Back to Journals » International Journal of General Medicine » Volume 17
Magnetic Resonance Imaging Findings and Their Association with Electroencephalographic Data in Children with Epilepsy at Tertiary Care Hospital in Mogadishu Somalia
Authors Elmi AM , Ibrahim AA, Hassan MS , Osman FAO , Çelik C , Dirie AM, Ibrahim IG
Received 26 November 2023
Accepted for publication 21 January 2024
Published 23 January 2024 Volume 2024:17 Pages 253—261
DOI https://doi.org/10.2147/IJGM.S448291
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Abdinasir Mohamed Elmi,1 Abdiwahid Ahmed Ibrahim,2 Mohamed Sheikh Hassan,2,3 Faisal Abdi Osoble Osman,1 Cihan Çelik,1 Abdikadir Mohamed Dirie,1 Ismail Gedi Ibrahim1
1Radiology Department, Mogadishu Somali Turkish Training and Research Hospital, Mogadishu, Somalia; 2Neurology Department, Mogadishu Somali Turkish Training and Research Hospital, Mogadishu, Somalia; 3Faculty of Medicine and Surgery, Mogadishu University, Mogadishu, Somalia
Correspondence: Abdinasir Mohamed Elmi, Radiology Department, Mogadishu Somali Turkish Training and Research Hospital, Mogadishu, Somalia, Email [email protected]
Introduction: Epilepsy is a neurological disorder characterized by abnormal, fast, synchronous neuronal discharge from the cerebral cortex. This abnormal excitation of the brain is usually short and self-limiting and can last anywhere from a few seconds to a few minutes. Neuroimaging and electroencephalography (EEG) are two widely used techniques to differentiate, verify, or exclude the diagnosis of epilepsy. The study aims to identify the frequency of EEG and MRI abnormalities in pediatric epilepsy and their correlations, aiming to improve diagnostic and treatment methods for these children.
Materials and Methods: In this cross-sectional retrospective study, we evaluated pediatric patients aged 0– 18 years who visited the Neurology Polyclinic between July 2022 and July 2023, were diagnosed with epilepsy in accordance with the ILAE 2014 epilepsy guidelines, and had undergone neuroimaging at the hospital’s radiology department. Demographic information and clinical data, including the patient’s age, gender, history of trauma, and congenital infection, were assessed. In all patients, a surface electroencephalogram (EEG) and brain magnetic resonance imaging (MRI) were performed.
Results: Our study recruited 102 pediatric patients aged between 0– 18 years, including 63 (61.8%) boys and 39 (37.2%) girls. An electroencephalogram (EEG) and MRI study have been done on all patients. An abnormal EEG study was seen in 79 (77.45%) participants, and an abnormal MRI was noted in 45 (44.1%) patients. The EEG and MRI were both abnormal in 34 cases (33.3%). The study found no significant correlation between magnetic resonance imaging and electroencephalographic findings (P =0.779).
Conclusion: We observed multiple abnormalities on neuroimaging in pediatric epileptic patients. Even though our sample size was small, our results demonstrated that there is no statistically significant relationship between EEG and MRI results.
