Back to Journals » International Medical Case Reports Journal » Volume 17
Kaposi’s Sarcoma with Primary Lymph Node Involvement in a Retroviral Infected (RVI) Patient
Authors Fenta BD , Aregawi AB , Geremew TT , Fenta BK
Received 21 January 2024
Accepted for publication 28 March 2024
Published 8 April 2024 Volume 2024:17 Pages 311—319
DOI https://doi.org/10.2147/IMCRJ.S458320
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xudong Zhu
Bizunesh Dires Fenta,1 Alazar Berhe Aregawi,2 Teketel Tadesse Geremew,1 Berhanu Kelemework Fenta3
1Department of Pathology, Hawassa University Comprehensive Specialized Hospital, Hawassa, Sidama, Ethiopia; 2Department of Surgery, Hawassa University Comprehensive Specialized Hospital, Hawassa, Sidama, Ethiopia; 3Department of Internal Medicine, Yanet Internal Medicine Specialized Center, Hawassa, Sidama, Ethiopia
Correspondence: Alazar Berhe Aregawi, Tel +251911914624, Email [email protected]
Abstract: One kind of angioproliferative disorder is Kaposi’s sarcoma (KS). Growth of spindle-shaped cells, edema, inflammation, and neoangiogenesis are its defining features. Because it lacks the typical indicators of malignancy, it is classified as an intermediate neoplasm. People who are immunocompromised, receiving organ transplants, or receiving antiretroviral therapy are linked to KS. Although lymph node involvement by KS is extremely uncommon, when it does occur, it usually manifests as either the epidemic form in (Human Immuno-deficiency) HIV-positive patients or the endemic form in Africans. There are four primary clinical manifestations of KS that have been documented: endemic, epidemic, iatrogenic, and classic. The diagnosis of KS is made by history, physical examination, and tissue biopsy. When treating localized disease, highly active antiretroviral therapy (HAART) may be sufficient to either improve or completely eradicate the illness. Nonetheless, chemotherapy and HAART would be necessary in the case of widespread illness. Here, we present the case of a 28-year-old female patient who is HIV positive and has a viral load that is not detected. She presented with generalized lymphadenopathy of 8 months duration. She had no cutaneous manifestations. The lymphadenopathy involved the tonsils, axilla, inguinal, and an unusual site, intraparotid on both sides. After a pathologic examination of the lymph nodes, she was found to have epidemic-type KS and was treated with HAART and chemotherapy. In our nation, we are not aware of any published case reports pertaining to a case like this. The purpose of this case report is to raise physicians’ awareness of this uncommon ailment and to encourage them to suspect KS when HIV patients exhibit generalized lymphadenopathy. The early initiation of systemic treatment is lifesaving for these patients.
Keywords: Kaposi’s sarcoma, KS, Human immune-deficiency Virus, HIV, human herpes virus-8, Acquired Immuno-deficiency Syndrome, AIDS, generalized lymphadenopathy
Introduction
Kaposi’s sarcoma (KS) is an angioproliferative disorder. It is characterized by the proliferation of spindle-shaped cells, inflammation, edema, and neo-angiogenesis.1 Moritz Kaposi first described KS in 1872. KS is categorized as an intermediate neoplasm due to the absence of conventional features of malignancy.1 KS is associated with stage 3 human immunodeficiency virus (HIV) infection, which is known as acquired immunodeficiency syndrome (AIDS). It is the most common malignancy associated with AIDS.2 KS is associated with patients under antiretroviral therapy, organ transplantation, or immunocompromised.2 KS has been reported among all groups at risk for HIV infection.3 It generally occurs in patients with a low CD4 count, particularly below 100 cells/mm3, and a high viral load count of > 10,000 copies/mL.3–6 KS can present at any time during an HIV infection.3
The risk for KS in acquired immunodeficiency syndrome (AIDS) patients is estimated to be 20,000 times greater than in the general population and 300 times greater than in other immunocompromised populations.4
While HHV-8 does not cause symptoms in immunocompetent hosts, it can cause neoplastic illnesses like KS in immunocompromised hosts.1 Viral DNA from HHV-8 is present in all KS.2,4,5,7–11 As one of the diseases that characterise acquired immunodeficiency syndrome (AIDS), KS has a very varied clinical course, ranging from asymptomatic to quickly advancing disease.3,8,12 Stomatognathic manifestations take the form of violet or purple macules, papules, or tumours.13
