Back to Journals » Drug Design, Development and Therapy » Volume 8
Mapracorat, a selective glucocorticoid receptor agonist, causes apoptosis of eosinophils infiltrating the conjunctiva in late-phase experimental ocular allergy
Authors Baiula M, Bedini A, Baldi J, Cavet M, Govoni P, Spampinato S
Received 18 February 2014
Accepted for publication 22 March 2014
Published 10 June 2014 Volume 2014:8 Pages 745—757
DOI https://doi.org/10.2147/DDDT.S62659
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Monica Baiula,1 Andrea Bedini,1 Jacopo Baldi,1 Megan E Cavet,2 Paolo Govoni,3 Santi Spampinato1
1Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy; 2Global Pharmaceutical R&D, Bausch & Lomb Inc., Rochester, NY, USA; 3Department of Biomedical, Biotechnological and Translational Sciences, University of Parma, Parma, Italy
Background: Mapracorat, a novel nonsteroidal selective glucocorticoid receptor agonist, has been proposed for the topical treatment of inflammatory disorders as it binds with high affinity and selectivity to the human glucocorticoid receptor and displays a potent anti-inflammatory activity, but seems to be less effective in transactivation of a number of genes, resulting in a lower potential for side effects. Contrary to classical glucocorticoids, mapracorat displays a reduced ability to increase intraocular pressure and in inducing myocilin, a protein linked to intraocular pressure elevation. Allergic conjunctivitis is the most common form of ocular allergy and can be divided into an early phase, developing immediately after allergen exposure and driven primarily by mast cell degranulation, and a late phase, developing from 6–10 hours after the antigen challenge, and characterized by conjunctival infiltration of eosinophils and other immune cells as well as by the production of cytokines and chemokines.
Methods: In this study, mapracorat was administered into the conjunctival sac of ovalbumin (OVA)-sensitized guinea pigs 2 hours after the induction of allergic conjunctivitis, with the aim of investigating its activity in reducing clinical signs of the late-phase ocular reaction and to determine its mechanism of anti-allergic effects with respect to apoptosis of conjunctival eosinophils and expression of the chemokines C-C motif ligand 5 (CCL5), C-C motif ligand 11 (CCL11), and interleukin-8 (IL-8) and the proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α).
Results: Mapracorat, administered into the conjunctival sac of OVA-sensitized guinea pigs 2 hours after allergen exposure, was effective in reducing clinical signs, eosinophil infiltration, and eosinophil peroxidase activity in the guinea pig conjunctiva; furthermore, it reduced conjunctival mRNA levels and protein expression of both CCL5 and CCL11. Mapracorat was more effective than dexamethasone in increasing, in conjunctival sections of OVA-treated guinea pigs, apoptotic eosinophils.
Conclusion: Mapracorat displays anti-allergic properties in controlling the late phase of ocular allergic conjunctivitis and is a promising candidate for the topical treatment of allergic eye disorders.
Keywords: allergic conjunctivitis, late-phase response, eosinophil
Introduction
Allergic conjunctivitis is the most common form of ocular allergy and is usually associated with type 1 hypersensitivity reactions, characterized by early-phase and late-phase responses. Common symptoms include ocular itching, hyperemia, tearing, and chemosis. The early-phase response is driven primarily by mast cell degranulation and develops immediately after exposure to the allergen. This is followed by the late-phase response after 6–10 hours, which is characterized by infiltration of eosinophils, neutrophils, and lymphocytes into the conjunctiva.1,2 Activation and recruitment of inflammatory cells and the release of cytokines, chemokines, adhesion molecules, and proteases contribute to more serious chronic forms.3,4
Topical glucocorticoids are among the most effective drugs for the treatment of allergic eye disease,2 as they potently inhibit a wide range of cytokines and chemokines that contribute to eosinophil accumulation and activation.5 However, glucocorticoid use is associated with increased intraocular pressure, risk of cataract formation, and decreased resistance to infections. There is, therefore, a pressing need for compounds with the anti-inflammatory potency of standard glucocorticoids but fewer or less troublesome side effects.6
Most of the effects of glucocorticoids on target cells are mediated by the regulation of transcription of steroid-responsive genes. It is thought that anti-inflammatory effects of glucocorticoids are largely mediated by a mechanism known as transrepression.7 The glucocorticoid receptor is recruited to DNA via direct or indirect interactions with other transcription factors, notably, members of the activating protein 1 and nuclear factor-kB families, and inhibits transcriptional activation by impairing recruitment of transcriptional coactivators or by promoting recruitment of corepressors.8,9 There is also evidence that glucocorticoid-mediated repression of inflammatory genes involves significant posttranscriptional and/or translational mechanisms,9 and the requirement for de novo protein synthesis has been highlighted.11,12 In contrast, certain side effects seem to be mediated mainly through transactivation, in which the glucocorticoid-bound glucocorticoid receptor directly binds to DNA through simple palindromic glucocorticoid response elements.7,8
