARPi to abiraterone in patients without HRR alterations.
Other PARPi that showed a good antitumor activity in Phase II trials are Rucaparib and Talazoparib.
In the TRITON2 phase II trial, 115 patients with mCRPC and with a deleterious germline or somatic BRCA1, BRCA2 or ATM mutation, who progressed after ARATs and taxane therapy, received rucaparib 600 mg twice daily.17 Rucaparib demonstrated a good objective response rate (ORR per independent radiology review: 43.5%; per investigator assessment: 50.8%).17 Phase III results are needed.
Talazoparib is another PARP inhibitor under study. TALAPRO-1 is an open-label, phase II trial that included 128 mCRPC patients with HRR alteration, that had received one or two taxane-based chemotherapy regimens for metastatic disease, and progressed on enzalutamide or abiraterone, or both, mCRPC. Those patients received Talazoparib at dose of 1 mg per day.18 After a median follow-up of 16.4 months, the ORR was 29.8%.18
PI3K/AKT/mTOR Inhibitors
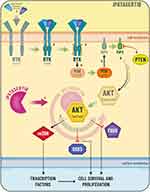
PI3K/AKT pathway and phosphatase and tensin homolog (PTEN) defects play a key role in prostate cancer and androgen signaling. Indeed, activation of PI3K signaling correlates with resistance to castration, cancer progression and poor outcomes.49 Inhibition of androgen receptor is associated with an increase phosphorylation of AKT and PTEN loss brings to decrease androgen receptor activity.49
Aberrantly active PI3K pathway has been described in 40% early PCa and 70% advanced PCa.49 Meanwhile, about 30% of primary and 60% of CRPC were found a defect of PTEN.50 Ipatasertib binds and inhibits the activity of AKT in an ATP-competitive manner, which may result in the inhibition of the PI3K/AKT signaling, tumor cell proliferation and the induction of tumor cell apoptosis (Figure 3).51
IPAtential150, a randomized phase III trial, studied the association of ipatasertib plus abiraterone acetate in 1101 patients. Patients were randomized to receive ipatasertib-abiraterone-prednisone or placebo-abiraterone-prednisone.19 Two groups were considered: PTEN-loss-by-immunohistochemistry group and intention-to-treat group. In the PTEN-loss group, the median radiological PFS was 16.5 months in the placebo-abiraterone group vs 18.5 months in the ipatasertib-abiraterone group (hazard ratio [HR] 0.77 [95% CI 0.61–0.98]; p = 0.034; significant at α=0.04). Meanwhile, in the intention-to-treat population median, PFS was 16.6 vs 19.2 months, respectively, without a statistically significant difference.19
Another AKT inhibitor, capivasertib, was investigated in a randomized, phase II trial, called ProCAID.20 In this trial, 150 mCRPC patients received up to 10 cycles of docetaxel and prednisolone and were randomly assigned to receive capivasertib or placebo.20. The addition of capivasertib to docetaxel did not show a benefit in composite PFS (the primary end point that included prostate-specific antigen progression events). The median OS (secondary endpoint) was 10.88 months longer in patients receiving capivasertib. However, this result will require validation in future prospective trials to address potential for bias.
Capivasertib was also studied in combination with enzalutamide in Phase I trial.52 mCRPC patients previously treated with abiraterone and/or enzalutamide, received escalating dose (320 mg, 400 mg and 480 mg b.i.d.) of capivasertib and enzalutamide (160 mg).52 The primary end-points were safety and tolerability, maximum tolerable dose and recommended phase II dose. The recommended phase II dose identified for capivasertib was 400 mg b.i.d. with 1/6 patients experiencing a dose limiting toxicity (maculopapular rash) at this level. The most common grade ≥3 adverse events were hyperglycemia (26.7%) and rash (20%).52
Other Targeted Therapies
Adenocarcinoma is the most common histological type of PCa, but an aggressive variant, neuroendocrine prostate cancer (NEPC), may develop de novo or as a progression of mCRPC.53 N-myc is involved in the progression of NEPC.53 N-myc belongs to the family of MYC proteins that bind the DNA. This family covers many functions such as gene transcription modulators of cell growth, cell cycle, differentiation, apoptosis, angiogenesis, metabolism, DNA repair, protein translation, and immune response.54 Alisertib may inhibit the N-myc pathway signaling, preventing the bond between N-myc and its stabilizing factor Aurora A.55 In a phase II trial, Alisertib, an Aurora Kinase A inhibitor, was studied in 60 men with metastatic PCa and at least one of NEPC histology, ≥50% NEPC marker expression, new liver metastases without PSA progression or elevated serum NEPC markers.55 The primary endpoint (radiologic PFS) was not met (13.4%), and median OS was 9.5 months with no significant differences between NEPC and adenocarcinoma (9.5 vs 8.6 months, respectively).55 On the other hand, exceptional responders were identified, including complete resolution of liver metastases and prolonged stable disease, with tumors suggestive of N-myc and Aurora-A overactivity.