Mucocutaneous areas, which include the skin of the lower limbs, face, trunk, genitalia, and oropharyngeal mucosa, are often where KS presents itself.6,14 Additionally susceptible are the lymph nodes and visceral organs, most frequently the gastrointestinal and respiratory systems.4,6,14
A tissue biopsy, physical examination, and history are used to diagnose KS.5,12,15
The severity of the skin lesions, the systemic symptoms of the illness, and the involvement of various organs all influence the choice of therapy for treating KS, which can be either systemic or local.1,16 When treating a localised disease, HAART medication by itself may be sufficient to improve or even cure the condition. The reduction of HIV replication and a drop in viral load accompanied by a rise in CD4 lymphocyte count are likely to be directly responsible for this. However, widespread illness would necessitate HAART in addition to chemotherapy.1,16
The purpose of this case report is to educate doctors about epidemic KS, an uncommon illness that affects people with HIV. When an HIV-positive patient initially appears with generalised lymphadenopathy without any cutaneous manifestation, doctors should be highly suspicious of this illness. Doctors also need to consider chemotherapy and HAART once the diagnosis is made. Overall, extracutaneous KS, including lymph nodes, accounts for 5.4% of the KS cases but up to now, we have not found a published case report of a similar case in our country.
Case Description
A 28-year-old known RVI female patient on HAART (Tenofovir, Lamivudine, and Dolutegravir) for the past 8 months, presented with swellings over the bilateral cervical, tonsillar, intraparotid, axillary, and inguinal areas of 8 months duration. The CD4 count of the patient was 378 cells/mm3 and the viral load was not detectable. Initially, the swellings were small and pea-sized and progressively increased to attain the size at presentation. Associated with the swellings, she had easy fatigability to the extent that she was unable to perform her daily activities. She had a low-grade intermittent fever, a loss of appetite, and a significant but unquantified weight loss. Otherwise, she did not have a skin rash, discoloration, or ulcer. She did not have a cough or shortness of breath. She did not have a history of diabetes or organ transplantation.
On physical examination
Her vital signs: Blood pressure was −115/70 mmHg, pulse rate was - 68, Temperature was- 37.4 °C, and oxygen saturation- 96%.
She had generalized lymphadenopathy over the bilateral cervical, tonsillar, intra-parotid, axillary, and groin areas; the largest lymphadenopathy measured 3x4 cm and was located in the right inguinal area. It was firm in consistency and non-tender. There were no other pertinent findings.
She was investigated with laboratory and imaging.
Investigations
On Serology
Complete blood count: WBC: 8400/uL with the differential of granulocyte (74.1%), lymphocytes (19.8%), and mixed (6.1%). Hemoglobin: 12.4 mg/dl, platelet count: 256×103 mm3; ESR of 15mm/hr;
Renal and Liver Function Tests Were Normal.
She was screened for hepatitis B and C, and they were negative.
On imaging
An ultrasound of the neck revealed several enlargements in the deep cervical, submandibular, intraparotid, and submental nodes on both sides. The hilum was compressed, the flow was disorganized, and there were hypoechoic echogenicity findings, all of which strongly suggested lymphoma.
Abdominopelvic ultrasound showed multiple inguinal, common femoral, and femoral nodal enlargements with compressed hilum, disorganized flow, and hypoechoic echogenicity. There was no intra-abdominal lymphadenopathy, calcification, or necrosis. All of which strongly suggested lymphoma.
Pathologic Examination
FNAC taken from bilateral cervical, axillary, and right groin showed a hemorrhagic background containing cohesive clusters of slender spindle cells with taper-ended nuclei. They were set in pinkish stromal fragments, along with small, mature lymphocytes and plasma cells in the background. The conclusion was a vascular tumor suggestive of Kaposi sarcoma (Figures 1–3).