A deeper understanding of the molecular mode of glucocorticoid action has led to the identification of novel selective glucocorticoid receptor agonists that should preserve the beneficial anti-inflammatory activity but offer a better side effect profile.12 However, the utility of dissociated glucocorticoid ligands, as more effective anti-inflammatory compounds with fewer side effects, is still debated,9–11 and studies aimed to investigate their pharmacological profile are needed. Schäcke et al13 have reported the pharmacological characterization of mapracorat (also known as BOL-303242-X or ZK 245186), a nonsteroidal selective glucocorticoid receptor agonist, for the topical treatment of inflammatory disorders (Figure 1). Mapracorat binds with high affinity and selectivity to the human glucocorticoid receptor and possesses potent anti-inflammatory activity, but seems to be less effective in transactivation of a number of genes,13 resulting in a lower potential for metabolic and ocular side effects. Physicochemical and pharmacokinetic properties of mapracorat suggest that it is particularly suited for topical treatment, displaying a low systemic activity. In fact, in vitro mapracorat is rapidly metabolized by liver microsomes and, in vivo, it shows a high hepatic clearance and a high volume of distribution. Mapracorat has a short half-life (2 hours) after intravenous administration and a low bioavailability (9%) after topical administration.13 These properties suggest that mapracorat meets the criteria to be considered a “soft” glucocorticoid agonist as it behaves like budesonide.14
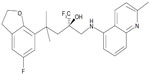  | Figure 1 Chemical structure of mapracorat. |
Ocular pharmacokinetics of mapracorat were investigated by Proksch et al15 after topical administration over a wide range of doses in rabbits and monkeys. Mapracorat is rapidly absorbed and widely distributed into ocular tissues after conjunctival administration, with measurable levels up to 24 hours after dosing. Higher concentrations are detected in tears, followed by conjunctiva and cornea, with lower levels observed in iris/ciliary body and aqueous humor. Plasma levels are lower than those found for other glucocorticoids like dexamethasone. Taken together, these data suggest that mapracorat has a favorable profile of activity in comparison to classical glucocorticoids and may be suitable for topical ocular administration adopting a daily dosing regimen.
Mapracorat, topically administered as eye drops, displays a reduced ability to increase intraocular pressure in normotensive rabbits when compared to dexamethasone16 and behaves as a partial glucocorticoid receptor agonist in inducing myocilin, a protein linked to intraocular pressure elevation, in monkey trabecular meshwork cells.17 Thus, it would be expected to possess an ocular safety profile superior to other glucocorticoids.
Previously, we reported that mapracorat promotes in vitro eosinophil spontaneous apoptosis and inhibits eosinophil migration and release of proinflammatory cytokines and chemokines from eosinophils,18 mast cells,17 or conjunctival epithelial cells, fibroblasts,19 and corneal epithelial cells.20
Exacerbations of allergic conjunctivitis cause eosinophils to infiltrate the conjunctiva, where they contribute to tissue derangement, inflammation, and remodeling.3,21 The chemokines C-C motif ligand 5 (CCL5) and C-C motif ligand 11 (CCL11) play a key role in recruiting eosinophils and other immune cells to the inflammation area22 and are expressed in the conjunctiva of patients with vernal keratoconjunctivitis.23,24 Eosinophil response to inflammatory stimuli leads to increased production of proinflammatory cytokines like interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) and chemokines including interleukin-8 (IL-8).25
Together with other interventions, the removal of eosinophils from the conjunctival epithelium and stroma should contribute to the resolution of the conjunctivitis. To accomplish cell removal, investigations are focused on the pharmacology of apoptosis. This process is considered a noninflammatory model of cell death because apoptotic cells are eliminated through phagocytosis.26
Glucocorticoid-mediated eosinophil apoptosis has been widely investigated with the adoption of in vitro cell models;27–29 however, its occurrence in the airway tissue after glucocorticoid treatment of allergic diseases is still debated.30–32 Studies demonstrating conjunctival eosinophil apoptosis, caused by glucocorticoid treatment at the conjunctival level, are scarce. To date, the potential anti-allergic activity of mapracorat in the eye, and whether eosinophil apoptosis and the chemokines CCL5 and CCL11 are the targets of its action, has been explored very little in vivo. This study specifically addressed these questions, adopting a guinea pig model of allergic conjunctivitis. Mapracorat was tested alongside dexamethasone, a traditional glucocorticoid used to treat ocular inflammation.2 Both drugs were topically administered 2 hours after antigen challenge.
Materials and methods
Materials
Mapracorat was provided by Bausch & Lomb Inc. (Rochester, NY, USA) and dissolved in 10% polyethylene glycol 3350/1% polysorbate 80 in phosphate-buffered saline (PBS) pH 7.0 (vehicle). Dexamethasone 21 disodium phosphate was obtained from Visufarma (Rome, Italy) and diluted in phosphate-buffered saline (PBS). Ovalbumin (OVA) grade V, aluminum hydroxide, o-phenylenediamine, hydrogen peroxide 30%, Triton X-100™, peroxidase acidic isoenzyme from horseradish, RNAlater®, and TRI Reagent® were obtained from Sigma-Aldrich (St Louis, MO, USA). RNase-free DNase was from Thermo Fisher Scientific (Waltham, MA, USA). Rabbit specific horseradish peroxidase (HRP)/diaminobenzidine (DAB) detection kit, anti-CCL11, and anti-CCL5 antibodies were from Abcam (Cambridge, UK).
