Back to Journals » Research and Reports in Urology » Volume 15
It is Possible to Reduce Ureteral Stent Clogging and Stent-Related Symptoms to Soothe the Pain of the Patient: A Case Report
Authors Vogt B , Dove-Rumé J
Received 31 March 2023
Accepted for publication 28 June 2023
Published 4 July 2023 Volume 2023:15 Pages 315—319
DOI https://doi.org/10.2147/RRU.S413199
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Benoît Vogt,1 Janine Dove-Rumé2
1Department of Urology, Polyclinique de Blois, La Chaussée Saint-Victor, France; 2English Department, François-Rabelais University, Tours, France
Correspondence: Benoît Vogt, Department of Urology, Polyclinique de Blois, 1 Rue Robert Debré, La Chaussée Saint-Victor, 41260, France, Tel +33 254906511, Fax +33 254906566, Email [email protected]
Introduction: Ureteral stent obstruction hinders the management of malignant diseases. Adequate stent insertion through an obstructed ureter does not necessarily guarantee renal decompression and stent-related symptoms adversely affect patient comfort. There are two major problems associated with ureteral stents: obstruction and intolerance to the stents.
Case Presentation: A 45-year-old woman was treated for cervical cancer with metastatic lymph nodes and ureteral obstruction with chemotherapy, radiotherapy, immunotherapy, and bilateral retrograde stenting. After recurrent stent obstruction, stent replacement was attempted more than 18 times over two years. In addition, stent-related symptoms adversely affected patient comfort. The patient was finally fitted with Superglide 8-French reinforced ureteral stents. Their replacement every six months was viewed by the patient as a relief compared to the all too frequent replacement of the previous stents. Moreover, the customized changes in the shape of Superglide stents improved patient comfort.
Discussion: Recent publications tend to indicate that large-lumen ureteral stents are most likely to remain permeable over time. Various modifications of the bladder or endo-ureteral part of double-pigtail stents have been increasingly reported, with the aim of improving their tolerance while maintaining effective drainage.
Conclusion: Adaptation of the internal lumen and shape of stents to the characteristics of the tumor and patient measurements appears to be important for increasing the drainage and tolerance of ureteral stents. The top priority for future ureteral stents suitable for malignant diseases should be to integrate these characteristics based on state-of-the-art data.
Keywords: malignant ureteral obstruction, ureteral stent, renal failure, stent failure, stent-related symptoms
Introduction
Ureteral obstruction due to disease progression can occur for patients with cancer and can reduce mean survival to 15 months.1–4 Renal obstruction caused by malignant diseases can bring about acute kidney injury, renal colic, or pyelonephritis, which induce additional suffering for the patient, who then feels that his/her plight is never-ending. In addition, stent-related symptoms adversely affect patient comfort and have been widely described, including in malignant diseases.2,5,6
Case Presentation
From 2015 to 2017, a 45-year-old woman was treated for cervical cancer with metastatic lymph nodes, including a 5-cm supraclavicular lymph node and ureteral obstruction, with chemotherapy, radiotherapy, and bilateral retrograde stenting.
The therapeutic effectiveness of such treatment was poor but immunotherapy with nivolumab resulted in a clinical response and complete remission by PET/CT and allowed treatment to be continued with surgery.
Concomitantly, recurrent stent obstruction occurred every two months or less and stent replacement was attempted more than 18 times over two years. Moreover, acute kidney injury appeared with a serum creatinine concentration of 2.7 mg/dL.
In 2017, the patient was fitted with 8-French reinforced Superglide Stents (Teleflex Medical, Ireland) on both sides and renal function improved, with the serum creatinine concentration dropping to 1.3 mg/dL. Following the change to these stents the ureteral stents were changed only every six months.