Cabozantinib, a tyrosine-kinase inhibitor, in addition to atezolizumab, a PD-L1 inhibitor was evaluated in a cohort of the phase Ib COSMIC-021 study.56 A total of 132 men with mCRPC progressed on ARATs treatment were enrolled. The combination of cabozantinib plus atezolizumab showed promising anti-tumor activity and manageable safety profile. The objective response rate was 23% with three complete responses and 28 partial responses.56
Immunotherapy
Sipuleucel-T
The prostatic acid phosphatase (PAP) is a glycoprotein with enzymatic activity whose levels increase in advanced PC.57 Sipuleucel-T is an autologous cancer “vaccine”.58,59 To prepare Sipuleucel, the white blood cell fraction containing antigen-presenting cells from each patient is collected.28 After that, the cells are exposed to the prostatic acid phosphatase-granulocyte macrophage colony stimulating factor (PAP-GM-CSF recombinant fusion protein) and subsequently reinfused. The resulting compound, infused in the patients, stimulates an antigen-presentation activity with recruitment of immunity cells against PCa.58
The use and efficacy of Sipuleucel-T was evaluated in a double-blind phase 3 trial that randomized 512 patients with minimally symptomatic or asymptomatic mCRPC to receive Sipuleucel-T or placebo. The median OS was 25.8 months in Sipuleucel-T arm vs 21.7 months in placebo arm, with a 22% death risk reduction (HR: 0.78; 95% CI: 0.61–0.98, P = 0.03).10
Nowadays, Sipuleucel-T is not available in Europe, but only in the USA as treatment of mCRPC, and only in specific cases: asymptomatic or minimally symptomatic patients, no liver metastases, life expectation >6 months, ECOG performance status 0–1.4
Novel Immunotherapy
In recent years, immunotherapy has become an important standard of care for several malignancies. However, in PCa, the effectiveness of immunotherapy seems to be limited compared to other cancers, such as non-small-cell lung cancer, renal cell carcinoma and melanoma. The cause may be related to the strong immunosuppressive PCa microenvironment, lower infiltration of T-cells in prostate tumors, and lower mutation burden.60 Therefore, PCa is generally considered as immunologically “cold tumor”. However, a subset of PCa patients, such as those carrying CDK12 mutations, high microsatellite instability (MSI) and mismatch repair-deficient (dMMR), may still benefit from an immunotherapy approach.60 Indeed, these tumor characteristics have been related to higher responses to immune checkpoint inhibitors (ICIs).60
Immune checkpoints (ICs) are proteins involved in the complex mechanism of self-recognition and prevention of the immune response against self-tissues, preventing their damage.60 While IC protects host tissue from autoimmune response, tumor cells exploit these mechanisms to hide from immune cells and escape to immune surveillance.61 CTLA-4 is an immune checkpoint receptor, expressed by activated T-cells, to send out inhibitory signal to T-cells and regulatory T-cells to control cytotoxic T-cell activation.60
Programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) are immune checkpoints playing an important role in inhibiting immune responses and promoting self-tolerance through modulating the activity of T-cells, activating or inhibiting apoptosis of regulatory T cells.60 Several types of tumors showed an incrementation level of PD-1/PD-L1 and PD-L1 are expressed in a subgroup of patients with primary prostate cancer, with increased levels in aggressive and metastatic disease.61
Anti-CTLA-4 or anti-PD-1/PD-L1 monotherapy failed to show a significant benefit in the treatment of unselected mCRPC patients.62
Pembrolizumab is a humanized, anti–PD-1 monoclonal antibody. In the multicohort, phase II KEYNOTE-199 study, 258 mCRPC patients were previously treated with docetaxel and one or more ARATs were included in 3 cohorts. 133 patients in cohort 1 and 66 patients in cohort 2 were enrolled with RECIST-measurable PD-L1 positive and PD-L1 negative, respectively.22 Fifty-nine patients in cohort 3 were enrolled with predominance of bone metastases, regardless of PD-L1 expression.22 Objective response rate was 5% (95% CI, 2% to 11%) in cohort 1 and 3% (95% CI, <1% to 11%) in cohort 2. Median duration of response was not reached (range, 1.9 to ≥21.8 months) and 10.6 months (range, 4.4 to 16.8 months), respectively. Median OS was higher in the cohort 3 (14.1 months) compared to cohorts 1 and 2 (9.5 and 7.9 months, respectively).22 The Food and Drug Administration (FDA) approved pembrolizumab for the treatment of mCRPC only in patients with high levels of MSI-H and dMMR.63 Interestingly, however, in KEYNOTE-199 there were patients who responded to Pembrolizumab with no MSI-H.22
The association of anti-CTLA-4 (Ipilimumab) and anti-PD-1 (nivolumab) was tested in phase II CHECKMATE650 trial.62 Ninety patients with mCRPC were stratified into two cohorts: pre-chemotherapy cohort and post-chemotherapy cohort.62 In cohorts 1 (pre-chemotherapy) and 2 (post-chemotherapy), objective response rate was 25% and 10%, and median OS was 19.0 and 15.2 months, respectively. Four patients, two per cohort, had a complete response.62 However, several grade 3–4 toxicities were reported; thus, dose/schedule optimization is needed to preserve safety.
