Back to Journals » Medical Devices: Evidence and Research » Volume 17
Lesson Learned from Mass Antibody Rapid Diagnostic Used in the Early COVID-19 Pandemic in Indonesia Contributors
Authors Indrati AR, Budiailmiawan L , Markus L , Johanis J , Logito V , Aryati
Received 7 November 2023
Accepted for publication 12 February 2024
Published 5 March 2024 Volume 2024:17 Pages 113—122
DOI https://doi.org/10.2147/MDER.S444025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Agnes Rengga Indrati,1 Luhung Budiailmiawan,2 Louisa Markus,3 Johanis Johanis,4 Verina Logito,1 Aryati5
1Department of Clinical Pathology, Faculty of Medicine Padjadjaran University/ Dr. Hasan Sadikin General Hospital, Bandung City, West Java, Indonesia; 2Department of Clinical Pathology, Pelabuhan Ratu Hospital, Sukabumi, West Java, Indonesia; 3Department of Clinical Pathology, Sidawangi Lung Hospital, Cirebon, West Java, Indonesia; 4Department of Clinical Pathology, Cengkareng Hospital, West Jakarta, Indonesia; 5Department of Clinical Pathology, Faculty of Medicine Airlangga University / Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia
Correspondence: Agnes Rengga Indrati, Email [email protected]
Introduction: Laboratory examination is extremely important in handling the COVID-19 pandemic. In the first era of the pandemic, the molecular and antigen tests were limited. Hence, at that time, it was necessary to carry out antibody Rapid Diagnostic Tests (RDT). However, many antibody RDTs were yet to obtain Food and Drug Authorization (FDA)’s approval.
Purpose: Therefore, The Indonesian Association of Clinical Pathology and Medical Laboratory (PDS PatKLIn) decided to conduct a validity test of RDT antibodies to find out the quality of SARS-CoV-2 diagnosis performance based on these RDTs used.
Patient and Methods: This is a descriptive observational design with diagnostic analysis. The retrospective secondary data were collected from 34 provinces in Indonesia from May to June 2020. Data analysis was carried out on the sensitivity and specificity values of each antibody RDT brand to the RT-PCR result and analyzed descriptive data.
Results: The amount of secondary data of antibody RDT and RT-PCR results collected was 139,908, consisting of 59 RDT brands of which 44% were authorized by The Indonesian COVID-19 Response Acceleration Task Force (Gugus Tugas Percepatan Penanganan COVID-19 Indonesia). There were huge variations of SARS-CoV-2 antibody RDT performance between total antibody types (sensitivity 59.18%, specificity 62%), IgM RDT (sensitivity 16– 100%, specificity 7– 97%), and RDT IgG (sensitivity 33– 96%, specificity 19– 100%).
Conclusion: The variations in the RDT antibodies’performance can cause errors in diagnosis leading to significant material and immaterial losses. Therefore, cooperation from various parties is needed for the pre- and post-marketing surveillance process to assess the performance and the characteristics of each RDT kit and other diagnostic methods to assist the rapid pandemic response process.
Keywords: antibody rapid diagnostic test, SARS-CoV-2, COVID-19
Introduction
At the end of 2019, there was an outbreak of a novel coronavirus infection from China which caused a worldwide COVID-19 pandemic.1 In February 2020, the World Health Organization (WHO) named the virus as Severe-Acute-Respiratory-Syndrome-Coronavirus-2 (SARS-CoV-2). For the past three years, the world has made various efforts to get over the pandemic by conducting laboratory tests, isolation, and vaccination.2 In March 2020, the WHO recommended conducting laboratory examinations on every patient suspected of COVID-19 with antibody RDT and PCR. Therefore, laboratory examinations have become one of the important pillars in establishing a diagnosis of COVID-19 to control and prevent infection transmission.
