Back to Journals » Research and Reports in Urology » Volume 15
Maintaining Serum Hemoglobin Levels Within the Physiological Range Prevented Bladder Tamponade Recurrence Due to Radiation-Induced Hemorrhagic Cystitis: A Case Report
Authors Ueda N, Sato M , Matsukawa A, Oki Y, Mizuno R, Akiyama M, Tei N, Miyake O
Received 7 May 2023
Accepted for publication 15 August 2023
Published 21 August 2023 Volume 2023:15 Pages 395—401
DOI https://doi.org/10.2147/RRU.S420329
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Norichika Ueda,1 Mototaka Sato,1 Atsuki Matsukawa,1 Yuta Oki,2 Ryoya Mizuno,1 Mai Akiyama,1 Norihide Tei,1 Osamu Miyake1
1Department of Urology, Toyonaka Municipal Hospital, Toyonaka, Japan; 2Department of Urology, Osaka Rosai Hospital, Sakai, Japan
Correspondence: Mototaka Sato, Department of Urology, Toyonaka Municipal Hospital, 4-14-1 Shibahara-cho, Toyonaka, 560-8565, Japan, Tel +81-6-6843-0101, Fax +81-6-6858-3531, Email [email protected]
Abstract: Radiation-induced hemorrhagic cystitis is a refractory disease that can cause severe hematuria and bladder tamponade. Bladder tamponade due to radiation-induced hemorrhagic cystitis can often recur repeatedly and markedly reduce the quality of life. However, no blood test parameter has been studied yet regarding the prevention of bladder tamponade recurrence. An 84-year-old patient with a history of radiation therapy for cervical cancer was repeatedly hospitalized for bladder tamponade due to radiation-induced hemorrhagic cystitis. At each hospitalization, blood transfusions were performed to treat severe anemia as the first treatment, resulting in hematuria improvement, and the patient was discharged without invasive treatments such as transurethral coagulation. However, anemia developed gradually after each discharge. The anemia progression was obviously unrelated to macrohematuria because macrohematuria did not appear during that period. When the serum hemoglobin level decreased below the physiological range, bladder tamponade recurred. Based on these findings, we posited that the monitoring of the serum hemoglobin level could be useful to predict the occurrence of bladder tamponade. We hypothesized that if the serum hemoglobin level did not fall below the physiological range, bladder tamponade would not occur. We treated chronic anemia after determining its cause and kept serum hemoglobin levels within the physiological range. Since the treatment was initiated, bladder tamponade has not recurred in over 27 months. In this case, the monitoring of the serum hemoglobin level was useful to predict the occurrence of bladder tamponade due to radiation-induced hemorrhagic cystitis. By maintaining serum hemoglobin levels within the physiological range, we successfully prevented the recurrence of bladder tamponade due to radiation-induced hemorrhagic cystitis.
Keywords: hematuria, blood tests, prevention, recurrence, monitoring
Introduction
Radiation therapy to the pelvis is commonly performed for urologic, gynecologic, and gastrointestinal malignancies.1–4 Radiation therapy is effective for these diseases, but toxicity to the bladder, especially radiation-induced hemorrhagic cystitis (RIHC), remains a disastrous side effect. RIHC is a refractory disease that often causes severe hematuria, resulting in bladder tamponade.1–4 Bladder tamponade is urinary retention resulting from obstruction of the bladder outlet by blood clots formed by intense hematuria. Bladder tamponade causes severe pain and progressive anemia and further exacerbates hematuria, leading to a negative vicious cycle of recurrent bladder tamponade.1,2 Persistent, intractable bleeding can lead to life-threatening hypovolemic shock.2 Hence, the vicious cycle of bladder tamponade requires repeated hospitalization and treatment, which drastically reduces the quality of life.1,2
Common treatments and recurrence prevention strategies for bladder tamponade due to RIHC include manual or continuous bladder irrigation, hemostatic agent administration, transurethral coagulation (TUC), intravesical instillation, hyperbaric oxygen therapy (HBOT), vascular embolization, enterocystoplasty, total cystectomy, and urinary diversion.1,2,4 However, implementing these treatments can be difficult depending on the patient’s general condition and hospital facilities. Unfortunately, prophylactic treatment and optimal management protocols for bladder tamponade due to RIHC have not been fully established. Herein, we report an interesting case in which bladder tamponade due to RIHC was successfully prevented by maintaining serum hemoglobin levels within the physiological range.