Induction of allergic conjunctivitis by active immunization and eosinophil detection
The experimental protocol was approved by the Ethical Committee for Animal Experiments of the University of Bologna, Bologna, Italy, and conformed to the EU Directive 2010/63/EU statement for animal experiments.
Allergic conjunctivitis was induced in male Dunkin-Hartley guinea pigs (250–300 g), purchased from Charles River Laboratory Inc. (Calco, Italy), as previously described.33 Guinea pigs were intraperitoneally (ip) treated, except the negative controls, once a week for 2 weeks with 2 mL of a saline solution containing 100 μg/mL OVA and 20 mg/mL aluminum hydroxide as adjuvant. Three weeks after the first OVA treatment, all animals were examined to ensure that there was no sign of preexisting ocular inflammation and then challenged with 30 μL per eye of saline solution containing 100 mg/mL OVA or with 30 μL per eye of saline solution (controls). Over 97% of the sensitized animals treated with topical OVA developed an allergic ocular reaction; guinea pigs that did not respond to the challenge were excluded from the study. Mapracorat and dexamethasone (0.1%, 0.25%, 0.4%; w/v) or the vehicle alone were administered in the conjunctival sac of both eyes (30 μL/eye), 2 hours after OVA challenge (groups of five guinea pigs). Both compounds were administered in a range of doses, including those of dexamethasone used in clinical studies (0.1% or 0.2%) and that of mapracorat previously employed in another in vivo study (0.4%).16
Conjunctival clinical symptoms were rated in a masked fashion on both eyes using the following scale: 0, no symptoms; 1, slight conjunctival redness with or without tears; 2, mild conjunctival redness with or without tears and mild chemosis; 3, mild conjunctival redness with or without tears and moderate chemosis; 4, severe conjunctival redness with tears and partial lid eversion; 5, severe conjunctival redness with tears and lids more than half closed. Pictures of both eyes were taken to evaluate the clinical score 30 minutes before and 1, 4, 6, 8, and 24 hours after OVA administration. The animals were euthanized 8 or 24 hours after OVA challenge by ip injection of Tanax® (3 mL/kg; Hoechst AG, Frankfurt am Main, Germany). Tarsal conjunctiva of both eyes was carefully excised and divided into separate samples for subsequent investigations. One sample was fixed in 10% buffered paraformaldehyde solution and paraffin embedded. Slides, 6 μm thick, were stained to determine the number of eosinophils33 or to carry out immunohistochemistry. To evaluate accumulation and distribution of eosinophils in the tarsal conjunctiva, slides were stained with Luna’s eosinophil stain. Slides were desiccated in xylene, stained with hematoxylin–Biebrich scarlet solution, differentiated in 1% acid alcohol, and subsequently stained with lithium carbonate. Eosinophil granules stain red-orange.33 The number of eosinophils in each field was counted under light microscopy (500× magnification).
In separate conjunctival specimens, eosinophil peroxidase activity was measured as previously described.33 Eosinophil peroxidase activity was also measured in ophthalmic lavage fluid (OLF) as previously described.34 OLF was collected as follows: a 30 μL aliquot of buffer consisting of 0.3 M sucrose in 50 mM sodium acetate buffer pH 5.4 with 10 units/mL heparin was applied to the eye using a micropipette, without touching the eye. After two or three forced blinks, 30 μL of lavage fluid was collected. The lavage was repeated three times in each eye and the OLF was collected in the same tube.
Immunohistochemistry
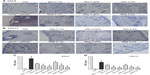
On conjunctival slices, after paraffin removal and rehydration, heat-mediated antigen retrieval was performed with citrate buffer (pH 6.0) for 20 minutes and the sections were washed three times in Tris-buffered saline containing 0.025% of Tween 20® (TBS-T) (Cayman Chemical Company, MI, USA). Immunohistochemical staining was made using rabbit specific HRP/DAB detection kit (Abcam), following the manufacturer’s instructions. Briefly, specimens were incubated with Protein Block solution (Abcam) for 5 minutes at room temperature to block nonspecific background staining. After washing once in TBS-T, tissue sections were incubated with antibodies (100 μL) that recognized human CCL11 or CCL5 (diluted 1:250) overnight at 4°C. The specimens were washed in TBS-T and incubated with biotinylated goat anti-rabbit immunoglobulin G for 10 minutes at room temperature. Slides were then developed with streptavidin–biotin complex/HRP and DAB substrate, according to the manufacturer’s instructions. Hematoxylin was used as background staining. Sections were examined with a light microscope in a blinded manner and photomicrographs were taken with a 20× objective. Coded slides were further analyzed using a validated semiquantitative scoring method based on the percentage of conjunctival tissue showing positive immunoreactive staining.35,36 Immunohistochemical scores were assigned according to five categories (0: no staining; 1: <25% staining; 2: 25%–50% staining: 3, 50%–75% staining; and 4: >75% staining).