The Phase 1b/2 KEYNOTE-365 Cohort B Study evaluates the efficacy and safety of pembrolizumab plus docetaxel and prednisone in patients with mCRPC who were chemotherapy naïve and who experienced failure of or were intolerant to ≥4 week of abiraterone or enzalutamide for mCRPC.64 Notwithstanding the limitations of the single-arm design and small sample size, the results demonstrated antitumor activity with confirmed PSA response rate of 34% and confirmed ORR (RECIST v1.1) of 23%.64
Another experimental study is KEYNOTE-046, a phase I/II, open-label, multicenter trial in which ADXS31142 was administered alone or in association with pembrolizumab to mCRPC patients who have progressed after 2 or fewer prior systemic treatment regimens.65 ADXS31-142 is an immunotherapy that uses live-attenuated Listeria monocytogenes able to produce an antigen adjuvant fusion protein (tLLO-PSA) formed by a fragment of the listeriolysin toxin (tLLO) and prostate-specific antigen (PSA).65 Among the 37 RECIST-evaluable patients, no objective responses were detected. Promising OS benefit was observed in subset patients previously treated with docetaxel and with visceral metastasis, which needs to be confirmed in future trials.65
Bone Metastases and Preventing Treatment
Bone metastases are a common cause of morbidity in patients with mCRPC.4,66 Complications include pathological fractures, spinal cord compression, surgery or radiotherapy (RT) for bone pain or a change in anticancer treatment of bone pain, defined as skeletal-related events (SREs).4,67 Many compounds have been tested for the prevention of SRE in patients with mCRPC.
Zoledronic Acid
Zoledronic acid, a bisphosphonate, was initially investigated when only docetaxel was available for mCRPC treatment. In a randomized trial, 643 patients with mCRPC and bone metastases were randomly assigned to receive 4-mg zoledronic acid, 8-mg zoledronic acid (subsequently reduced to 4 mg due to renal toxicity) or placebo every 3 weeks for 15 months.68 Fewer patients in the 4-mg zoledronic acid group than in the placebo group had at least one SRE (38% versus 49%, P = 0.028), and the median time to the first SRE was approximately 6 months longer in the 4-mg zoledronic acid group than in the placebo group (488 days versus 321 days; P = 0.009).67 Zoledronic acid did not show a benefit in median time to cancer progression and survival compared to placebo.66 Considering the ability to prolong time to first SREs, zoledronic acid is recommended by current guidelines in patients with bone metastases and mCRPC at risk for clinically significant SREs.39
Rank Ligand Inhibitors
Receptor Activator of Nuclear Factor κ B ligand (RANKL) has a significant role in bone resorption in normal and pathological states. It is expressed on the membrane of different cells, such as osteoblasts, osteocytes and T-cells, or as soluble forms.69 RANKL is the main driver of osteoclast formation, function, and survival, by binding to its receptor, RANK, expressed on osteoclasts and their precursor membrane.69 In prostate cancer, significant interactions between tumor cells and bone cells exist. In the bone microenvironment, tumor cells produce growth factors that stimulate stromal cells and osteoblasts to express RANKL on their membrane, with consequent activation of osteoclasts, increased bone turnover and release of factors potentially promoting the development of bone metastases.70 Denosumab is a human monoclonal antibody, which is administered subcutaneously, that specifically inhibits RANKL.