A biopsy from the cervical LAP was sent for histopathologic confirmation.
Lymph node tissue looked completely effaced under a microscope. It was made up of interlacing fascicles of hyperchromatic and pleomorphic spindle cells. They formed vascular slit-like (longitudinal section) and sieve-like (cross-section) blood-filled spaces. Accompanying mononuclear inflammatory infiltrates and extravasated RBCs were also seen. Occasional mitotic figures, including abnormal forms, were noted. Subcapsular remnant lymphoid aggregates were also noted (Figures 4–7). The histomorphology was consistent with the FNAC finding, which is Kaposi sarcoma.
 |
Figure 4 Under10X magnification, shows a hemorrhagic background containing a cohesive cluster of slender spindle cells set in a pinkish stroma. |
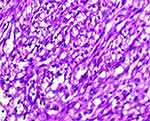 |
Figure 7 Under 100X magnifications, shows hyperchromatic spindle cells forming vascular slits and sieve-like blood-filled spaces. Accompanying mononuclear inflammatory infiltrates. |
Subsequently, the patient continued the HAART (Tenofovir, Lamivudine, and Dolutegravir) and was started on paclitaxel (175mg daily) and dexamethasone (8mg daily). She showed a partial response following the treatments. After 12 weeks, the patient’s performance status started to improve. The swellings started to decrease in size (the right groin LAP decreased to 2×2 cm), some disappeared (axillary lymphadenopathies), she started to perform her routine daily activities, and she gained weight (3kg). Currently, she is on follow-up every month.
Discussion
KS is a significant opportunistic illness that is typified by severe immunodeficiency and commonly affects patients who are HIV-positive.8,9 The incidence of KS in the general population is one case per 100,000 people, while in the HIV-positive population, it is one case per 20 people.17 KS was described in the literature as an uncommon cancer in central-eastern Europe and the Mediterranean in the late 19th and early 20th centuries. It was evident by the mid-1900s that KS moved from central Africa into southern Africa, forming a belt of countries that spanned the equator.18 An estimated 44,247 new cases of KS were diagnosed in 2012, with 85% of those cases occurring in Africa. An estimated 26,974 deaths globally are attributed to KS, with approximately 25,000 of those deaths taking place in sub-Saharan African nations.19 Compared to AIDS patients without KS, the mortality rate for patients with KS is significantly higher.20 When KS does occur in women, it progresses more aggressively than in men, despite the fact that female-to-male ratios in women range from 1: 3–15.21–23
KS is categorised into four distinct forms: iatrogenic disease, which impacts individuals who have received organ transplants and are undergoing immunosuppressive therapy; epidemic disease, which is the most prevalent opportunistic cancer in people infected with HIV-1; and the classical variant, which mainly affects older men of Jewish and East European ancestry. Conversely, the African endemic variant mainly affects children, teens, and adults in sub-Saharan Africa and has many extracutaneous manifestations.1,23–27 Patients who have a high viral load count of more than 10,000 copies/mL and a low CD4 count, especially fewer than 100 cells/mm3, are susceptible to KS.3–6 But our patient had a CD4+ count of 378 mm3, and the viral load was undetectable, which makes our case more atypical. Compared to the general population, the frequency and severity of epidemic KS have considerably decreased since the introduction of antiretroviral medication, going from a standardised incidence ratio of 22,100 to 3640. In our case, however, the occurrence of what is known as the immunological reconstitution inflammatory syndrome—a condition that arises in HIV patients who have begun antiretroviral therapy—can account for the development of KS.28
HHV-8, which infects KS spindle cells, is linked to KS.8,23,29 HHV8 infection is not ubiquitous. Although it affects less than 5% of the general population in the USA and Europe, men who have sex with men (MSM) and certain regions of the world, like sub-Saharan Africa and the countries bordering the Mediterranean, have much higher prevalence rates of it.30 Although the exact mechanisms of HHV8 transmission remain unknown, it has been demonstrated that saliva and intimate sexual contact account for the observed differences between population groups. Although less likely, blood transmission is also a possibility.30 Regardless of the region or population group, a high prevalence of HHV8 is linked to HIV infection, according to another meta-analysis that looked at the relationship between the two variables across the five continents.31 Our case is a known HIV patient, so she is categorized under AIDS-related epidemic KS, but what makes our case atypical is that she presented with generalized lymphadenopathy over bilateral cervical, tonsillar, axillary, inguinal, and even more atypical areas, intraparotid, without any cutaneous manifestation.