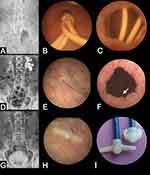
Nonetheless, the indwelling stents impaired the quality of life due to stent-related symptoms, despite the use of various stent shapes such as Polaris™ Loop stents (Boston Scientific) (Figure 1A–C). To improve the quality of life, the patient was fitted with 8-French pigtail-suture stents, with replacement of the bladder-loops by a thin suture thread (Figure 1D–F). In 2021, the patient was fitted with customized ureteral stents sectioned to the exact length of the ureter, with replacement of the bladder-loops by an end-piece that prevents the stent from slipping off into the ureter (Figure 1G–I). The pigtail-suture stents were replaced by customized stents with an end-piece for sudden ureteral orifice obstruction by post-radiation stricture. Replacement of the bladder-loops of the Superglide double-pigtail stents by these new devices decreased the urinary symptom score of the Ureteral Stent Symptom Questionnaire from 44 to 27.
Discussion
This case highlights the obstruction and intolerance of ureteral stents, which are the two major regularly reported problems and cause persistent patient suffering.4,5
Stent Obstruction
Ureteral stent obstruction hinders the management of malignant diseases. The failure of a single kidney may worsen acute kidney injury and is a barrier to several therapies, including chemotherapy. Stent obstruction divides survival by 2- or even 4-fold and if obstruction is not controlled during infection, death is very likely to occur within a month.3,7
Options for renal decompression include percutaneous nephrostomy or retrograde stenting.1 Percutaneous nephrostomy is a procedure in which a drainage tube can be placed under sedation, but dislodgement of the tube, with recurrent obstruction, is common. Most authors favor retrograde stenting. However, most studies have reported a mean stent-failure of 28% due to stent obstruction within an average of three months after placement. Stent obstruction can recur in under two months, even in under two days.1,2,8 Drainage of the malignant ureteral obstruction thus becomes a challenge for the urologist.3–5,8
Metal-mesh and Resonance® metallic ureteral stent (hereafter, “Resonance stent;” Cook Medical, Bloomington, IN, USA) have been developed as alternatives to simple ureteral stents1,8 but they are expensive and evaluation of their success in extrinsic ureteral obstruction compared to double-pigtail stents is difficult.8
In cases of extrinsic ureteral obstruction, Miyazaki et al reported that 50% of patients with a Resonance stent had died within eight months.9
On the contrary, Eaton Turner et al reported that the Memokath-051 stent allowed financial savings over five years,10 but for Miyazaki et al, only 14% of patients were still alive with a permeable stent at three years.10
Thus, Elsamra et al concluded that patients with extrinsic ureteral obstruction have a limited life expectancy and this fact could negate any purported cost-savings of metal-mesh or Resonance stents over double-pigtail or tandem stents.8
Finally, Ho et al noted that female sex and involvement of the ureteral orifice are poor prognostic factors, leading to obstruction of the Resonance stent in five months.11 In addition, the use of this type of stent did not appear to be optimal for this patient.
Recent studies comparing the stiffness and the lumen of several reinforced commercialized stents showed that these parameters appear to be important factors to maintain patency with respect to radial compression forces.12,13
Shilo et al examined the impact of ureteral deformation and external compression forces over a stented ureter to evaluate stent failure. By using an in vitro experimental setup with saline solution and double-pigtail stents (4.8 to 7-French), compression and high deformation of the stent, particularly under realistic degrees of compression, did not result in failure. The authors speculated that urine viscosity combined with aggregation of debris and large molecules are a common actual cause of stent failure.14 In another experiment using colloidal fluid, the authors found that colloids alone do not cause stent failure over time. Rather, it was the combination of colloid concentration, external pressure, and deformation that can lead to it. In these conditions, only the 8-French ureteral stent with a large lumen showed no change in renal unit pressure.15 In a recent experiment, only the 8-French stent remained patent consistently throughout the experiment duration, while tandem 7-French stents and Resonance stent failed. The authors concluded that larger luminal stents offer excellent resistance to external pressure and allow adequate colloidal flow.16 Moreover, in a clinical study including 156 patients over 10 years, the obstruction of an 8-French stiff stent in less than 6 months motivated the switch to 8-French tandem stents and thus made it possible to extend patency.4
Thus, it has been suggested that the likelihood of stent failure may decrease for larger lumen stents and tandem configurations because the likelihood of reaching >95% stent occlusion is lower relative to small lumen stents.17
This finding can help industrial manufacturers to integrate the physical notions in the concept of future stents by favoring stents with a large lumen and devoid of surface irregularities, which appear to favor encrustation.12–17
In the present case, stent replacement with larger lumen reinforced stents every six months was viewed by the patient as a relief compared to the all too frequent replacements of before.