The first cases in Indonesia were on March 2, 2020. There were 10 confirmed cases of COVID-19 in Indonesia, then increased sharply on April 6, 2020 (1968 confirmed cases).3 The increasing case finding is related to the increase in COVID-19 testing laboratories which were initially focused on laboratories of the research and development agency of the Indonesian Ministry of Health. Then, on March 19, 2020, the Indonesian Ministry of Health determined 41 laboratories with surveillance functions, and 29 laboratories without surveillance functions.4
The world is trying to develop various laboratory diagnostic techniques to increase the screening capacity of the SARS-CoV-2-virus. On January 23, 2020, WHO developed a reverse- transcriptase-real-time polymerase-chain-reaction (RT-PCR) technique to detect the SARS-CoV-2-virus. Subsequently, on February 2, 2020, WHO distributed 250,000 examination kits to each country. Molecular tests other than RT-PCR are also being developed up to approximately 14 commercial products of nucleic-acid-amplification-tests (NAAT) to detect the SARS-CoV-2 virus. This NAAT technique is the standard in diagnosing SARS-CoV-2.5 The use of NAAT has become the gold standard in testing to detect unique sequences of the SARS-CoV-2 genome. The diagnostic accuracy of this technique is very importance.6 However, it has several disadvantages including the difficulty of obtaining adequate specimens (nasopharyngeal and oropharyngeal swabs), long processing time, requires sophisticated laboratory facilities, more expensive reagents, and particular trained human resources. At the beginning of the pandemic, there was a shortage of trained clinical laboratory manpower. The diagnostic center laboratories were forced to hire additional personnel who had limited experience and technical knowledge and molecular test skills, especially in processing specimens, interpreting results, identifying errors, and troubleshooting, to meet increased testing demands. This leads to a vulnerability to diagnostic errors, including cross-contamination, which is increased with the tendency for generating false-positive results that can compromise the health of the patient and disrupt the efficacy of public health policies and public health response, surveillance programs, and restrictive measures for containing the outbreak.6 Another test that has been developed is the SARS-CoV-2 antigen test, which is promising for screening tests but has limitation in sensitivity. Both molecular and antigen tests have limitations in detecting in the window period and cannot detect past infections. Since antibody examination can overcome these limitations, it was used to complement the COVID-19 examination strategy.7,8 The lateral flow immunoassay (LFIA) examination method, known as the rapid-diagnostic-test (RDT), was the only antibody examination method available during the earlier pandemic.
Since its first emergence at the end of 2019, the SARS-CoV-2 virus has mutated to give rise to several variants of concern (VOC) that rapidly spread globally. The emergence of SARS-CoV-2 variants makes it important for continuous monitoring of variants circulating in the population and the assessment of their sensitivity to neutralization by immune sera. Several in vitro immunoassays have been developed to study the neutralizing activity of the antibodies produced during infection or after vaccination.9 For example, prior to the omicron variant, monoclonal antibody therapy was shown to be very effective in preventing death, but may not be as effective as the omicron variant. The Omicron variant contains a mutation in the RBD previously thought to be highly conserved and a target for monoclonal antibodies. Among the 15 RBD substitutions in the Omicron variant, the K417N substitution is responsible for the most significant disruption to the known mAbs.10
At the beginning of the pandemic, there are little pieces of information that were known about the characteristics of COVID-19 such as the rate of transmission, the level of infection in asymptomatic patients, or the immunological response of patients infected with SARS-CoV-2. Therefore, serological tests are urgently needed to expand the screening strategy.11 On April 1, 2020, the first SARS-CoV-2 antibody RDT examination received an emergency use authorization (EUA).12 This antibody test is used to detect the SARS-CoV-2 humoral response and past SARS-CoV-2-virus infections. However, it cannot be used as the only diagnostic tool to detect SARS-CoV-2 virus infection. In the first week of April 2020, dozen brands of SARS-CoV-2 antibody RDT were spread in the market without authorization. Most of these antibody RDTs failed to detect the humoral immune response of SARS-CoV-2 infection and were not authorized by the Food and Drug Administration (FDA).13
Capacity constraints are a lack of reagents and equipment and limited human resources. As a result of these obstacles, most laboratories cannot operate. The main obstacle is dependence on imported supplies and associated procurement times. Expanding real-time polymerase-chain reaction testing capacity, through increased numbers of laboratories and optimization of existing facilities, was clearly the main priority. Results of potential assessment of the use of rapid molecular testing machines in referral hospitals in Indonesia. Even if this potential can be exploited, several provinces still do not receive adequate diagnostic services if a spike in cases occurs.14
Meanwhile, the spread of the SARS-CoV-2 antibody RDT has been massive and difficult to control, causing concern, especially in the Indonesian community of clinical pathologists, regarding the performance of the antibody RDT to detect SARS-CoV-2 infection. The Indonesian Association of Clinical Pathology and Medical Laboratory (PDS PatKLIn) considered the need to assess the performance and carry out a validity test on the SARS-CoV-2 antibody RDT that had been spread and widely used in the community. This prompted PDS PatKLIn to conduct a post-marketing survey of 59 brands of SARS-CoV-2 antibody RDTs, circulating in 34 provinces through 30 organizational chapters. Therefore, this study assessed the sensitivity and specificity of the SARS-CoV-2 antibody RDT as well as the difficulties encountered in carrying out the test. The performance of the SARS-CoV-2 antibody RDT and other results are expected to be used for the management of other pandemics that are likely to emerge in the future.