Case Presentation
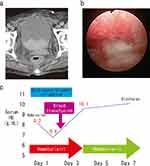
An 84-year-old woman was urgently admitted to our hospital because of bladder tamponade in July 2019. She had received radiotherapy for cervical cancer at another hospital in 2000 (irradiation method; dose unknown). A urinary catheter was placed due to urinary retention caused by a hypoactive bladder. Blood test results revealed anemia with a hemoglobin concentration of 8.2 g/dL. There were no abnormalities in the platelet count and coagulation profile, including prothrombin time-international normalized ratio (PT-INR) and activated partial thromboplastin time (APTT). She was not taking any anticoagulants or antiplatelet agents. Computed tomography (CT) showed large blood clots in the bladder (Figure 1a). Cystoscopy revealed dilated hemorrhagic vessels, mainly in the bladder neck (Figure 1b). Urinary cytology was negative. No other pathological cause of hematuria was identified, and a diagnosis of bladder tamponade due to RIHC was made. Bladder irrigation was performed to remove the blood clots, hemostatic agents (carbazochrome sodium sulfonate hydrate 25 mg/day and tranexamic acid 2000 mg/day) were administered, and continuous bladder irrigation was initiated. However, the hematuria did not improve, and the serum hemoglobin level dropped to 6.8 g/dL on day 2. Therefore, a blood transfusion was performed on day 3. Although the patient was scheduled to undergo TUC for hemostasis management, it was not performed because the serum hemoglobin level improved to 10.0 g/dL after the transfusion, and the hematuria resolved spontaneously. Thereafter, hematuria did not recur, and the patient was discharged on day 7 (Figure 1c).
However, bladder tamponade recurred approximately 2 months after discharge, and the patient was urgently hospitalized. After manual bladder irrigation to remove blood clots, hemostatic agent administration (carbazochrome sodium sulfonate hydrate 25–100 mg/day and tranexamic acid 1000–2000 mg/day), and blood transfusion, macrohematuria disappeared, and the bladder tamponade obviously improved again. When the macrohematuria improved, the administration of these hemostatic agents was terminated, and there was no recurrence of macrohematuria during hospitalization. Eventually, the patient was discharged without undergoing TUC. This clinical course repeated five times in one year. There were no abnormalities in the platelet count and coagulation profile including PT-INR and APTT each time. CT, urine cytology, urine culture, and cystoscopy were performed each time, but no other cause of hematuria could be identified, and a diagnosis of bladder tamponade due to RIHC was made. There was no evidence of local recurrence of cervical cancer or invasion of the urinary tract. The patient also had no history of previous use of anticancer drugs such as cyclophosphamide or ifosfamide. Therefore, drug-induced bleeding was also negative. Since significant anemia was observed at each admission, blood transfusions were performed as the first treatment. After the transfusion, the hematuria obviously disappeared each time. The patient was discharged each time without an invasive treatment such as TUC. Despite this frequent occurrence of bladder tamponade, the patient was unable to receive preventive treatment, such as HBOT, intravesical instillations, or vascular embolization, due to inadequate facilities. The hospital did not have the tools or equipment to perform intravesical injection therapy such as alum or formalin, vascular embolization of the bladder, and HBOT. To make matters worse, the patient was bedridden with deterioration in the activities of daily living and found it difficult to visit another hospital for preventive treatment. Moreover, the patient had chronic heart failure and poor surgical tolerance. The cardiothoracic ratio of the patient was 75% on chest x-ray, with a left ventricular ejection fraction of 39% on cardiac ultrasound. Although her cardiac function was stabilized with antihypertensive drug therapy and no drinking restrictions were needed, the invasive load from anesthesia or surgery was deemed dangerous by a cardiologist. Hence, the patient could not undergo invasive surgical procedures such as total cystectomy and urinary diversion.
