Back to Journals » International Medical Case Reports Journal » Volume 17
Lower Motor Neuron Facial Palsy Following COVID-19 Infection and COVID-19 AZD1222 Vaxzervria (AstraZeneca) Vaccine Administration: Two Case Reports
Authors Abbasher Hussien Mohamed Ahmed K , Siddig A, Abbashar A, Abbasher M, Abbasher AA, Hussien A, Alemam A Manhal G
Received 4 December 2023
Accepted for publication 12 March 2024
Published 23 March 2024 Volume 2024:17 Pages 215—219
DOI https://doi.org/10.2147/IMCRJ.S453158
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xudong Zhu
Khabab Abbasher Hussien Mohamed Ahmed,1 Amira Siddig,2 AlHussien Abbashar,3 Mohammed Abbasher,4 Abubaker Alsedig Abbasher,5 Abbasher Hussien,6 Gaffar Alemam A Manhal1
1Faculty of Medicine, University of Khartoum, Khartoum, Sudan; 2Department of Community Medicine, Faculty of Medicine, AlNeelain University, Khartoum, Sudan; 3AlYarmouk College, Khartoum, Sudan; 4Faculty of Medicine, AlNeelain University, Khartoum, Sudan; 5Zamzam University College, Khartoum, Sudan; 6Department of Internal Medicine and Neurology, Faculty of Medicine, University of Khartoum, Khartoum, Sudan
Correspondence: Khabab Abbasher Hussien Mohamed Ahmed, Faculty of Medicine, University of Khartoum, Khartoum, 11111, Sudan, Tel +249907712134, Email [email protected]
Abstract: Bell’s palsy is a lower motor neuron lesion rarely associated with COVID-19 infection or vaccinations. We documented two cases of Bell’s palsy in this report, one after contracting COVID-19 infection and the other after administration of AZD1222 Vaxzervria (AstraZeneca) Vaccine. After excluding all possible causes of Bell’s palsy in both cases, we determined that COVID-19 infection and the AZD1222 Vaxzervria (AstraZeneca) vaccine were the causes. Thus, we believe COVID-19 and the AZD1222 Vaxzervria (AstraZeneca) vaccine should be considered as causes of Bell’s palsy.
Keywords: COVID-19, SARS-CoV-2 virus, Bell’s palsy, AZD1222 Vaxzervria (AstraZeneca) vaccine, Sudan
Introduction
The coronavirus disease of 2019 (COVID-19) is defined as an illness caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Most people infected with the virus will experience mild to moderate respiratory symptoms and recover without needing treatment.1 Fever, sore throat, cough, shortness of breath, diarrhoea, and widespread weariness are the most prevalent symptoms. Acute respiratory distress syndrome, myocarditis, heart failure, renal failure, and recurrent pulmonary embolism are all complications of COVID-19.2
The most frequent neurological symptoms of COVID-19 infection are anosmia, ageusia, and headache. However, case series and observational studies show data on a large number of patients who develop cerebrovascular accidents (CVD), Guillain-Barré syndrome (G.B.S.), de novo status epilepticus and encephalopathy.3 In clinical terms, lower motor neuron lesion facial palsy is called Bell’s palsy. Bell’s palsy is usually idiopathic unilateral, acute weakness of the face and may be partial or complete, occurring with equal frequency on either side of the face.4 Additionally, tumours, trauma, infection, autoimmune illnesses, vasculitis, pregnancy and medicines can also cause Bell’s palsy.
After the COVID-19 pandemic, there was a documented link between COVID-19 and Bell’s palsy.5 Although, there was no clear explanation at the time; possible explanations include it could be caused by the direct action of the virus, an autoimmune response, or the recurrence of a coexisting herpes zoster infection.5 Additionally, in numerous countries, including the United States, a relationship between COVID-19 vaccines and lower motor neuron lesion facial palsy has been observed, although the causative link has yet to be proven.6 Although the precise mechanism of the neurological difficulties induced by COVID-19 vaccines is unknown, numerous theories have been proposed to classify these neurological disorders, including vascular, immunological, infectious, and functional causes.7
Cases’ Presentation
Case 1
A 65-year-old Sudanese woman was admitted to Omdurman Teaching Hospital with a high-grade fever and dry irritating cough. Clinical examination indicated a feverish patient with a pulse rate of 100 beats per minute and blood pressure of 100/70 mm Hg. Apart from the previous findings, clinical examination of the respiratory, cardiovascular, neurological and abdominal systems were normal. Her COVID-19 real-time polymerase chain reaction (RT-PCR) test was positive.