Material and Methods
This is a descriptive observational design with a diagnostic test analysis. The data were collected retrospectively to assess the validity, which includes sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the RDT antibody results against that of the SARS-CoV-2 RT-PCR. The secondary data are obtained from the community and hospitals that carry out antibody tests collected from clinical pathologists in 34 provinces, from May to June 2020. These data were the results of the SARS-CoV-2 antibody RDT and RT-PCR on patients with criteria for people without symptoms or asymptomatic people (AP), people under surveillance (PeUS) are people who has contact with confirmed patient, and patients under surveillance (PaUS) are patients with mild or severe symptoms who are in contact with confirmed patient. We can see Schematic Diagram Outlining the patient recruitment Process and Data Analysis Flow in Figure 1. Subsequently, the collected data were analyzed by diagnostic tests to calculate the sensitivity, specificity, PPV, NPV, and accuracy of the SARS-CoV-2 antibody RDT results against that of the RT-PCR examination. Statistical analysis was carried out using SPSS software version 20, and the results are presented in the form of tables and graphs.
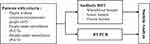 |
Figure 1 Schematic Diagram Outlining the patient recruitment Process and Data Analysis Flow. |
This study was approved by the Ethical Committee of the Faculty of Medicine, Padjadjaran University, and Dr. Hasan Sadikin General Hospital (No: 1003/UN6.KEP/EC/2022). All procedures followed were in accordance with the ethical standards put forth by the Helsinki Declaration of 1975, as revised in 2000.
Results and Discussion
A total of 139.908 secondary data of SARS-CoV-2 antibody RDT and RT-PCR collected between May-June 2020 were obtained from communities and hospitals in 34 provinces of Indonesia. The characteristics of the data such as patient status and type of sample are presented in the form of numbers and percentages as summarized in Table 1.
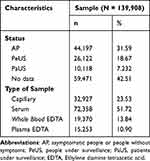 |
Table 1 Characteristics of Sample Data |
Most of the data were collected from a total of 44,197 asymptomatic people (AP), which is 31.59% of all data. The examination with unknown patient status was 42.51%; therefore, it cannot be concluded that the majority population passed through the examination at that time. The type of samples used mostly was a serum, in line with WHO recommendation (51.72%).15
The distribution of the SARS-CoV-2 antibody RDT was obtained 59 brands. The top 10 brands are presented in terms of number and percentage as shown in Table 2 and Figure 2. The most widely used brand was Wondfo, which was 61,793 examinations and reached 44.17% of all data collected.
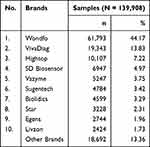 |
Table 2 The Top 10 SARS-CoV-2 Antibody RDT Brands in Indonesia |
 |
Figure 2 The top 10 SARS-CoV-2 antibody RDT brands in Indonesia (Antibody SARS-CoV-2 RDT that were used in Indonesia in early period of COVID-19 pandemic). |
All brands of SARS-CoV-2 antibody RDT were further investigated regarding their marketing authorization as shown in Table 3 and Figure 3. The recommendations by The Indonesian COVID-19 Response Acceleration Task Force (Gugus Tugas Percepatan Penanganan COVID-19 Indonesia) for RDT antibody SARS-CoV-2 Brand. Approximately 44% of all SARS-CoV-2 antibody RDTs have received recommendations from The Indonesian COVID-19 Response Acceleration Task Force (Gugus Tugas Percepatan Penanganan COVID-19 Indonesia), while the remaining 56% were not given recommendations.