After reviewing the clinical course of this patient, we noticed that the serum hemoglobin level, which had been elevated by blood transfusion at the time of discharge, gradually decreased after discharge. The anemia progression was apparently unrelated to hematuria because the patient and her family monitored the color of her urine several times a day, and macrohematuria did not appear during that period. In other words, the anemia was slowly progressing despite the absence of any macrohematuria. When the serum hemoglobin level dropped below the physiological range, bladder tamponade suddenly appeared every time (Figure 2). Based on this, we hypothesized that monitoring of serum hemoglobin levels could predict the occurrence of bladder tamponade due to RIHC; that is to say, if the serum hemoglobin level did not fall below the physiological range, bladder tamponade would not occur. Therefore, the patient was treated to maintain a physiological serum hemoglobin level by treating chronic anemia. We identified chronic renal failure and iron deficiency as the causes of the patient’s chronic progressive anemia. Thus, erythropoiesis-stimulating agent (ESA) and darbepoetin alfa plus sodium ferrous citrate (SFC) were administered for renal anemia and iron deficiency anemia, respectively. In addition, based on the clinical course, it was suggested that the risk of developing bladder tamponade increased when the serum hemoglobin level was below 9.0 g/dL (Figure 3). Therefore, the serum hemoglobin level was monitored regularly, once every two weeks, and the dosage and administration intervals of ESA and SFC were adjusted. The oral dose of SFC was one 50 mg tablet daily; ESA administered 60 μg at 4-week intervals. However, due to a mild increase in serum erythropoietin levels, the ESA dose was increased to 120 μg at 2-week intervals beginning in June 2020. This treatment prevented a decrease in serum hemoglobin levels below 9.0 g/dL and maintained them in the physiological range. Complete blood count and coagulation profile, including PT-INR and APTT, remained stable regardless of serum hemoglobin level (Figure 4). As a result, the patient has not had a single recurrence of bladder tamponade, which had previously been frequent, in more than 27 months since then (Figure 3).
Discussion
RIHC may occur as a late complication of radiation therapy for cancer of the pelvic organs.1,3,5,6 Radiation to the bladder causes fibrosis of the vascular endothelium, resulting in vascular occlusion and submucosal/muscular fibrosis. Subsequently, hypoxia and ischemia due to hypovascularization induce neovascularization in the form of hemorrhage-prone capillary dilatations, resulting in bladder tamponade.1,2,6–9
HBOT is the only well-researched and validated method for RIHC treatment.1,4 HBOT is hypothesized to increase the partial pressure of oxygen in serum, creating a steep oxygen gradient between the blood present in the bladder and the hypoxic bladder tissue, thus increasing the amount of oxygen supplied from hemoglobin to the bladder tissue.1,2 Hyperoxia induces primary neovascularization, secondary growth of healthy granulation tissue, and short-term vasoconstriction which may help control active bleeding.6 In contrast, when the serum hemoglobin level is elevated, as in this case, the amount of hemoglobin transported to the bladder increases. As indicated by the oxygen-hemoglobin dissociation curve, hemoglobin can dissociate more easily from oxygen at sites where the oxygen partial pressure is low,10 as in irradiated bladder tissue. Therefore, maintaining the serum hemoglobin level within the physiological range was thought to increase the amount of oxygen supplied to the bladder tissue and improve the ischemic state, resulting in the prevention of bladder tamponade. To the best of our knowledge, this is the first report on the recurrence prevention of RIHC-induced bladder tamponade by maintaining the serum hemoglobin level within the physiological range.