In all forms of KS, mucosal and cutaneous manifestations predominate. The lesions are classical, which means that these features primarily permit a clear clinical diagnosis. Just 5.4% of KS in a sizable AIDS-associated KS cohort were found to be non-cutaneous KS, indicating how uncommon non-cutaneous KS is. The prognosis for epidemic KS is poor due to increased visceral involvement.21,28 The gastrointestinal system and lungs are the most commonly involved visceral sites. Visceral involvement or diffuse dermatological lesions are frequently linked to lymph node involvement.1,21 For an HIV-positive patient with numerous cervical lymphadenopathies, concurrent lymphoma is an uncommon but potential diagnosis. The first clinical-radiologic diagnosis made for our patient was lymphoma; however, non-revealing CBC profiles, FNAC, and tissue biopsy ruled it out.21 Clinical manifestations of KS are typically described as raised plaques, papules, or macules that are pink, red, purple, or violaceous; however, nodular and clearly neoplastic-looking forms have also been reported.1,2,5,6,32 Mucocutaneous sites, which include the skin of the trunk, face, lower limbs, genitalia, and oropharyngeal mucosa, are where KS often presents itself.9,10,24,27 But since the introduction of HAART, the symptoms of KS have evolved significantly. Only a tiny percentage of KS patients exhibit lymph node and visceral disease as a result of HAART; our case falls into this tiny percentage of KS patients.4
The histopathological appearance of all clinical subtypes of KS is comparable.1 Histologically, the anaplastic variety is thought to be the most aggressive, and the slowest-growing type is the spindle cell variant. The growth rate of the mixed cell is in the middle.23 Our case’s histologic findings are consistent with the spindle cell variant. Early lesions are typically identified by an increase in tiny veins and capillaries surrounding one or more dilated vessels.1 It is common to observe a significant infiltration of mononuclear inflammatory cells. Spindle cell perivascular proliferation may also occur, but cellular atypia is not very noticeable.1
According to histology, the tumor’s nodules usually arise in the lymph node’s periphery, much like metastases from carcinomas that involve local glands. This pattern is not seen in cases of KS with generalized lymph node involvement; in these cases, the tumor appears to develop in the medulla of the node and gradually expand from within.22 In our case, the biopsy showed a similar pattern with the involvement of the medulla as well as the periphery of the node. The type of lymph node involvement in cases where the disease is in isolated glands is unclear because the tumor will completely replace the nodes, but the tumor likely originated from the medulla.22
Immunohistochemistry is not mandatory in the diagnosis of KS, but it is strongly recommended in cases where it is difficult to differentiate from other differentials. On immunohistochemistry, KS is characterized by expression of CD34, CD31, and CD2-40.10,12,15,32 For our patient, IHC was not done because the histomorphologic findings were classic and convincing for Kaposi sarcoma.
The management of KS is contingent upon factors such as the type of lesions, the extent of their spread, and the organs affected. When treating a localised disease, HAART medication by itself may be sufficient to improve or even cure the condition.1,4,11 This impact is believed to be directly caused by the inhibition of HIV replication, resulting in a reduction of viral load and an increase in CD4 cell count.4 Local therapeutic methods, such as surgical and laser removal, cryotherapy, radiation, injections of vinblastine or vincristine directly into the affected area, and the application of vinca alkaloids, bleomycin, or retinoids on the skin, are mostly recommended for small, specific lesions. Systemic therapy encompasses several treatment options for different forms of the disease. These include highly active antiretroviral therapy (HAART), which is effective against HIV, IFN-alpha, known for its ability to modulate the immune system and inhibit blood vessel growth, used for minimal cutaneous disease, and chemotherapeutic agents such as Paclitaxel, bleomycin, vincristine, and liposomal anthracyclines, which are used for disseminated and symptomatic forms of the disease. The most effective treatment for KS patients has not yet been determined, however recent advancements in comprehending the disease process may produce a universally accepted procedure.1,23 Our patient was started on paclitaxel (175mg daily) and dexamethasone (8mg daily) because she was diagnosed with one of the aggressive forms of Kaposi sarcoma which is epidemic type KS and she was symptomatic.
