Back to Journals » International Medical Case Reports Journal » Volume 17
Merkel Cell Carcinoma Masquerading Clinically as a Cyst in a Young Patient
Authors Ashby HE, Jones GN, Leedhanachoke O, Jen P, Helphenstine N, Al Akhrass F
Received 13 November 2023
Accepted for publication 21 March 2024
Published 4 April 2024 Volume 2024:17 Pages 289—293
DOI https://doi.org/10.2147/IMCRJ.S449543
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xudong Zhu
Harty Elouise Ashby,1 Grayson N Jones,1 Oon Leedhanachoke,2 Phillip Jen,3 Noah Helphenstine,3 Fadi Al Akhrass4
1Department of Pathology, Pikeville Medical Center, Pikeville, KY, USA; 2Department of General Surgery, Pikeville Medical Center, Pikeville, KY, USA; 3Department of BioMedical Science, University of Pikeville, Pikeville, KY, USA; 4Department of Infectious Diseases, Pikeville Medical Center, Pikeville, KY, USA
Correspondence: Phillip Jen, Department of BioMedical Science, University of Pikeville, 147 Sycamore Street, Pikeville, KY, 41501, USA, Tel +1 704 488 8258, Email [email protected]
Abstract: Merkel cell carcinoma (MCC) is an extremely rare and aggressive tumor. Here we report an unusual MCC that manifested as an abruptly enlarging, painful skin lesion over the right antecubital fossa and masqueraded as an epidermal cyst in a 42-year-old male. The lesion was surgically excised and subjected to histopathologic and immunohistochemical examinations. The subsequent analysis allowed for the diagnosis of MCC. Clinicians should always be cognizant of MCC, which can be easily misdiagnosed. Early diagnosis and appropriate treatment are keys to improving the survival rates of MCC patients.
Keywords: Merkel cell carcinoma, histopathologic, immunohistochemical, polyomavirus
Introduction
First described as the ‘trabecular carcinoma of the skin, Merkel cell carcinoma (MCC) is now recognized as a rare and aggressive malignant tumor with a mortality rate approximately three times higher than malignant melanoma (46% versus 16%, receptively).1 The global incidence of MCC is estimated to be 0.13–1.6 per 100,000 individuals each year. In the United States, especially due to the aging Baby Boomer population, the number of new MCC cases is predicted to reach approximately 3234 cases/year by 2025.2–5 Rising incidence of MCC maybe result of increased ultraviolet radiation (UVR) exposure, improved diagnostic markers, immunosuppression, and the increased spread of Merkel cell polyomavirus (MCPyV). As reported by the Surveillance, Epidemiology, and End Results (SEER) database, the 5-year survival rate for localized MCC is 75%, regional MCC is 61%, and Distant MCC is 24% (all SEER combined at 65%).6
The histogenesis of MCC is presently uncertain and has been a topic of much debate in recent years. What was known as trabecular carcinoma was renamed MCC due to its similarities to Merkel cells, a type of cell in the amine precursor uptake and decarboxylation (APUD) category. These similar features include immunopositivity for neuron-specific enolase, neurofilaments, cytokeratins (CK20), CD56, and the existence of biogenic amines and peptides in its osmiophilic granules.7,8 It was assumed that the biogenic amines and peptides might be neurotransmitters at chemical synapses between the Merkel cell and sensory nerve.9 Due to these similarities, most clinicians and scientists refer to MCC as a type of tumor with neuroendocrine differentiation.10,11 Other hypotheses suggest that the origin of MCC may be from dermal totipotent/pluripotent stem cells,8,11–13 precursor B cells, or developed from neural crest since they display transcription factors only seen in nervous tissues.7,14–16
Regardless of the histogenesis of this cancer, recent reports have characterized two subsets of MCC: MCPyV induced MCC and UVR-induced (MCPyV-negative) MCC. It is interesting to note that MCPyV-negative MCCs have high mutation burden, in contrast with MCPyV-positive MCC.17,18 It has been reported that the majority of the MCC (~80%) possesses clonally MCPyV DNA, expressing viral T antigen transcripts and proteins, and exhibit viral large T and small t antigen oncoproteins. The large T antigen includes MCC tumor-specific mutations that preserved its oncogenic function but lost its replication ability. On the other hand, the small t antigen provides the necessary impetus for cap-dependent translation. MCPyV negative MCC are generally caused by damages induced by UV radiation.19
Typically, MCC develops in fair-skinned individuals, sun-exposed skin, elderly (mean age at initial diagnosis ~70 years), chronically immunosuppressed individuals (eg, organ transplant recipients or patients with AIDS), and patients that suffer from other malignancies such as chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma, and multiple myeloma. It has also been reported that patients with MCC may have an increased risk of developing cancers of the salivary gland, stomach and small intestines, biliary sites other than the liver and gallbladder, and malignant brain tumors in men.8,20