 |
Table 3 Indonesian COVID-19 Response Acceleration Task Force Recommendation for SARS-CoV-2 Antibody RDT Brands (April 28, 2020) |
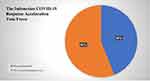 |
Figure 3 The proportion recommendations of SARS-CoV-2 antibody RDT. |
The various brands of SARS-CoV-2 antibody RDT were grouped based on the type of antibody examined into total, IgG, and IgM. The performance of each group was analyzed against the results of RT-PCR as the standard. The RT-PCR examination consists of Reverse-Real-Time-Polymerase-Chain-Reaction (rRT-PCR) and Molecular Rapid Test (TCM); using swab samples of the nasopharynx and oropharynx.
Collected antibody RDT results accompanied by RT-PCR results were 11,975 data (8.56%). The only brand of total antibody RDT was the brand with the highest number of uses, Wondfo with a performance of 59.18% sensitivity, 62% specificity, and 61.24% accuracy (Table 4). The SARS-CoV-2 total antibody RDT only provides one indicator, which is a combination of overall IgM and IgG antibodies value and is unable to differentiate the individual value of specific antibodies (IgM or IgG). Thus, explaining the low sensitivity and specificity of this type of RDT, it cannot determine whether the antibodies detected are from the past or the current infection. Since the detection is based on total antibodies, those formed from the current infection might not be adequate to give reactive results from the RDT examination.5 The PPV value of this RDT was 36.38% (33.85%–37.92%, 95% CI) and an NPV value of 80.52% (79.26%–81.79%, 95% CI). This indicated that the test is better used to rule out the possibility than to make a diagnosis of COVID-19.16
 |
Table 4 SARS-CoV-2 Total Antibody (IgG and IgM) RDT Performance |
The diagnostic performance of the SARS-CoV-2 IgG antibody RDT is presented in Table 5, which specifically only detects IgG-type antibody. The most widely of IgG antibody RDT data was Acro, which was 1389 data (39.66%), with the best diagnostic value. The diagnostic value of a total of 9 brands of SARS-CoV-2 IgG antibody RDT has a fairly wide range, which was 22.42–98.99%, 33.33–87.5%, and 16.08–99.06% for accuracy, sensitivity, and specificity. Several positive results from the IgG test that are generally considered markers for past infection indicate agreement with the results of the RT-PCR examination. Therefore, it revealed that IgG antibodies in COVID-19 can appear during acute infection. IgM antibodies, considered markers of current infection, showed a wider sensitivity range (16–100%) compared IgG antibody.8
 |
Table 5 The Performance of SARS-CoV-2 IgG Antibody RDT |
This secondary data also reveals problems in the examination process using the SARS-CoV-2 antibody RDT as shown in Table 6 and Figure 4. The most common problem encountered is that the results are unclear, making it difficult to determine whether they are reactive or non-reactive. This can be overcome by issuing a color grading card by the manufacturer to indicate at which level of color gradient the result is reactive. Another significant problem is the invalid results, where the SARS-CoV-2 antibody RDT and RT-PCR results from the same patient do not match. False-positive results were discovered mainly in the geriatric patient population with comorbidities and the pregnant patient population. The technical complaints in this study correspond to that of other investigations, where there are difficulties in reading the line and inconsistency of results in repeated examinations (reproducibility).15
 |
Table 6 The Performance of SARS-CoV-2 IgM Antibody RDT |
 |
Figure 4 Problems Reported relate to the Antibody Covid RDT kit. |
Generally, IgM antibodies appear earlier and have a shorter half-life than IgG. In the first week, IgG and IgM antibodies have low antibody levels and pass through seroconversion 7–12 days after the appearance of symptoms. IgM levels remain elevated for up to 3 weeks post symptoms and decreased rapidly. Meanwhile, IgG levels increased sharply compared to IgM in the first week after post-symptoms. The peak is at fifth week and persists until the seventh week.5,17 IgM levels were found to be higher in patients with severe cases than in moderate, mild, and asymptomatic cases, while IgG was still in asymptomatic, mild, moderate, or severe cases.18 This condition is probably the main cause of better IgG antibody RDT performance than the IgM antibody RDT group, considering that the data collected were mostly from the population without symptoms. The antibody RDT examination based on the principle of lateral flow immunochromatography (LFIA) method is the easiest, cheapest, and can be conducted anywhere. However, the performance of an antibody RDT depends on many factors such as endogenous, exogenous, and examination factors, which come from the patients, antibody products, and pre and post-analytic, respectively.18 Comorbid and physiological conditions also appear to affect the test results. The RDT of the SARS-CoV-2 antibody specificity was mostly reported to have a specificity above 95%, with various numbers of sensitivity.