Three days after admission she complained of incomplete left eye closure and right-sided mouth deviation. A lower motor neuron injury affecting the facial nerve was discovered during cranial nerves and higher functions examination (facial nerve palsy). (Figure 1) Other cranial nerves were found to be normal. She did not experience skin eruptions, parotid enlargement, or tongue fissures. Upper and lower limb examinations were performed and found to be normal. She has no truncal or neck weakness and no area of hypoesthesia. Complete blood count (C.B.C), blood urea, serum creatinine, chest X-ray, and CT-brain were among the tests performed. All the tests were within normal limits. Following her COVID-19 infection, a diagnosis of Bell’s palsy was made. She was treated with prednisolone 60 mg daily for five days, then reduced by 10 mg daily. After ten days of corticosteroids, she exhibited significant improvement. No antiviral therapy nor physiotherapy was used for her condition.
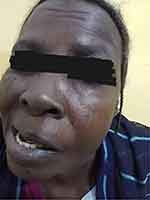 |
Figure 1 An incomplete left eye closure and a right-sided mouth deviation (Bell’s palsy) following COVID-19 infection. |
Case 2
A 45-year-old Sudanese man with no history of diabetes or hypertension presented to our private neurology clinic with an inability to close his right eye. On neurological examination, facial paralysis on the right side and a leftward displacement of the mouth were found. (Figure 2) All other neurological studies were within the normal range (muscle tone, reflexes).
 |
Figure 2 Facial paralysis on the right side, inability to close the right eye and leftward displacement of the mouth following AstraZeneca vaccine administration. |
The findings appeared three days after receiving the AZD1222 Vaxzervria (AstraZeneca) COVID-19 vaccination. Facial damage, ear pain and ear skin eruption did not precede the paralysis. His sense of taste was intact, and no transitory neurological symptoms preceded the event he described. He appeared ill, pale, and anxious during the assessment. His pulse rate was 87 beats per minute and his blood pressure was 130/75 mm Hg. There were no abnormalities found on systemic evaluation. He was diagnosed with a right-sided lower motor neuron lesion of the seventh cranial nerve, and the abnormalities were confined to the peripheral nervous system. Blood urea, serum creatinine, urine analysis, and a brain MRI were all performed and the results came back normal. Due to the absence of any apparent symptoms or signs that could specify the cause, a diagnosis of right-sided lower motor neuron lesion facial nerve palsy caused by the COVID-19 AZD1222 Vaxzervria (AstraZeneca) vaccine was made. He was treated with prednisolone 60 mg daily for five days, then reduced by 10 mg daily. After ten days of corticosteroids, he exhibited significant improvement. No antiviral therapy nor physiotherapy was used for his condition.