Detection of apoptotic cells with the terminal deoxynucleotidyl transferase dUTP nick end labeling technique
Sections of tarsal conjunctiva (6 μm) were deparaffinized, rehydrated, and permeabilized with proteinase K (20 μg/mL) for 20 minutes at room temperature. Apoptotic cells were visualized by a fluorescein isothiocyanate-linked terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay37 with the FragEL™ DNA Fragmentation Detection Kit (Merck Millipore, Billerica, MA, USA), following manufacturer instructions. No staining was evident in negative controls when omitting the terminal deoxynucleotidyl transferase enzyme. Slides were counterstained with the DNA-binding stain DAPI (4′,6-diamidino-2-phenylindole) to reveal pyknotic nuclei as well as the total number of cells. Apoptotic cells were defined as TUNEL-positive cells exhibiting apoptotic morphology (small cells with condensed nuclei). Specimens were analyzed with a microscope (Nikon Eclipse E800 [Nikon Instruments Europe BV, Amsterdam, the Netherlands]); TUNEL-positive cells per mm2 were counted and digital images were captured using a standard fluorescein filter (465–495 nm) and a filter for DAPI (330–380 nm).
Detection of apoptotic eosinophils
Combined staining with TUNEL and chromotrope-2R identifying apoptotic eosinophils was performed as previously described.31,38,39 After deparaffinization, tissue sections were incubated with chromotrope-2R solution for 30 minutes. Slides were washed twice with tap water and then stained with the FragEL™ DNA kit, as described in the previous paragraph. Apoptotic eosinophils were defined as both chromotrope-2R-positive and TUNEL-positive cells exhibiting apoptotic morphology (small cells with condensed nuclei).
Quantitative reverse transcriptase polymerase chain reaction
Tarsal conjunctiva specimens were stored in RNAlater at −20°C. Total RNA was extracted, after homogenization, with TRI Reagent and quantified using a spectrophotometer. For each sample, 1 μg of total RNA was treated with RNase-free DNase for 15 minutes at 25°C, followed by incubation at 65°C for 10 minutes to inactivate DNase. The RNA samples were then converted into cDNA using oligo(dT), random primers, and the High-Capacity cDNA Reverse Transcription Kits (Life Technologies Italia), according to the manufacturer’s instructions. Real-time PCR was performed using GoTaq® qPCR Master Mix obtained from Promega Corporation (Madison, WI, USA). The protocol consisted of denaturation at 95°C for 10 minutes, followed by 40 cycles of 95°C denaturation (20 seconds) and 58°C annealing (1 minute). No-template controls and DNA melting curve analysis were used as controls to ensure the lack of contaminating DNA in the RNA preparations and to rule out primer-dimer formation, respectively. Induction of mRNA was determined from the threshold cycle (Ct) values normalized for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression and then normalized to the value derived from conjunctivae of non-immunized guinea pigs. Primer sequences of GAPDH, IL-8, IL-1β, TNF-α, CCL11, and CCL5 have been previously published40,41 and are listed in Table 1. Primers were synthesized by Sigma-Aldrich.
Data analysis
Data are expressed as mean ± standard error of the mean or median ± standard error of the mean for the number of experiments indicated. Statistical comparisons were made by analysis of variance and post hoc Newman–Keuls test. Nonparametric analysis of the scores assigned to the conjunctival symptoms was done using the Friedman test followed by Dunn’s post hoc comparison. Significant differences among immunohistochemical scores were tested by the Kruskal–Wallis test followed by Dunn’s post hoc comparison. Data analysis was done using GraphPad Prism (v 4.0; GraphPad Software, Inc., La Jolla, CA, USA). P-values <0.05 were considered significant.
Results
Mapracorat and dexamethasone eye drops reduce late-phase allergic reaction in guinea pigs
Guinea pigs were actively immunized by ip injection of OVA and challenged with OVA instilled into the conjunctival sac. One hour after challenge, during the early-phase reaction, clinical observations revealed typical early-phase symptoms of allergic conjunctivitis: tearing and discharge, conjunctival redness, and chemosis. These clinical symptoms showed a second rise from 4–8 hours after allergen challenge in the late-phase reaction and then progressively decreased;42 24 hours after challenge, conjunctiva did not present any relevant clinical symptom.
Mapracorat and dexamethasone (0.1%, 0.25%, and 0.4%) administered in the conjunctival sac of both eyes 2 hours after OVA challenge were equally effective in reducing inflammatory signs observed in the late phase. Both drugs caused a dose-dependent response and a 0.4% dose was the most effective (Figure 2).
In addition to the clinical response, late-phase allergic reaction was evidenced by a significant increase in eosinophil peroxidase activity in the OLF collected 4, 6, and 8 hours after topical OVA challenge, taken as an index of the occurrence of eosinophils in tear fluids; conversely, 30 minutes before and 24 hours after OVA challenge, eosinophil peroxidase activity was not increased in the collected OLF. These data are indicative of conjunctival infiltration of circulating eosinophils that can also reach the tears. Mapracorat and dexamethasone were equally effective in reducing, in a dose-related manner, eosinophil peroxidase activity in tears (Figure 3).