In a phase III study, 1904 patients with CRPC were randomized to receive 120 mg denosumab plus placebo or 4 mg zoledronic acid plus placebo, every 4 weeks.71 The median time to first SRE was 20.7 months with denosumab compared to 17.1 months with zoledronic acid (HR 0.82, 95% CI 0.71–0.95; p = 0.0002 for non-inferiority; p = 0.008 for superiority).71 Hypocalcemia occurred more frequently in the denosumab group than in the zoledronic acid group (13% versus 6%), and even if osteonecrosis of the jaw occurred infrequently in both arms, the rate was slightly higher for denosumab than zoledronic acid (2.3% versus 1.3%).71 Considering these results, denosumab is recommended by current guidelines in patients with bone metastases and mCRPC at risk for clinically significant SREs.39
Predictive Biomarkers
The natural history of PCa is characterized by progression from a hormone sensitive phenotype to a castration resistant one. Furthermore, a subset of PCa might differentiate into neuroendocrine phenotype (NEPCs) with AR signaling loss and androgen-independence. Understanding the mechanism of castration resistance and neuroendocrine differentiation and exploring novel targets in order to develop personalized treatment strategies are major challenges in mCRPC.
The ability of mCRPC to progress and become more aggressive has been associated with specific molecular features, such as defects in at least two of the three tumor suppressor gene (PTEN, TP53, RB1), defined as aggressive-variant prostate cancer molecular signature (AVPC-MS).29 In post-hoc analyses of a randomized phase 1–2 trial comparing the combination of carboplatin-cabazitaxel to cabazitaxel alone in mCRPC, the presence of AVPC-MS as assessed by immunohistochemistry (aberrant results for at least two of TP53, RB1, and PTEN) or circulating tumor DNA (ctDNA) (genomic alterations in at least two of TP53, RB1, and PTEN) was associated with clinically meaningful benefit in PFS and OS with the addition of carboplatin to cabazitaxel. On the contrary, there was no advantage for the combination therapy compared to cabazitaxel monotherapy in AVPC-MS-negative tumors.29
Androgen receptor splice variant 7 (AR-V7) is an androgen receptor mutation resulting in the truncation of the ligand-binding domain with a consequent overactivation of receptor, regardless of androgen ligand binding.72 AR-V7 mRNA detected in circulating tumor cells (CTCs) may be used as a predictive biomarker.72 In a study by Antonarakis et al, the impact of AR-V7 in mCRPC patients receiving ARATs (enzalutamide or abiraterone) was investigated.73 Worse clinical outcomes (lower PSA response, shorter PSA PFS, clinical- or radiographic-PFS and OS) in the AR-V7 positive group were found. A subsequent study by Antonarakis et al showed that the detection of AR-V7 in CTCs was not associated with primary resistance to taxane chemotherapy in mCRPC. This study suggested that in AR-V7-positive tumors, taxanes seemed to be more efficacious than enzalutamide or abiraterone, while in AR-V7-negative tumors, taxanes and enzalutamide or abiraterone may have comparable outcomes.74
Moreover, the prevalence of AR-V7 seemed to be influenced by a previous treatment with abiraterone or enzalutamide, with higher cases of AR-V7 positivity in patients previously treated with ARATs.74 This result was corroborated by Sharp et al who demonstrated the expression of AR-V7 in less than 1% of primary PCa and a significant increase after treatment with ARATs.75
Overall, these results suggest the research of AR-V7 splice mutation in PCa as a potential strategy to support choice of treatment between ARATs and taxanes, suggesting AR-V7 detection in CTCs a negative predictor of response to ARATs. However, this strategy is not yet approved for clinical practice and still needs large scale prospective validation.
Liquid biopsy represents a new diagnostic method that is emerging alongside the better known and traditional tissue biopsy, and it is gaining great interest in mCRPC. Liquid biopsy may have different clinical applications such as early diagnosis, prognosis, assessment of treatment response, and mechanisms of drug resistance. Liquid biopsy consists in withdrawal from blood samples of CTCs, circulating free DNA (cfDNA), circulating tumor RNA (ctRNA), or extracellular vesicles (EVs) containing tumor materials.76 CTCs are tumor cells deriving from a primary or metastatic tumor into the peripheral blood and lymphatic system. Meanwhile, the tumor DNA can be found in the peripheral blood after cell death (necrosis and apoptosis).77
The presence of cfDNA in plasma is usually higher in cancer patients than in non-cancer patients, with average concentrations of 19 ng/mL in first-line mCRPC patients.78 cfDNA analysis represents an important test potentially allowing disease molecular stratification, evaluation of treatment response and the study of emerging resistant clones. A phase II trial (TOPARP-A) showed a reduction ≥50% in cfDNA concentration after 8-weeks with olaparib treatment, independently associated with longer OS.79. Quantity variation of cfDNA during treatment is closely related to tumor responses and may be important for detection of mechanisms of resistance. cfDNA can be more sensitive and specific than PSA, particularly when the PSA level is too low or there is early rising PSA.80
Other promising biomarkers for PC are circulating free non-coding RNAs (long non-coding RNA, micro-RNA), due to their high stability and important role in oncogenesis and spread of metastases.76,81 Mitchell et al first demonstrated the presence of PC-derived miRNA from PCa cells into the bloodstream.82 Several subsequent studies have correlated miRNA with disease stage, aggressiveness, and response to therapy. In their study, Urabe et al identified a diagnostic model constructed with the combined use of 2 miRNA (miR-17-3p and miR-1185-2-3p) as biomarkers to identify PCa, with sensitivity and specificity of 90%.83 In another study, the authors presented a pairwise model of five circulating miRNAs with sensitivity 99% and specificity 100% in early detection of PCa.84 In their study, Al Qatati et al demonstrated a correlation between the presence of high-risk PCa and increasing plasma levels of miR-16 (p = 0.002), miR-148a (p = 0.006) and miR-195 (p = 0.006).85 Further studies are needed to evaluate the role of miRNA as diagnostic and prognostic biomarkers.