Conclusion
KS must be suspected in HVI patients presenting as generalized lymphadenopathies as an initial manifestation without any skin lesions, regardless of their viral load. High index suspicion is needed by physicians for KS as its presentation mimics lymphoma and early initiation of chemotherapy and HAART is considered to be lifesaving.
Data Sharing Statement
The data used to substantiate the study’s conclusions will be made available by the corresponding author upon reasonable request.
Ethical Review
The patient granted written informed consent for the publication of this case report, after obtaining authority from the Institutional Review Board (IRB) at Hawassa University.
Acknowledgments
The authors would like to acknowledge all the managing teams who are directly or indirectly involved in the management of this patient and the authors would like to acknowledge the patient who allowed us to undertake this case report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No particular grants from public, private, or nonprofit funding organizations were received for this paperwork.
Disclosure
Regarding the materials or procedures utilized in this study or the conclusions presented in this paper, none of the authors disclose any conflicts of interest.
References
1. Zoubeidi H, Aydi Z, Daoud F, et al. Kaposi’s sarcoma presenting as lymphadenopathy in an immunocompetent patient. Eur J Case Rep Intern Med. 2016;3(7):1.
2. Chakraborty R, Pandya D, Kar P, Sethi J. Kaposi’s sarcoma associated with advanced HIV infection: a case report. J Family Med Prim Care. 2020;9(8):4463. doi:10.4103/jfmpc.jfmpc_913_20
3. Tang ASO, Teh YC, Chea CY, Yeo ST, Chua HH. Disseminated AIDS-related Kaposi’s sarcoma. Oxf Med Case Rep. 2018;2018(12):452–454. doi:10.1093/omcr/omy107
4. Hamed KA, Muller KE, Nawab RA. Kaposi’s Sarcoma of the breast. AIDS Patient Care STDS. 2000;14(2):85–88. doi:10.1089/108729100318019
5. Goyack LE, Heimann MA. Disseminated Kaposi sarcoma. Clin Pract Cases Emerg Med. 2021;5(4):491–493. doi:10.5811/cpcem.2021.9.53692
6. Warpe BM. Kaposi sarcoma as initial presentation of HIV infection. N Am J Med Sci. 2014;6(12):650. doi:10.4103/1947-2714.147984
7. Costa R, Silva L, Monteiro R, Santos F, Mota M. Kaposi sarcoma as presentation of HIV – a clinical case. Cureus. 2021;13(10):e18936. doi:10.7759/cureus.18936
8. Ramos AL, Granado J, Calderón AI, André S, Nogueira F. Pulmonary Kaposi’s sarcoma-an atypical clinical presentation. Int J Infect Dis. 2022;115:185–188. doi:10.1016/j.ijid.2021.11.031
9. Pittore B, Pelagatti CL, Deiana F, et al. Isolated kaposi sarcoma of the tonsil: a case report and review of the scientific literature. Case Rep Otolaryngol. 2015;2015:874548. doi:10.1155/2015/874548
10. Rusu-Zota G, Manole OM, Galeș C, Porumb-Andrese E, Obadă O, Mocanu CV. Kaposi sarcoma, a trifecta of pathogenic mechanisms. Diagnostics. 2022;12(5):1242. doi:10.3390/diagnostics12051242
11. Htet KZ, Waul MA, Leslie KS. Topical treatments for Kaposi sarcoma: a systematic review. Skin Health Dis. 2022;2(2):e107. doi:10.1002/ski2.107
12. El Mawla Z, Ghannoum H, Saliba M, Michel Minari A, Kanaan HM. Visceral Kaposi’s sarcoma as a presentation in a newly diagnosed HIV-infected man: a case report. Cureus. 2022;14(3):e23339. doi:10.7759/cureus.23339
13. Shroff HJ, Dashatwar DR, Deshpande RP, Waigmann HR. AIDS-associated Kaposi’s sarcoma in an Indian female. J Assoc Physicians India. 1993;41(4):241–242.
14. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190. doi:10.1186/1471-2407-8-190
15. Radu O, Pantanowitz L. Kaposi Sarcoma. Arch Pathol Lab Med. 2013;137(2):289–294. doi:10.5858/arpa.2012-0101-RS
16. Odongo FCA. Fatal disseminated Kaposi’s sarcoma due to immune reconstitution inflammatory syndrome following HAART initiation. Case Rep Infect Dis. 2013;2013:546578. doi:10.1155/2013/546578
17. La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’ s sarcoma in HIV-positive patients: the state of art in the HAART-era. Eur Rev Med Pharmacol Sci. 2013;17(17):2354–2365.
18. El-Mallawany NK, McAtee CL, Campbell LR, Kazembe PN. Pediatric Kaposi sarcoma in context of the HIV epidemic in sub-Saharan Africa: current perspectives. Pediatric Health Med Ther. 2018;9:35–46. doi:10.2147/PHMT.S142816
19. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
20. Makombe SD, Harries AD, Jkl Y, et al. Outcomes of patients with Kaposi’s sarcoma who start antiretroviral therapy under routine programme conditions in Malawi. Trop Doct. 2008;38(1):5–7. doi:10.1258/td.2007.060023
21. Leung TL, Wong LY, Cheuk A. Kaposi’s sarcoma presenting with multiple cervical lymphadenopathies in a renal transplant recipient: a case report. Hong Kong Med J. 2020;26(2):146–148. doi:10.12809/hkmj197971
22. Bhana D, Templeton AC, Master SP, et al. Kaposi sarcoma of lymph nodes. Br J Cancer. 1970;24(3):464–470. doi:10.1038/bjc.1970.55
23. Borki R, Lkhoyaali S, Laadam K, et al. Lymphadenopathic kaposi sarcoma in an immunocompetent young patient: a case report. Pan Afr Med J. 2017;28. doi:10.11604/pamj.2017.28.20.7726
24. Osei N, Fletcher G, Showunmi A, Ahluwalia M. A case of non-cutaneous Kaposi sarcoma. Cureus. 2022;14(12):e32394. doi:10.7759/cureus.32394
25. Fernandes F, Eloy C, Carimo A, et al. Simultaneous presentation of Kaposi sarcoma and HHV8-associated large B-cell lymphoma in the same lymph node: a rare diagnosis in an HIV-negative patient. Am J Case Rep. 2013;14:263–266. doi:10.12659/AJCR.883980
26. Shetty K, Giannini P, Achong R. Kaposi’s sarcoma of lymph node leading to a diagnosis of HIV. Oral Oncol Extra. 2005;41(9):234–237. doi:10.1016/j.ooe.2005.06.008
27. Bisceglia M, Amini M, Bosman C. Primary Kaposi’s sarcoma of the lymph node in children. Cancer. 1988;61(8):1715–1718. doi:10.1002/1097-0142(19880415)61:8<1715::AID-CNCR2820610833>3.0.CO;2-P
28. Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20(12):1645–1654. doi:10.1097/01.aids.0000238411.75324.59
29. Hussein MR. Cutaneous and lymphadenopathic Kaposi’s sarcoma: a case report and review of literature. J Cutan Pathol. 2008;35(6):575–578. doi:10.1111/j.1600-0560.2007.00844.x
30. Engels E, Atkinson J, Graubard B, et al. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007;196(2):199–207. doi:10.1086/518791
31. Rohner E, Wyss N, Heg Z, et al. HIV and human herpesvirus 8 co-infection across the globe: systematic review and meta-analysis. Int, J, Cancer. 2016;138(1):45–54. doi:10.1002/ijc.29687
32. Mohanna BS, Sánchez L J, Ferrufino Ll JC, et al. Lymph node involvement in classic Kaposi sarcoma: report of three cases. Rev Med Chil. 2007;135(9):1166–1170. doi:10.4067/s0034-98872007000900011
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