However, it must be emphasized that there are strong limitations because this is only one case report; there have been no other similar case reports in the past, and we have never experienced a similar case. In addition, there have been no reports to date demonstrating this hypothesis in animal models. This is because an animal model of late radiation cystitis causing hematuria has not yet been established.3 Hence, we cannot conclude that this treatment method can be adapted to many patients with the same disease. To clarify this, it is necessary to establish an appropriate animal model of RIHC and to determine the efficacy of this treatment. Furthermore, it is very important to involve other institutions and more patients. In reality, there are a certain number of patients worldwide who, like the patient in this case, are unable to receive treatment to prevent recurrence due to poor facilities or poor surgical tolerance. For such patients, this treatment strategy may be useful. Although longer prospective studies and larger sample sizes are needed, our results suggest that serum hemoglobin level may be an important parameter related to bladder tamponade.
Conclusion
By maintaining serum hemoglobin levels within the physiological range, we successfully prevented the recurrence of bladder tamponade due to RIHC.
Ethics Approval and Informed Consent
Institutional approval was not required to publish this case report. In the case of case reports, our hospital requires written consent from the patient for the presentation and publication of case report. Written informed consent was obtained from the patient for publication.
Consent for Publication
Written informed consent was obtained from the patient for the publication of this case report.
Acknowledgments
The authors wish to thank the investigators, staff, and affiliated institutions for their important contributions to this study, which was conducted at Toyonaka Municipal Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Oscarsson N, Muller B, Rosen A, et al. Radiation-induced cystitis treated with hyperbaric oxygen therapy (RICH-ART): a randomized, controlled, Phase 2–3 trial. Lancet Oncol. 2019;20(11):1602–1614. doi:10.1016/S1470-2045(19)30494-2
2. Pascoe C, Duncan C, Lamb BW, et al. Current management of radiation cystitis: a review and practical guide to clinical management. BJU Int. 2019;123(4):585–594. doi:10.1111/bju.14516
3. Brossard C, Lefranc AC, Simon JM, et al. Understanding molecular mechanisms and identifying key processes in chronic radiation cystitis. Int J Mol Sci. 2022;23(3):1836. doi:10.3390/ijms23031836
4. Vanneste BGL, Van Limbergen EJ, Marcelissen TA, et al. Development of a management algorithm for acute and chronic radiation urethritis and cystitis. Urol Int. 2022;106(1):63–74. doi:10.1159/000515716
5. Zuppone S, Bresolin A, Spinelli AE, et al. Pre-clinical research on bladder toxicity after radiotherapy for pelvic cancers: state-of-the art and challenges. Front Oncol. 2020;10:527121. doi:10.3389/fonc.2020.527121
6. Helissey C, Cavallero S, Brossard C, et al. Chronic inflammation and radiation-induced cystitis: molecular background and therapeutic perspectives. Cells. 2020;10(1):21. doi:10.3390/cells10010021
7. Mendenhall WN, Henderson RH, Costa JA, et al. Hemorrhagic radiation cystitis. Am J Clin Oncol. 2015;38(3):331–336. doi:10.1097/COC.0000000000000016
8. Capelli-Schellpfeffer M, Gerber GS. The use of hyperbaric oxygen in urology. J Urol. 1999;162(3):647–654. doi:10.1097/00005392-199909010-00002
9. Dellis A, Deliveliotis C, Kalentzos V, et al. Is there a role for hyperbaric oxygen as primary treatment for grade 4 radiation-induced haemorrhagic cystitis? A prospective pilot-feasibility study and review of literature. Int Braz J Urol. 2014;40(3):296–305. doi:10.1590/S1677-5538.IBJU.2014.03.02
10. Arora S, Tantia P. Physiology of oxygen transport and its determinants in intensive care unit. Indian J Crit Care Med. 2019;23(Suppl 3):172–177. doi:10.5005/jp-journals-10071-23246
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.