Prostate Cancer Antigen 3 (PCA3) is a non-coding RNA, overexpressed only in PCa, that could be potentially used as a biomarker. Rodriguez et al in their meta-analysis compared five studies in which authors dosed urinary PCA3 after prostate massage in patients with histologically confirmed PCa, obtaining a sensitivity of 0.69 (95% CI: 0.61–0.75) and specificity of 0.65 (95% CI: 0.553–0.733). They concluded that the data obtained are not yet sufficient to use PCA3 as gold standard in the diagnosis of PCa but have the potential to reduce the unnecessary biopsies.86
PCA3 was also evaluated in a study from Merdan et al, combined with TMPRSS2-ERG, a specific fusion-gene, expressed in 50% of PCa. The use of PSA alone was compared with the use of PCA3 and TMPRSS2-ERG (T2/ERG) scores to determine whether patient should repeat biopsy. Authors demonstrated that the use of those two scores would have avoided 55.4–64.7% of repeat biopsies, without affecting 10-year survival.87
An additional mechanism for intercellular communication, involved in multiple processes, is the extracellular vesicles (EVs), a heterogeneous group of cell-derived membranous structures that includes exosomes, microvesicles and apoptotic bodies. EVs play an important role in tumorogenesis, spread of metastasis and tissue invasion, thanks to their protective double-layer membranes.88 In a recent trial, a urinary exosome gene expression assay was used for stratifying patients with PCa Gleason group (GG) ≥2 from GG = 1, in men aged ≥50 years with a PSA gray zone (PSA 2–10 ng/mL), scheduled for initial biopsy. Authors determined a score of >15.6 could be used to discriminate patients as high-risk.89,90
The importance of homologous recombination repair gene alterations as predictive biomarkers for PARP inhibition has been previously discussed.
PTEN loss, which is detected in 40–50% of mCRPC patients, increases AKT signaling, boosting tumor growth and leading to worse outcomes of androgen-receptor pathway blocking91 In the IPATential150 trial, the addition of ipatasertib, a competitive ATPase inhibitor of AKT, to abiraterone acetate resulted in longer PFS compared to abiraterone acetate alone in patients with PTEN loss by immunohistochemistry, supporting PTEN loss as a potential predictive factor of response to AKT inhibition strategy.19
Conclusion
In the last few years, the treatment landscape of mCRPC has drastically changed with the introduction of new treatments with various mechanisms, including ARATs, PARP inhibitors, and theragnostics. However, mCRPC still represents a lethal disease and newer treatment strategies aiming at durable response are highly needed. Moreover, the search of predictive biomarkers should be prioritized to allow tailored treatment strategies for patients with mCRPC.
Acknowledgement
We thank Dr Marco Mascitti for his fundamental contribution in the realization of the figures.