Keywords: pediatric epilepsy, electroencephalography, magnetic resonance imaging
Introduction
Epilepsy is a neurological disorder originating from the cerebral cortex and is defined by symptoms that arise from an excessive, aberrant, fast, and synchronous neuronal discharge. This abnormal, abrupt excitation of the brain happens occasionally, usually short, self-limiting, and can last anywhere from a few seconds to a few minutes.1 The diagnosis of epilepsy is considered when the individual had a minimum of two separate, unprovoked seizure attacks.2 Epilepsy is one of the most prevalent neurological disorders in children and adolescents. Its prevalence range of 4 to 6 cases per 1000 children.3 Roughly ten million children under the age of 15 have epilepsy globally, accounting for one-fourth of all epileptic patients. Nearly 1.12 million of these children dwell in developing and underdeveloped countries.2 According to recent studies, there are 7.0–14.8 cases of active convulsive epilepsy per 1,000 people in Sub-Saharan Africa (SSA). This represents nearly twice the estimated 4.5 −5.0 per 1,000 individuals in Europe. This significant burden is probably related to increased rates of perinatal insults, traumatic brain injury, and infectious diseases that affect the central nervous system.3
Neuroimaging and electroencephalography (EEG) are two widely used techniques to differentiate, verify, or exclude the diagnosis of epilepsy and characterize the underlying pathology. The role of neuroimaging is to detect underlying cerebral lesions that may be causally related to the seizure disorder, provide a prognosis, and plan appropriate care. For the majority of brain epileptogenic lesions, magnetic resonance imaging (MRI) is superior to computed tomography scans in identifying potential epileptogenic lesions. EEG is a crucial and common method for identifying seizures in children. In essence, both awake and asleep EEGs should be performed on any child who had repeated seizures.1,4
Some authors have questioned whether EEG should be a required component of the initial evaluation, given that the results of the EEG have little influence on treatment decisions. However, experts conclude that an EEG study is crucial to determining the seizure type and also to diagnose and differentiate epilepsy syndromes. Most researchers have suggested neuroimaging only in certain circumstances, but risk factors for abnormal imaging findings are not routinely apparent.5 Epilepsy is associated with a variety of structural brain abnormalities. They can be divided into congenital and acquired abnormalities. Among the acquired abnormalities are hippocampal sclerosis (HS) and abnormalities arising from insults to the brain, such as trauma, infection, and hypoxia.6 Malformations of the cortical development and low-grade cortical tumors are examples of congenital abnormalities. A disturbance in the neurodevelopment process, which can happen at the stages of neuronal proliferation, migration, or cortical structure, causes MCD.7 These disruptions can cause numerous MCDs, including lissencephaly, hemimegalencephaly, polymicrogyria, heterotropia, and schizencephaly.8
The aim of our study is to describe the frequency of EEG and MRI abnormalities among pediatric epilepsy patients and also to determine relationships between MRI and EEG abnormalities to find better diagnostic and treatment modalities for these children in Somalia context.
Materials and Methods
In this cross-sectional retrospective study, we evaluated pediatric patients aged 0–18 years who visited the Neurology Polyclinic between July 2022 and July 2023, were diagnosed with epilepsy in accordance with the ILAE 2014 epilepsy guidelines,9 and had performed neuroimaging at the hospital’s radiology department at the Mogadishu Somali Turkish Training and Research Centre.
Demographic information and clinical data, including patient’s age, gender, history of trauma, and congenital infection were assessed. In all patients, a surface electroencephalogram (EEG) and brain magnetic resonance imaging (MRI) was performed. EEG results were grouped into five different categories: Normal, Focal epileptiform, Focal slowing, Generalized epileptiform discharge, and Generalized slowing. Two neurologists with pediatric neurology proficiency interpreted the EEG data. In all patients, MRI examination was done using 1.5 Tesla (Siemens, Germany) with the standard MRI epilepsy protocol. MRI scans were assessed by a single radiologist without knowing the patient’s medical information or EEG results. MRI findings were classified into 18 categories from medical literature and personal experience: normal, trauma, tumor, vascular, atrophy, hydrocephalus, infection, encephalomalacia, hippocampal sclerosis, periventricular leukomalacia, cystic lesion, calcification, craniostenosis, cortical lesions, white-matter lesions, multiple lesions, corpus callosum, and other lesions.
Ethical Approval
Due to the fact that our hospital is a research hospital, a general written informed consent is obtained from every patient or patient guardian/parents to obtain their data for use of retrospective research purposes from the hospital medical records as long as any of their personal information is not disclosed. The ethics committee who approved this retrospective study waived such consent from individual study subjects. The ethics committee of Mogadishu Somali Turkish Training and Research Hospital approved our study (Ethics Protocol No.: MSTH/10511).
Statistical Analysis
Data were analyzed using SPSS (Statistical Package for Social Sciences, IBM Inc., Chicago, IL, USA) v27.0. Descriptive statistics were used to summarize the data in terms of frequencies and percentages. The chi-square test was used to analyze the association between EEG and MRI findings. P-values >0.05 were considered statistically significant.