Stent-Related Symptoms
Many stent designs have been reported to alleviate stent-related symptoms, but it is essential that stenoses be drained with a reinforced tube segment for malignant diseases.4,8 Symptoms are likely largely due to bladder irritation caused by the bladder loop (Figure 1C) and have been widely described, including in malignant diseases.2,6,18
Previous studies showed that the innovative pigtail-suture stent6 or a customized ureteral stent with a non-refluxing silicone end-piece18 significantly decreased stent-related symptoms. Indeed, the thin thread of the pigtail-suture stent (Figure 1E) or the small end-piece of the customized stent (Figure 1H) result in the presence of only minimal amounts of material in the bladder. Moreover, the sutures or the small end-piece are away from the bladder neck, avoiding any conflict with the bladder (Figure 1F). The pigtail-suture stents were replaced by customized stents with an end-piece for sudden ureteral orifice obstruction by post-radiation stricture. Indeed, the orifice obstruction had to be bypassed by a rigid stent segment and not by the suture. The end-piece is made up of two side wings that are flexible enough to bend when passing through the urethra during insertion (Figure 1I). The deployment of the wings prevents migration of the end-piece into the ureter when moving (Figure 1H). This customized stent with end-piece has shown encouraging results and multicenter studies with randomized, controlled trial should confirm the improvement in patients’ quality of life as reported and allow commercialization.
Ultimately, it has turned out that customized ureteral stents greatly improve patient comfort in terms of frequency and incontinence.
Current research to relieve irritation caused by stents and detect obstruction early is promising, with new innovations, such as the use of an exclusively intraureteral spiral stent19 or electronic screening of renal overpressure by a resonance-based wireless device.20
Conclusion
This present urgent message highlights the gravity of recurring stent failure and stent-related symptoms and shows how crucial active detection and subsequent treatment are to preserving renal function, overall survival, and health-related quality of life.
All actors currently fighting against cancer should be actively concerned by the choice of the appropriate stent: the patient, because he/she will live longer and with better comfort, the oncologist because the patient will obtain better renal function and a wider choice in his/her treatment, and the urologist, because drainage for the patients will be as rapid and efficient as possible, with the stent that suits them best.
The top priority for future stents suitable for malignant diseases should be to integrate dimensions of stent lumen and shape according to state-of-the-art data. Thus, it would be interesting to assess the impact of stent stiffness with wide lumen and customized stents with an end-piece on drainage efficiency and stent-related symptoms in patients in a prospective randomized controlled trial.
Ethics Approval and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (French Ethical Committee: 2015-09-11 and CPP 17-VOGT-01, National Medicine Safety Agency: 2015-A01181-48 and 2017-A00205-48) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent for the case details and accompanying images published was obtained from the patient included in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript are the following: Benoît Vogt received royalties from Rocamed for the treatment of ureteral stones but there are no financial competing interests in the manuscript. The authors report no other conflicts of interest in this work.