Disclosure
Dr Laura Marandino reports travel expenses from Janssen, personal fees for speaker compensation from Merck and Gilead, and research funding from AstraZeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
2. Bandini M, Pompe RS, Marchioni M, et al. Radical prostatectomy or radiotherapy reduce prostate cancer mortality in elderly patients: a population-based propensity score adjusted analysis. World J Urol. 2018;36:7–13. doi:10.1007/s00345-017-2102-9
3. Marchioni M, Di Nicola M, Primiceri G, et al. New antiandrogen compounds compared to docetaxel for metastatic hormone sensitive prostate cancer: results from a network meta-analysis. J Urol. 2020;203:751–759. doi:10.1097/JU.0000000000000636
4. Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–282. doi:10.1016/j.eururo.2020.09.046
5. Petrylak DP, Tangen CM, Hussain MHA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi:10.1056/NEJMoa041318
6. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi:10.1056/NEJMoa040720
7. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi:10.1016/S1470-2045(14)71205-7
8. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–154. doi:10.1016/j.eururo.2016.07.032
9. Armstrong AJ, Lin P, Tombal B, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol. 2020;78:347–357. doi:10.1016/j.eururo.2020.04.061
10. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi:10.1056/NEJMoa1001294
11. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi:10.1016/S0140-6736(10)61389-X
12. de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–2518. doi:10.1056/NEJMoa1911206
13. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi:10.1016/S1470-2045(12)70379-0
14. Nadal R, Taplin M-E, Bellmunt J. Enzalutamide for the treatment of prostate cancer: results and implications of the AFFIRM trial. Future Oncol. 2014;10:351–362. doi:10.2217/fon.13.275
15. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–223. doi:10.1056/NEJMoa1213755
16. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–2102. doi:10.1056/NEJMoa1911440
17. Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38:3763–3772. doi:10.1200/JCO.20.01035
18. De Bono JS, Mehra N, Higano CS, et al. TALAPRO-1: phase II study of talazoparib (TALA) in patients (pts) with DNA damage repair alterations (DDRm) and metastatic castration-resistant prostate cancer (mCRPC). JCO. 2021;39:93. doi:10.1200/JCO.2021.39.6_suppl.93
19. Sweeney C, Bracarda S, Sternberg CN, et al. Ipatasertib plus Abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398:131–142. doi:10.1016/S0140-6736(21)00580-8
20. Crabb SJ, Griffiths G, Marwood E, et al. Pan-AKT inhibitor capivasertib with docetaxel and prednisolone in metastatic castration-resistant prostate cancer: a randomized, placebo-controlled phase II trial (ProCAID). JCO. 2021;39:190–201. doi:10.1200/JCO.20.01576
21. Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi:10.1056/NEJMoa2107322
22. Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38:395–405. doi:10.1200/JCO.19.01638
23. Pienta KJ. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin Oncol. 2001;28:3–7. doi:10.1016/S0093-7754(01)90148-4
24. Duran GE, Wang YC, Francisco EB, et al. Mechanisms of resistance to cabazitaxel. Mol Cancer Ther. 2015;14:193–201. doi:10.1158/1535-7163.MCT-14-0155
25. Hager S, Ackermann CJ, Joerger M, Gillessen S, Omlin A. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol. 2016;27:975–984. doi:10.1093/annonc/mdw156
26. Regan MM, O’Donnell EK, Kelly WK, et al. Efficacy of carboplatin-taxane combinations in the management of castration-resistant prostate cancer: a pooled analysis of seven prospective clinical trials. Ann Oncol. 2010;21:312–318. doi:10.1093/annonc/mdp308
27. Ross RW, Beer TM, Jacobus S, et al. A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel. Cancer. 2008;112:521–526. doi:10.1002/cncr.23195
28. Schaeffer EM, Srinivas S, Adra N, et al. NCCN guidelines® insights: prostate cancer, version 1.2023. J Natl Compr Canc Netw. 2022;20:1288–1298. doi:10.6004/jnccn.2022.0063
29. Corn PG, Heath EI, Zurita A, et al. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1-2 trial. Lancet Oncol. 2019;20:1432–1443. doi:10.1016/S1470-2045(19)30408-5
30. Hikita K, Honda M, Shimizu R, et al. Efficacy of combination chemotherapy with docetaxel, estramustine and carboplatin in men with castration-resistant prostate cancer. Cancer Diagn Progn. 2021;1:451–457. doi:10.21873/cdp.10060
31. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi:10.1056/NEJMoa1014618
32. O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor Abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004;90:2317–2325. doi:10.1038/sj.bjc.6601879
33. Pal SK, Stein CA, Sartor O. Enzalutamide for the treatment of prostate cancer. Expert Opin Pharmacother. 2013;14:679–685. doi:10.1517/14656566.2013.775251
34. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi:10.1126/science.1168175
35. Loriot Y, Fizazi K, de Bono JS, Forer D, Hirmand M, Scher HI. Enzalutamide in castration-resistant prostate cancer patients with visceral disease in the liver and/or lung: outcomes from the randomized controlled phase 3 AFFIRM trial. Cancer. 2017;123:253–262. doi:10.1002/cncr.30336
36. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi:10.1056/NEJMoa1405095
37. Morris MJ, Corey E, Guise TA, et al. Radium-223 mechanism of action: implications for use in treatment combinations. Nat Rev Urol. 2019;16:745–756. doi:10.1038/s41585-019-0251-x
38. Smith M, Parker C, Saad F, et al. Addition of radium-223 to Abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:408–419. doi:10.1016/S1470-2045(18)30860-X
39. Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2020;31:1119–1134. doi:10.1016/j.annonc.2020.06.011
40. Pene F, Courtine E, Cariou A, Mira J-P. Toward theragnostics. Crit Care Med. 2009;37:S50–58. doi:10.1097/CCM.0b013e3181921349
41. Hofman MS, Emmett L, Violet J, et al. TheraP: a randomized phase 2 trial of 177 Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019;124:5–13. doi:10.1111/bju.14876
42. Congregado B, Rivero I, Osmán I, Sáez C, Medina López R. PARP inhibitors: a new horizon for patients with prostate cancer. Biomedicines. 2022;10:1416. doi:10.3390/biomedicines10061416
43. Mateo J, Lord CJ, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30:1437–1447. doi:10.1093/annonc/mdz192
44. Cleary JM, Aguirre AJ, Shapiro GI, D’Andrea AD. Biomarker-guided development of DNA repair inhibitors. Mol Cell. 2020;78:1070–1085. doi:10.1016/j.molcel.2020.04.035
45. Dan R, Van Allen EM, Y-M W, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi:10.1016/j.cell.2015.05.001
46. Hussain M, Mateo J, Fizazi K, et al. Survival with olaparib in metastatic castration-resistant prostate cancer. N Engl J Med. 2020;383:2345–2357. doi:10.1056/NEJMoa2022485
47. Saad F, Armstrong AJ, Thiery-Vuillemin A, et al. PROpel: phase III trial of olaparib (ola) and Abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). JCO. 2022;40:11. doi:10.1200/JCO.2022.40.6_suppl.011
48. Chi KN, Rathkopf DE, Smith MR, et al. Phase 3 MAGNITUDE study: first results of niraparib (NIRA) with Abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. JCO. 2022;40:12.
49. Braglia L, Zavatti M, Vinceti M, Martelli AM, Marmiroli S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: still a potential druggable target? Biochim Biophys Acta Mol Cell Res. 2020;1867:118731. doi:10.1016/j.bbamcr.2020.118731
50. Batra A, Winquist E. Emerging cell cycle inhibitors for treating metastatic castration-resistant prostate cancer. Expert Opin Emerg Drugs. 2018;23:271–282. doi:10.1080/14728214.2018.1547707
51. Zhang T, George DJ, Armstrong AJ. Precision medicine approaches when prostate cancer akts up. Clin Cancer Res. 2019;25:901–903. doi:10.1158/1078-0432.CCR-18-2491
52. Kolinsky MP, Rescigno P, Bianchini D, et al. A phase I dose-escalation study of enzalutamide in combination with the AKT inhibitor AZD5363 (capivasertib) in patients with metastatic castration-resistant prostate cancer. Ann Oncol. 2020;31:619–625. doi:10.1016/j.annonc.2020.01.074
53. Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–495. doi:10.1158/2159-8290.CD-11-0130
54. Duffy MJ, O’Grady S, Tang M, Crown J. MYC as a target for cancer treatment. Cancer Treat Rev. 2021;94:102154. doi:10.1016/j.ctrv.2021.102154
55. Beltran H, Oromendia C, Danila DC, et al. A phase II trial of the aurora kinase A inhibitor alisertib for patients with castration-resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin Cancer Res. 2019;25:43–51. doi:10.1158/1078-0432.CCR-18-1912
56. Agarwal N, McGregor B, Maughan BL, et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results from an expansion cohort of a multicentre, open-label, phase 1b trial (COSMIC-021). Lancet Oncol. 2022;23:899–909. doi:10.1016/S1470-2045(22)00278-9
57. Alpert E, Akhavan A, Gruzman A, et al. Multifunctionality of prostatic acid phosphatase in prostate cancer pathogenesis. Biosci Rep. 2021;41:BSR20211646. doi:10.1042/BSR20211646
58. Madan RA, Antonarakis ES, Drake CG, et al. Putting the pieces together: completing the mechanism of action jigsaw for sipuleucel-T. J Natl Cancer Inst. 2020;112:562–573. doi:10.1093/jnci/djaa021
59. Handy CE, Antonarakis ES. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 2018;14:907–917. doi:10.2217/fon-2017-0531
60. Fujii T, Naing A, Rolfo C, Hajjar J. Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2018;130:108–120. doi:10.1016/j.critrevonc.2018.07.010
61. Barbosa AM, Gomes-Gonçalves A, Castro AG, Torrado E. Immune system efficiency in cancer and the microbiota influence. Pathobiology. 2021;88:170–186. doi:10.1159/000512326