Results
Our study recruited 102 pediatric patients aged between 0–18 years (mean ± SD: 9.06 ± 5.2 years), including 63 (61.8%) boys and 39 (37.2%) girls. The age distribution consisted of 7 infants (1 to 12 months), 27 early childhood (1 to 5 years), 26 middle childhood (5 to 10 years), 24 late childhood (10 to 15 years), and 18 adolescents (15 to 18 years). 10 participants have a history of trauma. No history of congenital infections was documented in our study participants.
EEG Findings
An electroencephalogram (EEG) study has been done on all patients. An abnormal EEG study was seen in 79 participants (77.4%), including 48 males and 31 females. A normal EEG result was observed in 23 participants. The EEG findings were grouped into: normal 23 (22.5%) as demonstrated in Figure 1, focal epileptiform discharge 28 (27.5%), generalized epileptiform discharge 43 (42.2%) as seen in Figure 2, focal slowing 3 (2.9%), and generalized slowing 5 (4.9%).
 |
Figure 2 An electroencephalogram of 8-year-old child with idiopathic generalized epileptic seizure showing 2–3 HZ sharp and slow wave activity. |
MRI Findings
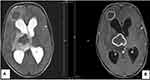
An abnormal MRI finding among children with epilepsy studied was 45 (44.1%), as demonstrated in Figure 3. The brain MRI abnormalities were classified as either acquired or congenital anomalies. Of these, hippocampal sclerosis was the most frequently seen acquired MRI abnormality, with 14 cases (13.7%), including 9 patients with right-sided HS, 3 patients with left-sided HS, and 2 with bilateral hippocampal sclerosis. The remaining acquired abnormal MRI findings consisted of atrophy in 12 cases (11.8%) as shown in Figure 4, encephalomalacia in 4 cases (3.9%), 3 cases (2.9%) with multiple lesions, 2 cases (2%) with white matter lesions, 2 cases (2%) with infections including meningoencephalitis and intracranial tuberculoma as shown in Figure 5, 1 case (1%) with brain tumor as shown in Figure 6 and 2 cases (2%) with periventricular leukomalacia. Among congenital MRI abnormalities, cortical malformations, including cortical heterotopia and polymicrogyria, as seen in Figure 7, and corpus callosum agenesis, as demonstrated in Figure 8, were the most frequent. Others include hydrocephalus secondary to aqueductal stenosis.
 |
Figure 3 Distribution of different MRI findings in pediatric patients with epilepsy. |
 |
Figure 4 Axial FlAIR (A) and T2 (B) weighted sequences showing atrophic changes in the left frontal and parietal lobes with surrounding gliosis. |
 |
Figure 7 Pachygyria-polymicrogyria in a 2-year-old child with epilepsy. Axial T2 shows broadly enlarged and thickened gyri in the bilateral cerebral hemisphere. |
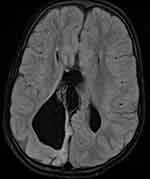 |
Figure 8 Axial FlAIR images demonstrating agenesis of the corpus callosum with parallel ventricles and colpocephaly. |
Relationship Between EEG and MRI Findings
In our study, 34 cases (33.3%) had abnormal EEG and MRI results, including 19 males and 15 females, while 12 cases (11.7%) had normal EEG and MRI findings, as indicated in Table 1. In addition, 45 (44.1%) cases showed normal MRI and abnormal EEG findings, while 11 (10.8%) cases revealed abnormal MRI and normal EEG. There is no statistically significant association between magnetic resonance imaging and electroencephalographic findings (P =0.779).
 |
Table 1 Comparison of EEG and MRI Findings |
Discussion
Epilepsy is a disease of the cerebral cortex and can cause severe long-term neurological illnesses with notorious physical and social limitations. Based on a self-reported survey, the Centers for Disease Control and Prevention of the United States (CDC) estimated that 1.1–2.2% of the population had epileptic seizures.10 To the best of our knowledge, studies about pediatric epilepsy and their EEG and MRI findings are not available in Somalia.