References
1. Pickersgill NA, Wahba BM, Vetter JM, et al. Factors associated with ureteral stent failure in patients with malignant ureteral obstruction. J Endourol. 2022;36(6):814–818. doi:10.1089/end.2021.0364
2. Goldfarb RA, Fan Y, Jarosek S, Elliott SP. The burden of chronic ureteral stenting in cervical cancer survivors. Int Braz J Urol. 2017;43(1):104–411. doi:10.1590/S1677-5538.IBJU.2016.0667
3. Rosenberg BH, Bianco FJ Jr, Wood DP Jr, Triest JA. Stent-change therapy in advanced malignancies with ureteral obstruction. J Endourol. 2005;19(1):63–67. doi:10.1089/end.2005.19.63
4. Vogt B, Blanchet LH. 10-year experience with reinforced ureteral stents for malignant ureteral obstruction. Res Rep Urol. 2021;13:581–589. doi:10.2147/RRU.S326274
5. Betschart P, Schmid HP, Abt D. Das ewige Leid mit dem Doppel-J-Katheter [Problems with Ureteral Stents – a Never-Ending Story]. Praxis. 2016;105:323–328. German. doi:10.1024/1661-8157/a002302
6. Vogt B, Desgrippes A, Desfemmes FN. Changing the double-pigtail stent by a new suture stent to improve patient’s quality of life: a prospective study. World J Urol. 2015;33:1061–1068. doi:10.1007/s00345-014-1394-2
7. Varnavas M, Bolgeri M, Mukhtar S, Anson K. The role of tandem double-J ureteral stents in the management of malignant ureteral obstruction. J Endourol. 2016;30(4):465–468. doi:10.1089/end.2015.0670
8. Elsamra SE, Leavitt DA, Motato HA, et al. Stenting for malignant ureteral obstruction: tandem, metal or metal-mesh stents. Int J Urol. 2015;22(7):629–636. doi:10.1111/iju.12795
9. Miyazaki J, Onozawa M, Takahashi S, et al. The resonance® metallic ureteral stent in the treatment of malignant ureteral obstruction: a prospective observational study. BMC Urol. 2019;19(1):137. doi:10.1186/s12894-019-0569-y
10. Eaton Turner E, Jenks M, McCool R, et al. The memokath-051 stent for the treatment of ureteric obstruction: a NICE medical technology guidance. Appl Health Econ Health Policy. 2018;16(4):445–464. doi:10.1007/s40258-018-0389-3
11. Bsh H, Chiu PKF, Lam W, et al. Risk factors in the prediction of long-term patency of resonance metallic ureteric stent in malignant ureteric obstruction. BJUI Compass. 2020;1(2):74–81. doi:10.1002/bco2.14
12. Vogt B, Blanchet LH. Analysis of ureteral tumour stents for malignant ureteral obstruction: towards reshaping an optimal stent. Res Rep Urol. 2021;13:773–782. doi:10.2147/RRU.S334277
13. Vogt B. Investigating the encrustation of reinforced ureteral stents by computational flow dynamic simulations. World J Urol. 2023;41:1451–1457. doi:10.1007/s00345-023-04356-5
14. Shilo Y, Modai J, Leibovici D, Dror I, Berkowitz B. The impact of ureteral deformation and external ureteral pressure on stent failure in extrinsic ureteral obstruction: an in vitro experimental study. J Endourol. 2020;34:68–73. doi:10.1089/end.2019.0465
15. Shilo Y, Modai J, Leibovici D, Dror I, Berkowitz B. Impact of colloidal fluid on stent failure under extrinsic ureteral obstruction: an in vitro experimental study. J Endourol. 2020;34:987–992. doi:10.1089/end.2020.0330
16. Shilo Y, Modai J, Leibovici D, Dror I, Berkowitz B. Comparative study of renal drainage with different ureteral stents subject to extrinsic ureteral obstruction using an in vitro ureter-stent model. BMC Urol. 2021;21:100. doi:10.1186/s12894-021-00865-w
17. Amitay-Rosen T, Shilo Y, Dror I, Berkowitz B. Influence of single stent size and tandem stents subject to extrinsic ureteral obstruction and stent occlusion on stent failure. J Endourol. 2022;36(2):236–242. doi:10.1089/end.2021.0426
18. Vogt B. A new customized ureteral stent with nonrefluxing silicone end-piece to alleviate stent-related symptoms in malignant diseases. Urology. 2020;137:45–49. doi:10.1016/j.urology.2019.12.022
19. Shilo Y, Willenz U, Berkowitz B. Design of a fully intraureteral stent and proof-of-concept in vivo evaluation. Transl Androl Urol. 2022;11:773–779. doi:10.21037/tau-22-41
20. Darestani MRY, Shalabi N, Lange D, Chew BH, Takahata K. Intelligent ureteral stent for early detection of hydronephrosis. Adv Mater Technol. 2021;2100652. doi:10.1002/admt.202100652
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.