62. Sharma P, Pachynski RK, Narayan V, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell. 2020;38:489–499.e3. doi:10.1016/j.ccell.2020.08.007
63. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi:10.1200/JCO.19.02105
64. Yu EY, Kolinsky MP, Berry WR, et al. Pembrolizumab plus docetaxel and prednisone in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort B study. Eur Urol. 2022;82:22–30. doi:10.1016/j.eururo.2022.02.023
65. Stein MN, Fong L, Tutrone R, et al. ADXS31142 immunotherapy ± pembrolizumab treatment for metastatic castration-resistant prostate cancer: open-label phase I/II KEYNOTE-046 study. Oncologist. 2022;27:453–461. doi:10.1093/oncolo/oyac048
66. Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177–183. doi:10.1038/pcan.2011.7
67. Saad F, Gleason DM, Murray R, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi:10.1093/jnci/djh141
68. Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–1468. doi:10.1093/jnci/94.19.1458
69. Blair JM, Zheng Y, Dunstan CR. RANK ligand. Int J Biochem Cell Biol. 2007;39:1077–1081. doi:10.1016/j.biocel.2006.11.008
70. Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi:10.1056/NEJMra030831
71. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi:10.1016/S0140-6736(10)62344-6
72. Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. 2016;19:231–241. doi:10.1038/pcan.2016.17
73. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and Abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi:10.1056/NEJMoa1315815
74. Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi:10.1001/jamaoncol.2015.1341
75. Sharp A, Coleman I, Yuan W, et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129:192–208. doi:10.1172/JCI122819
76. Wang Y, Wang Z, Gang X, Wang G. Liquid biopsy in prostate cancer: current status and future challenges of clinical application. Aging Male. 2021;24:58–71. doi:10.1080/13685538.2021.1944085
77. Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665.
78. Conteduca V, Wetterskog D, Sharabiani MTA, et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or Abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Ann Oncol. 2017;28:1508–1516. doi:10.1093/annonc/mdx155
79. Goodall J, Mateo J, Yuan W, et al. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017;7:1006–1017. doi:10.1158/2159-8290.CD-17-0261
80. González-Billalabeitia E, Conteduca V, Wetterskog D, Jayaram A, Attard G. Circulating tumor DNA in advanced prostate cancer: transitioning from discovery to a clinically implemented test. Prostate Cancer Prostatic Dis. 2019;22:195–205. doi:10.1038/s41391-018-0098-x
81. Szilágyi M, Pös O, Márton É, et al. Circulating cell-free nucleic acids: main characteristics and clinical application. Int J Mol Sci. 2020;21:6827. doi:10.3390/ijms21186827
82. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi:10.1073/pnas.0804549105
83. Urabe F, Matsuzaki J, Yamamoto Y, et al. Large-scale circulating microRNA profiling for the liquid biopsy of prostate cancer. Clin Cancer Res. 2019;25:3016–3025. doi:10.1158/1078-0432.CCR-18-2849
84. Liu H-P, Lai H-M, Guo Z. Prostate cancer early diagnosis: circulating microRNA pairs potentially beyond single microRNAs upon 1231 serum samples. Brief Bioinform. 2021;22:bbaa111. doi:10.1093/bib/bbaa111
85. Al-Qatati A, Akrong C, Stevic I, et al. Plasma microRNA signature is associated with risk stratification in prostate cancer patients. Int J Cancer. 2017;141:1231–1239. doi:10.1002/ijc.30815
86. Rodríguez SVM, García-Perdomo HA. Diagnostic accuracy of prostate cancer antigen 3 (PCA3) prior to first prostate biopsy: a systematic review and meta-analysis. Can Urol Assoc J. 2020;14:E214–E219. doi:10.5489/cuaj.6008
87. Merdan S, Tomlins SA, Barnett CL, et al. Assessment of long-term outcomes associated with urinary prostate cancer antigen 3 and TMPRSS2:ERG gene fusion at repeat biopsy. Cancer. 2015;121:4071–4079. doi:10.1002/cncr.29611
88. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi:10.1038/nrm.2017.125
89. McKiernan J, Donovan MJ, Margolis E, et al. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2–10ng/mL at initial biopsy. Eur Urol. 2018;74:731–738. doi:10.1016/j.eururo.2018.08.019
90. Margolis E, Brown G, Partin A, et al. Predicting high-grade prostate cancer at initial biopsy: clinical performance of the ExoDx (EPI) Prostate Intelliscore test in three independent prospective studies. Prostate Cancer Prostatic Dis. 2022;25:296–301. doi:10.1038/s41391-021-00456-8
91. Ferraldeschi R, Nava Rodrigues D, Riisnaes R, et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with Abiraterone acetate. Eur Urol. 2015;67:795–802. doi:10.1016/j.eururo.2014.10.027
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.