Back to Journals » Research and Reports in Urology » Volume 15
Indocyanine Green (ICG)-Guided One-Stage Delayed Bladder Closure and Radical Soft-Tissue Mobilization (Kelly Procedure) For Bladder Exstrophy Repair: The First Experience
Authors Paraboschi I, Gnech M, Minoli DG, De Marco EA, Parente G, Mantica G, Bagnara V, Manzoni G, Leclair MD, Berrettini A
Received 28 June 2023
Accepted for publication 3 August 2023
Published 9 August 2023 Volume 2023:15 Pages 375—380
DOI https://doi.org/10.2147/RRU.S423521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Video abstract of "Kelly Procedure for bladder exstrophy repair" [ID 423521].
Views: 591
Irene Paraboschi,1 Michele Gnech,1 Dario Guido Minoli,1 Erika Adalgisa De Marco,1 Giovanni Parente,2 Guglielmo Mantica,3,4 Vincenzo Bagnara,5 Gianantonio Manzoni,1 Marc-David Leclair,6 Alfredo Berrettini1
1Department of Pediatric Urology, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy; 2Department of Pediatric Surgery, ASST Papa Giovanni XXIII Hospital, Bergamo, 24127 Italy; 3IRCCS Ospedale Policlinico San Martino, Genoa, Italy; 4Department of Surgical and Diagnostic Integrated Sciences (DISC), University of Genoa, Genoa, Italy; 5Department of Pediatric Surgery, Policlinico G.B.Morgagni, Catania, Italy; 6Chirurgie Infantile, CHU de Nantes, Nantes, France
Correspondence: Guglielmo Mantica, Consultant Urologist, Dirigente Medico, IRCCS Ospedale Policlinico San Martino, Genova, Italy, Email [email protected]
Abstract: The vascular supply of the pelvic structures and the external genitalia can be easily injured during the one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure) for bladder exstrophy surgical repair. Aiming to help surgeons assessing and confirming tissue perfusion and viability, indocyanine green (ICG)-based laser angiography was incorporated into the operative approach to reduce the risk of ischemic injuries. The EleVision IR system (Medtronic Ltd) was adopted to confirm the identification of the vascular pedicles and assess the tissue perfusion in real-time in a 5-month-old with bladder exstrophy undergoing the one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure). ICG (0.15 mg/kg) was intravenously administered at 6 key steps during surgery with the ability to be re-dosed every 15 minutes. ICG-based laser angiography helped to confirm the correct identification of the vascular structures during surgery and to assess tissue perfusion in real-time. Blood flow did not change considerably after initial dissection or upon approximating the pubis symphysis. At the end of the procedure, good penile perfusion was shown, proving that no direct injury or substantial compression of the pudendal vessels had occurred following the mobilization and the reconstructive phase. ICG-based laser angiography proved to be safe, effective, and easy to employ and should be considered as a reasonable adjunct for tissue perfusion assessment and operative decision-making in patients undergoing bladder exstrophy Kelly repair.
Keywords: bladder exstrophy, radical soft-tissue mobilization, fluorescence-guided surgery, indocyanine green, children
Introduction
The most appropriate surgical technique for bladder exstrophy reconstruction has been strongly debated. Advocates of the one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure) highlight the potential benefits of minimizing surgical steps, favoring the development of the bladder plate, increasing bladder outlet resistance, and enhancing penile elongation.1,2 Nevertheless, the complete mobilization of the corpora cavernosa from the ischio-pubic rami has been associated in certain cases with irreversible injury to the tissue perfusion and consequent penile loss.3
Conventionally, the visual inspection of the bladder and penile vascularization is the only modality used to assess tissue viability. More recently, however, fluorescence-guided surgery (FGS) has been increasingly involved in surgical settings, being considered a very promising intraoperative imaging modality to help surgeons visualize the tissue vascular supply in real-time thanks to the administration of near-infrared (NIR) fluorescent dyes or fluorescently labeled molecules.4–8
Worth noting, FGS has also been extending its applications in many fields of pediatric urology, where it has shown its value as a fundamental intraoperative tool to improve surgical and functional outcomes, minimize anesthetic time, and lower the overall healthcare costs.4–8
In particular, indocyanine green (ICG)-guided FGS has gained great popularity in surgical procedures in which the visual inspection of the tissue perfusion may not be very reliable to determine its viability.4–8
In recently published studies, Kaefer et al9 and Rained et al10 have highlighted the role of ICG-FGS for penile perfusion measurement in infants with bladder exstrophy undergoing closure in both complete repairs and staged repairs. However, bladder and penile perfusion testing using intraoperative laser angiography in children with bladder exstrophy undergoing the one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure) for bladder exstrophy repair have never been reported.