It has been shown that MRI is effective for examining brain structure and imaging potential pathologic conditions that could be related to the etiology of seizures in pediatric epilepsy.11 Although MRI is a noninvasive form of imaging and very sensitive, it is not readily available or expensive.12 Because of its greater resolution and lack of radiation exposure to patients, MRI has been favored over computed tomography.11
We analyzed 102 pediatric patients with epileptic seizures aged between 0 −18 years. The prevalence of abnormal brain MRI findings in our study was 45 (44.1%), consisting of 40 (39.5%) participants with acquired lesions and 5 (4.9%) participants with congenital anomalies, which is a higher percentage than that noticed in most of the previous studies. For example, Anne et al found 15.8% of brain abnormalities detected with MRI in childhood epilepsy.13 A similar study in Spain reported a prevalence of 21.9%.14 The greatest percentage of children with epilepsy were in the 1–5 and 6–10-year age groups, and they had the highest frequency of structural brain abnormalities detected on MRI investigation. Hippocampal sclerosis, encephalomalacia, and atrophy secondary to prenatal brain injury were the most frequently acquired brain imaging abnormalities in our study. These findings are in keeping with a previous retrospective observational study conducted at a tertiary hospital in Kenya on children who presented with epilepsy. The study detected 33% of brain MRI abnormalities, with the most frequent findings being features related to hippocampal sclerosis, encephalomalacia secondary to chronic infarcts, periventricular leukomalacia, cerebral atrophy, and disorders of neuronal migration.4 A cross-sectional descriptive study involving children below 18 years with epilepsy at urban hospitals in Kampala-Uganda revealed MRI abnormalities in 74.15% of children, in contrast to our study. The most common abnormalities were hippocampal sclerosis (HS), Hypoxic Ischaemic Encephalopathy (HIE) and Periventricular Leukomalacia (PV),8 which was similar to our results. Two patients (2%) showed radiological findings of CNS infections, which were comparable to other studies.8 Radiological evidence of malformations of cortical development (MC) was observed in 2 cases (2%). Varying rates of 3.8 to 8.84% have been reported in previous studies.4,8 MCDs are epileptogenic and known to cause other complications, like global developmental delay.15 One participant had brain neoplasms (1% of all abnormalities). Brain tumors are linked to 4–5% of all cases presenting with epilepsy.16,17
In our study, EEG was done on all participants. Of these 79 (77.4) patients, including 48 males and 31 females, 48 had abnormal EEG results. Abnormal EEGs and MRIs were detected in 34 (33.3%) cases. This finding is in keeping with a previous retrospective study done by Dirik et al, which revealed MRI and EEG abnormalities in 39.2% of patients.18,19 In contrast to our study, Denise et al reported EEG and MRI abnormalities in 98% of cases8. This higher percentage in their study may be due to a methodological difference.
Even though our sample size was small, we assessed EEG and MRI findings in pediatric epilepsy. Our results demonstrated that there is no statistically significant relationship between EEG and MRI results. This implies that normal MRI scan results cannot be predicted from normal EEG readings. These findings highlight that an epileptic patient should not be placed in a low-risk category based on normal EEG readings, and these patients should have an MRI for additional assessment.
Conclusions
We observed multiple abnormalities on neuroimaging in pediatric epileptic patients, and the information given might help in directing medical or surgical management. Despite the fact that EEG and MRI are the best diagnostic equipment for evaluating patients presenting with seizures, our results did not demonstrate a correlation between EEG and neuroimaging findings. Rather, our findings showed that EEG information should not be the only condition for subsequent MRI evaluation. A large sample-sized population or prospective trial study should be used to confirm our study results.
Abbreviations
EEG, Electroencephalography; MRI, Magnetic Resonance Imaging; MCD, Malformations of cortical development; SSA, Sub-Saharan Africa; ILAE; International League Against Epilepsy.
Ethical Approval
The ethics committee of Mogadishu Somali Turkish Training and Research Hospital approved our study (Ethics Protocol No.: MSTH/10511). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Wright NB.; Wright NB. Imaging in epilepsy: a paediatric perspective. British J Radiol. 2001; 74(883):575–589. doi:10.1259/bjr.74.883.740575
2. Nm X, Tt T, Hq H, Nh S, Mx N. Magnetic resonance imaging findings and their association with electroencephalogram data in children with partial epilepsy. Cureus. 2020;12(5):1.