Discussion
COVID-19 is predominantly a respiratory illness, but it can cause multiple neurological symptoms such as headache, Guillain-Barre syndrome, transverse myelitis, epilepsy, and cranial nerve palsies.8 Bell’s palsy is an idiopathic, acute peripheral-nerve palsy involving the facial nerve, which supplies all the muscles of facial expression. The annual incidence of Bell’s palsy is 15 to 30 per 100,000 persons, with equal numbers of men and women affected. There is no preference for either side of the face. Bell’s palsy has been described in patients of all ages, with a peak incidence in the 40s.9
In this report, we documented two cases of Bell’s palsy, one after exposure to COVID-19 infection and the other after administration of COVID-19 AZD1222 Vaxzervria (AstraZeneca) Vaccine. Bell’s palsy is usually idiopathic; however, hypertension, diabetes, obesity, pregnancy, preeclampsia, trauma, tumours, infections, autoimmune illnesses and vasculitis have all been linked. According to the Clinical Practice Guidelines, which have identified Bell’s palsy as a diagnosis of exclusion, we considered all related causes of Bell’s palsy in our report.10 Other possible causes of Bell’s palsy such as trauma, malignancy, congenital causes, post-surgical and infectious etiologies were all negative after clinical evaluation. Thus, the diagnosis of Bell’s palsy for our two cases due to COVID-19 infection and COVID-19 AZD1222 Vaxzervria (AstraZeneca) vaccine was confirmed. The aetiology of Bell’s palsy following exposure to COVID-19 infection or vaccination requires further analysis, but it could be due to direct facial nerve inflammation and nerve compression inside the facial nerve canal. Another observed cause was immune-mediated damage to the facial nerve.7 Bell’s palsy is a severe unusual side effect of messenger R.N.A. (mRNA) COVID-19 vaccines. It is believed to be immune-mediated possibly via vaccine antigens mimicking host molecules or by activating autoreactive dormant T-cells, with a prevalence after mRNA-1273 (Moderna) vaccine not higher than the standard viral immunizations.11 According to a study by Wan et al in Hong Kong on the relationship between Bell’s palsy and the mRNA-based BNT162 b2 vaccine, patients who received the COVID-19 vaccine have a higher risk of getting Bell’s palsy than those who were not vaccinated.5 According to the US Food and Drug Administration and the UK Medicine and Healthcare Regulatory Agency, the observed prevalence of Bell’s palsy among vaccinated persons was no more significant than the expected background rate.12
Most of Bell’s palsy cases improved independently over time. Clinically important improvement occurs within 3 weeks in 85% of people and 3 to 5 months in the remaining 15%; however, some cases remain with residual facial weakness.4 Both patients in this report showed significant improvement after 10-day courses of corticosteroids.
Conclusion
COVID-19 infections have various clinical presentations including Bell’s palsy, a relatively rare symptom following COVID-19 infection as well as vaccination. This case report presented 2 cases of Bell’s palsy following COVID-19 infection and Vaccination. Nevertheless, the benefits of immunization outweigh the low reported incidence of similar vaccine’s adverse effects.
Data Sharing Statement
The data used in this report is available from the corresponding author upon reasonable request.
Consent for Publication
Both patients provided written informed consent for their case details and images to be published.
No institutional approval was required to publish this case report.
Acknowledgment
We acknowledge that this manuscript was released as a preprint in Authorea under the DOI: https://doi.org/10.22541/au.164787778.85729887/v1.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors themselves funded the study, and no funds were granted.
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. World Health Organization. Coronavirus; 2020. Available from: https://www.who.int/health-topics/coronavirus.
2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–Infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi:10.1001/jama.2020.1585
3. Camargo-Martínez W, Lozada-Martínez ID, Escobar-Collazos A, et al. Post-COVID 19 neurological syndrome: implications for sequelae’s treatment. J Clin Neurosci. 2021;88:219–225. doi:10.1016/j.jocn.2021.04.001
4. Holland NJ, Bernstein JM. Bell’s palsy. BMJ Clin Evid. 2014;2014:1204.
5. Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi:10.1016/s1473-3099(21)00451-5
6. Kyriakidis NC, López‐Cortés A, González EV, Barreto-Grimaldos A, Ortiz‐Prado E. SARS-CoV-2 vaccines strategies: a comprehensive review of Phase 3 candidates. Npj Vaccines. 2021;6(1). doi:10.1038/s41541-021-00292-w
7. Yang Y, Huang L. Neurological disorders following COVID-19 Vaccination. Vaccines. 2023;11(6):1114. doi:10.3390/vaccines11061114
8. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2021;43(1):3–40. doi:10.1007/s10072-021-05662-9
9. Tiemstra JD, Khatkhate N. Bell’s palsy: diagnosis and management. PubMed. 2007;76(7):997–1002.
10. Baugh RF, Basura GJ, Ishii LE, et al. Clinical Practice guideline: bell’s palsy. Otolaryngol Head Neck Surg. 2013;149(S3). doi:10.1177/0194599813505967
11. Renoud L, Khouri C, Revol B, et al. Association of facial paralysis with mRNA COVID-19 vaccines. JAMA Intern Med. 2021;181(9):1243. doi:10.1001/jamainternmed.2021.2219
12. Cirillo N, Doan R. Bell’s palsy and SARS-CoV-2 vaccines—an unfolding story. Lancet Infect Dis. 2021;21(9):1210–1211. doi:10.1016/s1473-3099(21)00273-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