A meta-analysis study reported a sensitivity range of 66–76%.19–21 Moreover, the diagnostic performance obtained showed a greater range than in other investigations. This is due to limitations in a retrospective examination of this secondary data, which include the heterogenecity of the overall examination process (pre- and post-analytical). The number of different samples for each group and brand of antibody RDT which affects the validity of each brand antibody RDT. The smaller samples than necessary will have insufficient statistical power to answer the main research question and lead to statistically insignificant results.21 The absence of data on the day of sampling from the onset of the disease. Additionally, these factors also become the main limitations of this study.
Conclusion and Suggestion
This study shows the general state of use of SARS-CoV-2 antibody RDTs at the beginning of the COVID-19 pandemic, where various brands flood the community with their respective advantages and limitations. A large variety of these SARS-CoV-2 antibody RDT brands were discovered. These include the type of antibody tested, completeness of recommendations and/or certification, diagnostic performance, and technical difficulties. These variations, especially in terms of diagnostic performance, can cause diagnostic errors; hence, there is a risk of being misdiagnosed or falsely diagnosed.
Therefore, cross-sectoral collaboration is needed, between regulators and implementers to overcome the pandemics that can emerge in the future such as the COVID-19 pandemic. Clinical Pathologists can have an important role in pre-, and post-marketing evaluation, as they are in charge of most of the clinical laboratory assays. This collaboration is expected to oversee the acceleration of the pre-market verification and validation process to guarantee the quality of diagnostic tools circulating in the community and limited diagnostic tools that have not been certified and/or whose diagnostic performance is unknown.
Abbreviations
RDT, Rapid Diagnostic Test; FDA, Food and Drug Authorization; PDS PatKLin, The Indonesian Association of Clinical Pathology and Medical Laboratory; SARS-CoV-2, Severe-Acute-Respiratory-Syndrome-Coronavirus-2; COVID-19, Coronavirus disease; WHO, World Health Organization; RT-PCR, Reverse Transcriptase-Real-Time Polymerase-Chain-Reaction; NAAT, Nucleic-Acid-Amplification-Tests; LFIA, The Lateral Flow Immunoassay; EUA, Emergency Use Authorization; PPV, positive predictive value; NPV, negative predictive value; AP, people without symptoms or asymptomatic people; PeUS, people under surveillance; PaUS, patients under surveillance; EDTA, Ethylene diamine tetraacetic acid.
Ethics Approval and Consent to Participate
This study was approved by the Ethical Committee of the Faculty of Medicine, Padjadjaran University, and Dr. Hasan Sadikin General Hospital Bandung, Indonesia (No: 1003/UN6.KEP/EC/2022). The data accessed complied with relevant data protection and privacy regulations. All procedures followed were in accordance with the ethical standards put forth by the Helsinki Declaration of 1975, as revised in 2000.
Acknowledgment
The authors would like to thank 543 contributors from 30 branch of The Indonesian Association of Clinical Pathology and Medical Laboratory (PDS PatKLIn) for providing the data. We are extremely grateful to the patients who participated in the study.
Funding
This research was funded by The Indonesian Association of Clinical Pathology and Medical Laboratory Specialists (PDS PatKLIn) and Padjadjaran University through grant-in-aid.