The occurrence of a late-phase allergic reaction was confirmed by histological analysis of tarsal conjunctiva specimens, obtained from guinea pigs sacrificed 24 hours after OVA challenge, containing numerous infiltrating eosinophils (Figure 4A). Similarly, a significant increase of conjunctival eosinophil peroxidase activity was measured (Figure 4B). Mapracorat and dexamethasone were equally effective in reducing, in a dose-related manner, eosinophil infiltration and eosinophil peroxidase activity in the guinea pig conjunctiva (Figure 4A and B).
Mapracorat and dexamethasone eye drops increase conjunctival eosinophil apoptotic cells in late-phase allergic reaction in guinea pigs
OVA challenge did not cause any significant elevation of apoptotic, TUNEL-positive cells lying scattered in the conjunctival tissue in comparison to controls at a 24-hour time point (Figure 5). Interestingly, we observed that mapracorat and dexamethasone, administered 2 hours after OVA, were effective in increasing, in a dose-related manner, the number of apoptotic TUNEL-positive cells in guinea pig conjunctiva in comparison to OVA-treated animals. Mapracorat eye drops caused a more marked conjunctival cell apoptosis compared to the same dose of dexamethasone (Figure 5).
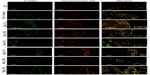
To characterize the nature of apoptotic cells detected in conjunctival specimens, we adopted a combined staining with TUNEL and chromotrope-2R that allows identification of tissue apoptotic eosinophils. As shown in Figure 6, histological analysis performed in guinea pigs sacrificed 24 hours after OVA challenge showed numerous eosinophils, evidenced as red cells by chromotrope-2R staining, infiltrating the tarsal conjunctiva. Under these conditions, only very rare apoptotic, non-eosinophilic cells (stained as green cells) were evidenced by the TUNEL procedure. In fact, adopting this procedure, TUNEL- and chromotrope-2R-positive apoptotic eosinophils appear as yellow cells. The number of apoptotic eosinophils was increased, in a dose-related manner, in mapracorat- or dexamethasone-treated guinea pig conjunctiva as compared to OVA-treated animals. TUNEL-positive green cells when counterstained with chromotrope-2R appear as yellow cells. Mapracorat was more effective than dexamethasone in increasing the number of apoptotic cells (Figure 6). No TUNEL staining was detected in control conjunctival sections or in absence of the deoxynucleotidyl transferase enzyme (data not shown). Thus, both glucocorticoid receptor agonists induce, 24 hours after OVA challenge, a significant apoptosis of conjunctival eosinophil.
As previously stated in this paper, late-phase allergic reaction is characterized by conjunctival eosinophil infiltration that starts 4 hours after allergen challenge. To investigate if apoptotic eosinophils are detected in the conjunctiva as early as 8 hours after OVA challenge, we performed a separate experiment in which we observed that mapracorat or dexamethasone (0.4%) did not elevate the number of TUNEL-positive cells in conjunctival specimens of OVA-challenged guinea pigs sacrificed 6 hours after drug treatment (8 hours after OVA), although a significant number of infiltrating chromotrope-2R-positive eosinophils was evidenced (Figure 7). Thus, eosinophil apoptosis induced by glucocorticoid receptor agonists is a late event that has been detected 22 hours after drug treatment and has also been observed in human eosinophils cultured in vitro for at least 24 hours.18
Mapracorat and dexamethasone eye drops reduce conjunctival chemokines and cytokines mRNA levels and CCL5 and CCL11 protein expression
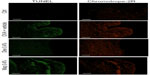
OVA challenge induced a significant elevation of mRNA levels and protein expression of CCL5 and CCL11 in tarsal conjunctival specimens after 24 hours. Mapracorat and dexamethasone, administered 2 hours after OVA, were equally effective in reducing, in a dose-related manner, conjunctival mRNA levels (Figure 8) and protein expression (Figure 9) of both chemokines.
Furthermore, both glucocorticoid agonists induced a dose-dependent decrease of conjunctival mRNA levels of IL-1β, IL-8, and TNF-α (Figure 8).