In this case report, we describe the first step-by-step protocol to perform ICG-FGS during the one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure) for bladder exstrophy repair in a 5-year-old boy. We hypothesize that the bladder and penile assessment of tissue perfusion at various points of the procedure may be a reasonable adjunct in patients undergoing closure of bladder exstrophy to lower the risk of intra- and post-operative complications while improving anatomical and functional surgical outcomes.
Case Presentation
This case report showed for the first time the step-by-step protocol for ICG-based one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure) for the repair of bladder exstrophy in a 5-year-old boy aiming to reduce the risks of intraoperative complications (Figure 1a).
The bladder and penile perfusion assessments were easy to evaluate, and no substantial technical difficulties were encountered.
ICG-based laser angiography helped to confirm the correct identification of the vascular structures during surgery (ie the neurovascular pedicle exiting the Alcock’s canal) and to assess tissue perfusion in real-time.
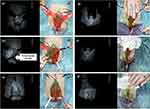
The first perfusion assessment (ICG, 0.15 mg/kg) was performed after the mobilization of the bladder plate and the exposure of both corpora (Figure 2a). This NIR fluorescent agent provided the real-time assessment of the tissue perfusion 80 seconds post-infusion with the ability to re-dose every 15 minutes.
The second measurement was performed after the full mobilization of the left corpus (Figure 2b). The periosteum of the left ischiopubic rami was incised and peeled away to allow the full mobilization of the ipsilateral corpus. Since ICG half-life is between 150–180 s, this helped to identify the neurovascular pedicle exiting the Alcock’s canal.
The third perfusion assessment was performed after the full mobilization of the bladder and both corpora cavernosa (Figure 2c). The right corpus together with the striated muscular structures linking the anterior pelvic ring were detached from the bones. This dissection was likely to induce reflex vasoconstriction and edema, factors that could predispose to ischemia. However, the blood flow did not change to any substantial degree.
The following assessment was performed after the separation of the epispadic urethral plate and corpora (Figure 2d). Penile disassembly epispadias repair allowed the freeing of the corpora from the short urethral plate and their external rotation. The urethral plate proved to be entirely mobilized.
The second-last perfusion assessment was performed at the end of the reconstructive phase (Figure 2e). Following the funnel-shaped cervicoplasty, the urethral plate was tabularized and the muscular structures previously mobilized were wrapped around the neo-urethra aiming to achieve physiological urinary continence. The bladder was then closed, the corpora were re-approximated, and the rectus abdominal muscles were sutured. Finally, also the pubic bones were re-approximated.
The intraoperative real-time laser angiography proved good tissue vascularization at the end of the mobilization and reconstructive phase. The bladder plate blood flow did not change to any substantial degree either after initial dissection or upon rotation of the hips with apposition of the symphysis pubis. This helped to proceed with the complete repair and deciding not to perform a staged approach.
The last ICG-based perfusion assessment was performed after the neo-urethra was transposed ventrally in a scrotal position, allowing for penile elongation (Figure 2f). The penile skin was then re-orientated dorsally to reconstruct the penile skin shaft (Figure 1b).
At the end of the procedure, good penile perfusion was shown, proving that no direct injury or substantial compression of the pudendal vessels had occurred following the mobilization and the reconstructive phase of the procedure.
The perioperative course was uneventful. Both esthetic and functional outcomes proved to be excellent also at one month’s follow-up (Figure 1c).