3. Ünver O, Sp K, Uysal S, Ünver A. The epidemiology of epilepsy in children: a report from a Turkish pediatric neurology clinic. J Child Neurol. 2015;30(6):698–702. doi:10.1177/0883073814539559
4. Samia P, Odero N, Njoroge M, et al. Magnetic resonance imaging findings in childhood epilepsy at a tertiary hospital in Kenya. Front Neurol. 2021;12:623960. doi:10.3389/fneur.2021.623960
5. Doescher JS, deGrauw TJ, Musick BS, et al. Magnetic resonance imaging (MRI) and electroencephalographic (EEG) findings in a cohort of normal children with newly diagnosed seizures. J Child Neurol. 2006;21(6):490. doi:10.1177/08830738060210061901
6. Cd W, Altmann A, Ja B, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain. 2018;141(2):391–408. doi:10.1093/brain/awx341
7. Aj B, Wb D, Guerrini R. Malformations of cortical development and epilepsy. Cold Spring Harbor Perspect Med. 2015;5(5). doi:10.1101/cshperspect.a022392
8. Apolot D, Erem G, Nassanga R, et al. Brain magnetic resonance imaging findings among children with epilepsy in two urban hospital settings, Kampala-Uganda: a descriptive study. BMC Med. Imaging. 2022;22(1):1–6. doi:10.1186/s12880-022-00901-7
9. Rs F, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. doi:10.1111/epi.12550
10. Centers for Disease Control and Prevention CDC. Prevalence of epilepsy and health-related quality of life and disability among adults with epilepsy--South Carolina, 2003 and 2004. MMWR. MMWR. Morbidity and Mortality Weekly Report. 2005;54(42):1080.
11. Wd G, Chiron C, Helen CJ, et al. Guidelines for imaging infants and children with recent‐onset epilepsy. Epilepsia. 2009;50(9):2147–2153. doi:10.1111/j.1528-1167.2009.02075.x
12. Wh T, Ss S, Wiebe S, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the international bureau for epilepsy, the international league against epilepsy, and the world health organization. Epilepsia. 2006;47(10):1700–1722. doi:10.1111/j.1528-1167.2006.00633.x
13. At B, Gw M, Ra B, et al. Frequency, prognosis and surgical treatment of structural abnormalities seen with magnetic resonance imaging in childhood epilepsy. Brain. 2009;132(10):2785–2797. doi:10.1093/brain/awp187
14. Durá‐Travé T, Me Y, Esparza‐Estaún J, Gallinas‐Victoriano F, Aguilera‐Albesa S, Sagastibelza‐Zabaleta A. Magnetic resonance imaging abnormalities in children with epilepsy. Eur J Neurol. 2012;19(8):1053. doi:10.1111/j.1468-1331.2011.03640.x
15. Fernández-Menéndez A, Casado A. Review and update on malformations of cortical development and neuronal migration disorders. Pediatr Adolescent Med. 2016;1(1):16.
16. Wa H, Jf A, Lt K. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34(3):453. doi:10.1111/j.1528-1157.1993.tb02586.x
17. Ali A, Akram F, Khan G, Hussain S. Paediatrics brain imaging in epilepsy: common presenting symptoms and spectrum of abnormalities detected on MRI. J Ayub Med Coll Abbottabad. 2017;29(2):215–218.
18. Sheikh HM, no S, Ali AB, Gökgül A, Hassan AF, Ih A. Epidemiology and risk factors of convulsive status epilepticus patients admitted in the emergency department of tertiary hospital in Mogadishu, Somalia. Int J Gene Med. 2022; 15:8567–8575. doi:10.2147/IJGM.S391090
19. Dirik MA, Sanlidag B. Magnetic resonance imaging and interictal electroencephalography findings in newly diagnosed epileptic children. J Clin Med. 2018;7(6):134. doi:10.3390/jcm7060134
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.