Disclosure
Agnes Rengga Indrati is an academic author, a clinical pathologist and a staff of the Immunoserology Department, Faculty of Medicine Padjadjaran University, Dr. Hasan Sadikin General Hospital. Luhung Budiailmiawan is a clinical pathologist and consultant of infectious disease. Johanis, Louisa Markus, and Verina Logito are clinical pathologists. Aryati is an academic author, a professor of clinical pathology and staff of the Infectious Disease and Immunoserology Department, Faculty of Medicine Airlangga University, Dr. Soetomo General Hospital, and the head of The Indonesian Association of Clinical Pathology and Medical Laboratory (PDS PatKLin). The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nguyen T, Wolff A. Rapid diagnostics for SARS-CoV-2 virus: point-of-care testing and lessons learned during the pandemic. Bioanalysis. 2021;13(15):1165–1167. doi:10.4155/bio-2021-0100
2. Nguyen T, Duong Bang D, Wolff A. 2019 novel coronavirus disease (COVID-19): paving the road for rapid detection and point-of-care diagnostics. Micromachines. 2020;11(3):306. doi:10.3390/mi11030306
3. World Health Organization. Indonesia: coronavirus Diseases (COVID-19) Dashboard with Vaccine [database on the Internet]. World Health Organization; 2023. Available from: https://covid19.who.int/region/searo/country/id.
4. Indonesian Ministry of Health. Decree of the Indonesian Minister of Health Number HK.0 1.07/MENKES/ 214/ 2O2O About Network of Examining Laboratories Coronavirus Disease 2019 (COVID-19). Jakarta: Indonesian Ministry of Health; 2020:1–11.
5. Smerczak E. SARS-CoV-2 Antibody Testing: where are we now. Lab Med. 2021;20:1–11.
6. Albano PM, Notarte KI, Macaranas I, Maralit B. Cross-contamination in molecular diagnostic laboratories in low- and middle-income countries: a challenge to COVID-19 testing. PJP. 2020;5(2):7–11. doi:10.21141/PJP.2020.09
7. Arevalo-RodriguezI I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. Pone. 2020;12:1–19.
8. Bohn MK, Loh TP, Wang CB, et al. IFCC interim guidelines on serological testing of antibodies against SARS-CoV-2. Clin Chem Lab Med. 2020;58(12):2001–2008. doi:10.1515/cclm-2020-1413
9. Chmielewska AM, Czarnota A, Bienkowska-Szewczyk K, Grzyb K. Immune response against SARS-CoV-2 variants: the role of neutralization assays. NPJ Vaccines. 2021;6(1):142. doi:10.1038/s41541-021-00404-6
10. Jary A, Marot S, Faycal A, et al. Spike gene evolution and immune escape mutations in patients with mild or moderate forms of COVID-19 and treated with monoclonal antibodies therapies. Viruses. 2022;14(2):226. doi:10.3390/v14020226
11. Bai Y, Yao L, Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi:10.1001/jama.2020.2565
12. U.S. FDA. Coronavirus (COVID-19) update: daily roundup April 2, 2020. U.S. FDA; 2020. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-daily-roundupapril-2-2020.
13. U.S. FDA. Coronavirus (COVID-19) update: FDA provides more regulatory relief during outbreaks, and continues to help expedite the availability of diagnostics. 2020. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19updatefda-provides-more-regulatory-relief-during-outbreak-continues-help.
14. Hendarwan H, Syachroni S, Aryastami NK, et al. Assessing the COVID-19 diagnostic laboratory capacity in Indonesia in the early phase of the pandemic. WHO South-East Asia Journal of Public Health. 2020;9(2):134–140. doi:10.4103/2224-3151.294307
15. World Health Organization. SARS-Cov-2 Antibody Detection Test Kit Performance Evaluation Protocol. Geneva: World Health Organization; 2020:1–16.
16. CDC. Interim Guidelines for COVID-19 Antibody Testing. Centers for Diseases Control and Prevention; 2022. Available from: https://www.cdc.gov/coronavirus/2019ncov/lab/resources/antibody-testsguidelines.
17. Iyer AS, Jones FK, Nodoushani A, et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. medRxiv. 2020;3:20.
18. Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response to COVID-19 severity. J Clin Invest. 2020;130(10):5235–5244. doi:10.1172/JCI138759
19. Lisboa Bastos M, Tavaziva G, Abidi SK, et al. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi:10.1136/bmj.m2516
20. Deeks JJ, Dinnes J, Takwoingi Y, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6(6):CD013652. doi:10.1002/14651858.CD013652
21. Andrade C. Sample size and its importance in research. Indian J Psychol Med. 2020;42(1):102–103. doi:10.4103/IJPSYM.IJPSYM_504_19
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