Discussion
Mapracorat is a selective glucocorticoid receptor agonist with demonstrated anti-inflammatory properties for the topical treatment of inflammatory conditions.13,16 Previously, we reported that this agent inhibits the release of cytokines and chemokines from human mast cells.18 In agreement with our findings, Zhang et al43 and Cavet et al19,20 reported that mapracorat may act, with similar activity and potency as dexamethasone, to prevent the release of cytokines and chemokines in various primary human ocular cells, including conjunctival epithelial cells and fibroblasts. These effects help to explain the action of mapracorat in reducing the conjunctival symptoms that we preliminarily observed in OVA-sensitized guinea pigs by administering mapracorat before allergen exposure and thus showing its activity in preventing the early phase of ocular allergy.18 In this former model18, a prophylactic treatment of mapracorat was effective in alleviating clinical signs of conjunctivitis as it may act upstream, preventing mast cell degranulation observed in the initial allergy cascade. Conversely, in the present study, topical mapracorat was administered after the induction of allergic conjunctivitis, when mast cells were already degranulated, with the aim of investigating its activity in the late-phase ocular reaction. We found that mapracorat, as well as dexamethasone, reduce conjunctival eosinophil accumulation and promote their apoptosis. We focused on eosinophils since these cells mediate unique cytotoxic and inflammatory effects and are crucial for the development of allergic disorders, including conjunctivitis.4 Besides selective migration, longer cell survival and decreased apoptosis are relevant to tissue-specific accumulation of these inflammatory cells.29
Glucocorticoids are the most effective anti-inflammatory drugs used to treat eosinophil disorders as they can prevent eosinophil accumulation and activation. In vitro models have suggested that glucocorticoids mediate their effects on eosinophil activity by an induction of eosinophil apoptosis;27,44,45 however, the in vivo therapeutic relevance of such explanations is still questionable. Apoptotic eosinophils have previously been observed in parasite-infected and glucocorticoid-treated rat gut45 whereas, as regards airway tissue, several studies have reported that glucocorticoids resolve established eosinophilic inflammation in vivo without inducing any detectable apoptosis of tissue eosinophils.31,38,39,46 These cited authors have proposed that eosinophils could efficiently be eliminated by egression into the airway lumen, where they may undergo apoptosis much more frequently than cells resident in tissue compartments. Interestingly, adopting the same histological procedures developed by Uller et al and Uller et al to ascertain any significant apoptosis in airway tissue,31,38 we show here, for the first time, that mapracorat and dexamethasone may cause a dose-dependent increase of eosinophil apoptosis in the conjunctiva. In agreement with our previous study carried out in vitro on human eosinophils,18 we confirm that mapracorat is more effective than dexamethasone in increasing spontaneous eosinophil apoptosis. This unique profile might be due to differences in mapracorat versus glucocorticoids such as dexamethasone in the binding to the glucocorticoid receptor, which leads to a change in receptor conformation and could induce differential binding with other cofactors and/or with glucocorticoid recognition elements residing in the promoter of target genes.47
Despite only one single glucocorticoid receptor gene having been discovered to date, several receptor isoforms may be generated via alternative splicing or via alternative translation initiation mechanisms.48 These isoforms have distinct tissue distribution patterns and are capable of regulating unique sets of genes and mediating glucocorticoid-induced apoptosis in distinct manners.49 For instance, the glucocorticoid receptor-C3 isoform induces the expression of proapoptotic molecules more efficiently than the D3 isoform.50 Therefore, it should be considered that mapracorat may induce apoptosis more efficiently than dexamethasone by binding to certain glucocorticoid receptor isoforms expressed in eosinophils. In addition, it has been reported that components of cytoskeleton may facilitate glucocorticoid-induced apoptosis.50 Thus, mapracorat could also influence these events more effectively that dexamethasone.
The reduced eosinophilia in mapracorat- and dexamethasone-treated groups may, in part, reflect a reduced recruitment of these cells to the conjunctiva. In fact, we noted that both glucocorticoid receptor agonists significantly suppress eosinophil accumulation in tear fluid collected 4–8 hours after allergen challenge when eosinophil apoptosis was not yet detected. Our findings, showing a reduced, dose-dependent reduction in mRNA and protein expression of CCL5 and CCL11 and in mRNA levels of IL-1β, IL-8, and TNF-α, suggest that these are related to the mapracorat- or dexamethasone-mediated reduction of conjunctival eosinophilia and confirm that these chemokines and cytokines may represent useful markers with which to monitor the anti-allergic efficacy of mapracorat and other glucocorticoids. Interestingly, CCL5 and CCL11 have been pointed out as two major chemokines elevated in the conjunctiva of patients with vernal keratoconjunctivitis.24,26
Development of therapeutic glucocorticoid receptor agonists with full anti-inflammatory properties and reduced potential for side effects is an active area of research.9,11,51 Transrepressive mechanisms are thought to account for the majority of anti-inflammatory effects mediated through the glucocorticoid receptor; however, recent studies highlight, in some cell types and inflammatory conditions, the necessity for induction of anti-inflammatory properties for maximal therapeutic effects.52 Induction of these anti-inflammatory proteins likely involves transactivation, whether this is via glucocorticoid responsive element binding, more complex tethering, or composite actions incorporating other transcription factors, remains to be elucidated. Therefore, it is essential to verify the anti-inflammatory activity of novel glucocorticoid receptor ligands in vivo in models, such as the one adopted in this study. As reported,9–11 it would be better to search for “differential” compounds that show the most favorable functional profiles than for glucocorticoid ligands that distinguish transrepression and transactivation.
Clinical interest for mapracorat is also supported by several clinical trials completed in the last few years testing for allergic conjunctivitis,53 dry eye syndrome,54 and the treatment of ocular inflammation and pain following cataract surgery.55 Trial results, which are not yet released by Bausch & Lomb, will be fundamental to confirm mapracorat’s therapeutic effects in clinical conditions involving ocular inflammation.