A high level of physician suspicion is generally required to diagnose this rare disease. MCC often appears as a small cystic lesion, acneiform eruptions, asymptomatic red violaceous papule, or nodule.17,21 MCC has often been mistaken for lipoma, dermatofibroma, fibroma, vascular lesions, or cysts. MCC has also been misdiagnosed with squamous cell carcinoma, malignant melanoma, small cell carcinoma of the skin, basal cell carcinoma, or lymphoma.13,21 In a 2008 publication by Heath and colleagues, they utilized clinical characteristics of MCC as identifying features to aid clinicians in recognizing MCC. They indicated that typical features of MCC are asymptomatic, expand rapidly, the patient may be immunocompromised, older than 50 years, and fair complexion person often exposed to UV radiation. These features allow Heath and colleagues to create the acronym AEIOU. Nonetheless, the indistinctness of MCC features emphasizes the importance of biopsy and histological analysis to verify the diagnosis.21
Case Report
A 42-year-old male presented with an enlarging, painful skin lesion that appeared abruptly in his right antecubital fossa. Initially, the lesion appeared as a pink papule but quickly developed into a nodule. The patient indicated that he has been suffering from the enlarging mass for over a month. Clinically, the attending physician initially thought the lesion could be an epidermal inclusion cyst and performed a surgical excision of the lesion (Figure 1).
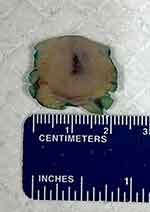 |
Figure 1 Pathologic macroscopic image of the tumor surgically excised. Measuring 1.7×1.2 x 1.2 cm. |
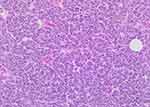
Intraoperatively, the lesion did not have the classic features of a cyst, and the specimen was sent to pathology for evaluation. Pathologic gross examination of the skin revealed a solitary, firm, white, roundish mass within the dermis and subcutaneous tissue measuring 1.7×1.2 x 1.2 cm. Histopathologic examination showed a tumor comprised of cells with large, angulated, pleomorphic, hyperchromatic nuclei with nuclear molding, granular chromatin, and scant cytoplasm (Figure 2). The immunohistochemical evaluation showed the tumor cells to stain positive for pancytokeratin (dot-like), CK20 (dot-like) (Figure 3), CD56 (Figure 4), and synaptophysin. Additional immunohistochemical staining indicates that the tumor cells are Merkel cell polyomavirus-immunoreactive (MCPyV-IR). The Ki-67 proliferation index was greater than 95% in the tumor cells. The patient was diagnosed with Merkel cell carcinoma that focally involved the resection margins.
 |
Figure 3 Tumor cells show positive, dot-like, staining for cytokeratin 20. (Immunohistochemical stain, original magnification x20). |
 |
Figure 4 Tumor cells show diffuse positive staining for CD56. (Immunohistochemical stain, original magnification x20). |
The patient underwent re-excision within four days with axillary sentinel lymph node biopsy. Sentinel lymph node biopsies are utilized to help determine whether the MCC has spread beyond the skin to the first lymph node on a direct lymphatic drainage pathway from the primary tumor. The re-excision specimen showed focal residual MCC with negative surgical margins (2 mm), and the sentinel lymph nodes were negative for metastatic carcinoma. As the current guidelines recommend a 1 to 2 cm negative margin, the patient will undergo additional wide re-excision. The patient’s PET scan in June this year was negative for MCC. An additional PET scan was performed in December, six months after the previous scan. Unfortunately, the result indicates a local recurrence of the MCC. Three core biopsies were taken from the lesion in the incisional scar, and the immunohistochemical evaluation positively identified MCC and these tumor cells are MCPyV-IR. After the finding, the patient elected to continue his future management at the Cleveland Clinic. Details of the patient’s future management have not been disclosed at this present time.
Discussion
Merkel cell carcinoma is a rare and very aggressive skin cancer associated with older age, sun exposure, and MCPyV. It carries a high risk of recurrence and metastasis, especially within the first three years following initial diagnosis. Our case was the first to be reported at Pikeville Medical Center, which serves as a Level II Trauma Center for southeastern Kentucky. Additionally, this case is unusual as the patient is of a younger age, the anatomic location is less sun-exposed, and he has a negative history of immunosuppression or malignancies (the patient only matched one of the AEIOU criteria). Additional examination using monoclonal anti-MCPyV large T-antigen antibodies (Millipore) found that the excised MCC was immunopositive for the polyomavirus. This immunopositivity indicates possible virus-driven oncogenesis.