Conclusion
We confirm that mapracorat is a promising candidate for the topical treatment of allergic eye disorders. It easily reaches conjunctival tissue when administered topically,15 and some of its cellular targets may contribute to eosinophil apoptosis and/or to prevention of eosinophil recruitment and activation, in addition to inhibiting the release of cytokines and chemokines.
Acknowledgments
This study was supported by a grant from the University of Bologna, Bologna, Italy (Ricerca Fondamentale Orientata) and an unrestricted grant from Bausch & Lomb (Rochester, NY, USA).
Disclosure
The authors report no conflicts of interest in this work. Megan E Cavet is an employee of Bausch & Lomb. Bausch & Lomb did not participate in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit this paper for publication.
References
Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153 Suppl 1:17–21. | |
Bielory BP, O’Brien TP, Bielory L. Management of seasonal allergic conjunctivitis: guide to therapy. Acta Ophthalmol. 2012;90:399–407. | |
Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478–485. | |
Irkec MT, Bozkurt B. Molecular immunology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2012;12:534–539. | |
Chauhan S, Leach CH, Kunz S, Bloom JW, Miesfeld RL. Glucocorticoid regulation of human eosinophil gene expression. J Steroid Biochem Mol Biol. 2003;84:441–452. | |
Bielory BP, Perez VL, Bielory L. Treatment of seasonal allergic conjunctivitis with ophthalmic corticosteroids: in search of the perfect ocular corticosteroids in the treatment of allergic conjunctivitis. Curr Opin Allergy Clin Immunol. 2010;10:469–477. | |
De Bosscher K, Haegeman G, Elewaut D. Targeting inflammation using selective glucocorticoid receptor modulators. Curr Opin Pharmacol. 2010;10:497–504. | |
Ratman D, Vanden Berghe W, Dejager L, et al. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol Cell Endocrinol. 2013;380:41–54. | |
Newton R, Holden NS. Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol. 2007;72:799–809. | |
Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134:54–67. | |
Newton R. Anti-inflammatory glucocorticoids: changing concepts. Eur J Pharmacol. 2014;724:231–236. | |
De Bosscher K. Selective glucocorticoid receptor modulators. J Steroid Biochem Mol Biol. 2010;120:96–104. | |
Schäcke H, Zollner TM, Döcke WD, et al. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol. 2009;158:1088–1103. | |
Bodor N, Buchwald P. Corticosteroid design for the treatment of asthma: structural insights and the therapeutic potential of soft corticosteroids. Curr Pharm Des. 2006;12(25):3241–3260. | |
Proksch JW, Lowe ER, Ward KW. Ocular pharmacokinetics of mapracorat, a novel selective glucocorticoid receptor agonist, in rabbits and monkeys. Drug Metab Dispos. 2011;39:1181–1187. | |
Shafiee A, Bucolo C, Budzynski E, Ward KW, López FJ. In vivo ocular efficacy profile of mapracorat, a novel selective glucocorticoid receptor agonist, in rabbit models of ocular disease. Invest Ophthalmol Vis Sci. 2011;52:1422–1430. | |
Pfeffer BA, DeWitt CA, Salvador-Silva M, Cavet ME, López FJ, Ward KW. Reduced myocilin expression in cultured monkey trabecular meshwork cells induced by a selective glucocorticoid receptor agonist: comparison with steroids. Invest Ophthalmol Vis Sci. 2010;51:437–446. | |
Baiula M, Spartà A, Bedini A, et al. Eosinophil as a cellular target of the ocular anti-allergic action of mapracorat, a novel selective glucocorticoid receptor agonist. Mol Vis. 2011;17:3208–3223. | |
Cavet ME, Volhejn S, Harrington KL, Zhang JZ. Anti-allergic effects of mapracorat, a novel selective glucocorticoid receptor agonist, in human conjunctival fibroblasts and epithelial cells. Mol Vis. 2013;19:1515–1525. | |
Cavet ME, Harrington KL, Ward KW, Zhang JZ. Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells. Mol Vis. 2010;16:1791–1800. | |
Chambless SL, Trocme S. Developments in ocular allergy. Curr Opin Allergy Clin Immunol. 2004;4:431–434. | |
Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr Opin Pulm Med. 2005;11:35–42. | |
Abu El-Asrar AM, Struyf S, Al-Kharashi SA, Missotten L, Van Damme J, Geboes K. Chemokines in the limbal form of vernal keratoconjunctivitis. Br J Ophthalmol. 2000;84:1360–1366. | |
Leonardi A, Jose PJ, Zhan H, Calder VL. Tear and mucus eotaxin-1 and eotaxin-2 in allergic keratoconjunctivitis. Ophthalmology. 2003;110:487–492. | |
Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136(1):11–20. | |
Duffin R, Leitch AE, Fox S, Haslett C, Rossi AG. Targeting granulocyte apoptosis: mechanisms, models, and therapies. Immunol Rev. 2010;236:28–40. | |
McColl A, Michlewska S, Dransfield I, Rossi AG. Effects of glucocorticoids on apoptosis and clearance of apoptotic cells. ScientificWorldJournal. 2007;7:1165–1181. | |