Conclusion
In conclusion, the incidence rate of MCC is increasing, and, as highlighted by our case, it can affect younger patients without the usual risk factors. These findings strongly advocate for clinicians to consider MCC in their differential diagnosis and to review its specific education and treatment. Early detection and appropriate management are essential to improve patient outcomes.
Abbreviation
MCC, Merkel cell carcinoma; APUD, amine precursor uptake and decarboxylation; MCPyV-IR, Merkel cell polyomavirus-immunoreactive.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report. As per institutional policy, no IRB is needed.
Disclosure
The authors have no conflict of interest to declare.
References
1. Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105(1):107–110. doi:10.1001/archderm.1972.01620040075020
2. Albores-Saavedra J, Batich K, Chable-Montero F, et al. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2009;37:20–27. doi:10.1111/j.1600-0560.2009.01370.x
3. Jacobs D, Huang H, Olino K, et al. Assessment of Age, Period, and Birth Cohort Effects and Trends in Merkel Cell Carcinoma Incidence in the United States. JAMA Dermatol. 2021;157:59–65. doi:10.1001/jamadermatol.2020.4102
4. Paulson K, Youn S, Vandeven N, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457–463. doi:10.1016/j.jaad.2017.10.028
5. Mistry K, Levell NJ, Hollestein L, et al. Trends in incidence, treatment and survival of Merkel cell carcinoma in England 2004–2018: a cohort study. Br J Dermatol. 2023;188(2):228–236. doi:10.1093/bjd/ljac044
6. SEER*Explorer: an interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. Accessed from: https://seer.cancer.gov/explorer/.
7. Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. Anat Rec a Discov Mol Cell Evol Biol. 2003;271:225–239. doi:10.1002/ar.a.10029
8. Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48. doi:10.3389/fonc.2018.00048
9. Winkelmann RK. The Merkel cell system and comparison between it and the neurosecretory or APUD cell system. J Invest Dermatol. 1977;69:41–46. doi:10.1111/1523-1747.ep12497864
10. Harold A, Amako Y, Hachisuka J, et al. Conversion of Sox2-dependent Merkel cell carcinoma to a differentiated neuron-like phenotype by T antigen inhibition. Proc Natl Acad Sci U S A. 2019;116(40):20104–20114. doi:10.1073/pnas.1907154116
11. Mazziotta C, Cervellera CF, Badiale G. Distinct retinoic gene signatures discriminate Merkel cell polyomavirus-positive from -negative Merkel cell carcinoma cells. J Med Virol. 2023;95(7):e28949. doi:10.1002/jmv.28949
12. Hoefler H, Kerl H, Lackinger E, Helleis G, Denk H. The intermediate filament cytoskeleton of cutaneous neuroendocrine carcinoma (Merkel cell tumour). Immunohistochemical and biochemical analyses. Virchows Arch a Pathol Anat Histopathol. 1985;406:339–350. doi:10.1007/BF00704303
13. Mulchan N, Cayton A, Asarian A, Xiao P. Merkel cell carcinoma: a case report and literature review. J Surg Case Rep. 2019;rjz322. doi:10.1093/jscr/rjz322
14. Leonard JH, Bell JR. Insights into the Merkel cell phenotype from Merkel cell carcinoma cell lines. Australas J Dermatol. 1997;38:S91–S98. doi:10.1111/j.1440-0960.1997.tb01019.x
15. Sauer CM, Haugg AM, Chteinberg E, et al. Reviewing the current evidence supporting early B-cells as the cellular origin of Merkel cell carcinoma. Crit. Rev Oncol Hematol. 2017;116:99–105. doi:10.1016/j.critrevonc.2017.05.009
16. Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Dev Biol. 2003;253(2):258–263. doi:10.1016/S0012-1606(02)00015-5
17. Gauci M-L, Aristei C, Becker JC, et al. Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline – update 2022. Eur J Cancer. 2022;171:203–231.
18. Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7(3):3403–3415. doi:10.18632/oncotarget.6494
19. Spurgeon ME, Lambert PF. Merkel cell polyomavirus: a newly discovered human virus with oncogenic potential. Virol. 2013;435(1):118–130. doi:10.1016/j.virol.2012.09.029
20. Howard RA, Dores GM, Curtis RE, Anderson WF, Travis LB. Merkel Cell Carcinoma and Multiple Primary Cancers. Cancer. Epidemiol Biomarkers Prev. 2006;15:1545–1549. doi:10.1158/1055-9965.EPI-05-0895
21. Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–381. doi:10.1016/j.jaad.2007.11.020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.