Druilhe A, Létuvé S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003;8:481–495. | |
Baiula M, Bedini A, Carbonari G, Dattoli SD, Spampinato S. Therapeutic targeting of eosinophil adhesion and accumulation in allergic conjunctivitis. Front Pharmacol. 2012;3:203. | |
Woolley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Woolley MJ. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:237–243. | |
Uller L, Emanuelsson CA, Andersson M, Erjefält JS, Greiff L, Persson CG. Early phase resolution of mucosal eosinophilic inflammation in allergic rhinitis. Respir Res. 2010;11:54. | |
Uller L, Persson CG, Erjefält JS. Resolution of airway disease: removal of inflammatory cells through apoptosis, egression or both? Trends Pharmacol Sci. 2006;27:461–466. | |
Qasem AR, Bucolo C, Baiula M, et al. Contribution of alpha4beta1 integrin to the antiallergic effect of levocabastine. Biochem Pharmacol. 2008;76:751–762. | |
Kato M, Imoto K, Miyake H, Oda T, Miyaji S, Nakamura M. Apafant, a potent platelet-activating factor antagonist, blocks eosinophil activation and is effective in the chronic phase of experimental allergic conjunctivitis in guinea pigs. J Pharmacol Sci. 2004;95:435–442. | |
Walker RA. Quantification of immunohistochemistry – issues concerning methods, utility and semiquantitative assessment I. Histopathology. 2006;49:406–410. | |
Kelly MM, King EM, Rider CF, et al. Corticosteroid-induced gene expression in allergen-challenged asthmatic subjects taking inhaled budesonide. Br J Pharmacol. 2012;165:1737–1747. | |
Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. | |
Uller L, Andersson M, Greiff L, Persson CG, Erjefält JS. Occurrence of apoptosis, secondary necrosis, and cytolysis in eosinophilic nasal polyps. Am J Respir Crit Care Med. 2004;170:742–747. | |
Uller L, Lloyd CM, Rydell-Törmänen K, Persson CG, Erjefält JS. Effects of steroid treatment on lung CC chemokines, apoptosis and transepithelial cell clearance during development and resolution of allergic airway inflammation. Clin Exp Allergy. 2006;36:111–121. | |
Kubo S, Kobayashi M, Masunaga Y, et al. Cytokine and chemokine expression in cigarette smoke-induced lung injury in guinea pigs. Eur Respir J. 2005;26:993–1001. | |
Lacy HM, Bowlin AK, Hennings L, Scurlock AM, Nagarajan UM, Rank RG. Essential role for neutrophils in pathogenesis and adaptive immunity in Chlamydia caviae ocular infections. Infect Immun. 2011;79:1889–1897. | |
Choi SH, Bielory L. Late-phase reaction in ocular allergy. Curr Opin Allergy Clin Immunol. 2008;8:438–444. | |
Zhang JZ, Cavet ME, VanderMeid KR, Salvador-Silva M, López FJ, Ward KW. BOL-303242-X, a novel selective glucocorticoid receptor agonist, with full anti-inflammatory properties in human ocular cells. Mol Vis. 2009;15:2606–2616. | |
Zhang X, Moilanen E, Kankaanranta H. Enhancement of human eosinophil apoptosis by fluticasone propionate, budesonide, and beclomethasone. Eur J Pharmacol. 2000;406:325–332. | |
Kawabori S, Soda K, Perdue MH, Bienenstock J. The dynamics of intestinal eosinophil depletion in rats treated with dexamethasone. Lab Invest. 1991;64:224–233. | |
Uller L, Persson CG, Källström L, Erjefält JS. Lung tissue eosinophils may be cleared through luminal entry rather than apoptosis: effects of steroid treatment. Am J Respir Crit Care Med. 2001;164:1948–1956. | |
Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29–43. | |
Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18:331–342. | |
Lu NZ, Collins JB, Grissom SF, Cidlowski JA. Selective regulation of bone cell apoptosis by translational isoforms of the glucocorticoid receptor. Mol Cell Biol. 2007;27:7143–7160. | |
Wu I, Shin SC, Cao Y, et al. Selective glucocorticoid receptor translational isoforms reveal glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis. 2013;4:e453. | |
Catley M. Dissociated steroids. ScientificWorldJournal. 2007;7:421–430. | |
Clark AR. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007;275:79–97. | |
Bausch & Lomb Incorporated. Mapracorat Ophthalmic Formulation in Subjects With Allergic Conjunctivitis. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01289431?term=mapracorat&rank=6. NLM identifier: NCT01289431. Accessed March 20, 2014. | |
Bausch & Lomb Incorporated. Study to Assess the Safety and Efficacy of BOL-303242-X Ophthalmic Suspension in Dry Eye Syndrome. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01163643?term=nct01163643&rank=1. NLM identifier: NCT01163643. Accessed March 20, 2014. | |
Bausch & Lomb Incorporated. Mapracorat Ophthalmic Suspension, 3% for the Treatment of Ocular Inflammation and Pain Following Cataract Surgery. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01591161?term=mapracorat&rank=3. NLM identifier: NCT01591161. Accessed March 20, 2014. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.