Discussion
In the last few decades, the management of infants born with bladder exstrophy has considerably improved, particularly, following the introduction of the radical soft-tissue mobilization (Kelly procedure) in the early nineties.1–3,11–13 This surgical technique has reached great recognition worldwide. Pediatric urologists dealing with the difficulties associated with the surgical reconstruction of this daunting congenital malformation early realized the remarkable advances provided by this procedure, including better functional, anatomical, and cosmetic outcomes.1–3,11–13 Despite the increasing global experience and information on their surgical treatment, however, infants born with bladder exstrophy still continue to represent a surgical challenge for pediatric urologists. In fact, intra- and post-operative complications remain important issues to deal with. In particular, skin dehiscence, urinary fistula formation, hemicorpora and glans loss have been reported as common short-term postoperative complications during the surgical repair of bladder exstrophy.3,14–16 Several causes for their occurrence have been evoked, including an inadequate blood supply, tension at the site of the surgical anastomosis, extensive mobilization of the soft tissues, and vascular compression during pubic approximation.3,14–16
Recently, ICG-based FGS has been introduced in several fields of pediatric surgery and urology, thanks to its ability to identify in real-time the tissue vascular perfusion.5–7,17 Initially developed for NIR photography by Kodak Research Laboratories in 1955, ICG was finally approved for clinical use by the Food and Drug Administration (FDA) in 1956.18 Intravenously administered, ICG has unique pharmacokinetics. In fact, by binding albumin, it is sequestrated in the vascular stream and completely excreted into the biliary tract within a few hours after its administration. Initially, its water solubility and fast biliary secretion made it a very promising tool for evaluating hepatic and cardiac function. In recent years, however, its clinical and surgical applications have considerably expanded, becoming a fundamental adjunct for several surgical procedures in both adults and children.18
In this regard, a recently published study performed by the Pediatric Urology Midwest Alliance (PUMA)9 has described the successful repair of 8 infants with bladder exstrophy who underwent either the Complete Primary Repair of Exstrophy (CPRE) or the first portion of a staged procedure incorporating penile perfusion testing using ICG-based laser angiography. The authors demonstrated that the closure of the bony pelvis resulted in decreased penile perfusion further accentuated in patients undergoing penile reconstruction. An objective and detailed method for penile perfusion assessment using ICG during CPRE has been also described in a 4-month-old boy by Raines et al.10 Further studies using this intraoperative imaging modality were advocated to develop individualized approaches in children with bladder exstrophy.
Aiming to reduce the risk associated with a bladder plate and penile poor vascular supply (eg skin dehiscence, urinary fistulas, penile corporal loss), we first incorporated ICG-laser angiography in the one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure) for the repair of bladder exstrophy.
In these infants, during the surgical procedure, it is fundamental to identify an adequate vascular supply of the bladder plate and maintain good vascular perfusion of the corpora and glans without tension or kink at the site of the surgical anastomosis. In fact, anatomic variations and congenital aberrations are extremely frequent in infants with bladder exstrophy, making the surgical procedure rather challenging.
The tremendous spatial resolution of even fine anatomical structures (eg the neurovascular pedicle exiting the Alcock’s canal), the great contrast, the high sensitivity, the lack of ionizing radiation, and the low cost are a few of the advantages provided by this novel intraoperative technique, we proved to be easily employed for the surgical treatment of infants with bladder exstrophy.
Our first case demonstrated that this novel intraoperative technique is a safe and effective intraoperative imaging adjunct in infants undergoing the one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure), and no adverse effects occurred.
The dose of ICG usually recommended ranges from 0.05 mg/kg to 0.15 mg/kg. We only used 0.15 mg/kg of ICG and achieved excellent ICG fluorescence, without reporting any side effects.
Regarding optical imaging devices, several cameras have been employed in pediatric surgery and urology to detect the fluorescence signal intraoperatively in real-time. To date, the Photodynamic Eye marketed by Hamamatsu Photonics Co (Hamamatsu, Japan), and the Image 1 S marketed by the Karl Storz GmbH & Co (Tuttlingen, Germany) have been the most commonly adopted devices.17
The EleVisionTM IR platform (Medtronic Ltd., UK) proved to be an excellent imaging system. It required a small amount of ICG and clearly delineate the tissue vascular perfusion in real-time. This device has two independent channels (one for visible and one for infrared light), which made the sensitivity of the NIR signal optimal for our goals.
Further studies involving more patients and longer experience with fluorophores and optical imaging devices are warranted to reach more firm conclusions. In fact, we are aware that a single case report and the fact that no numerical or objective way of measuring ICG change was presented represent the main limits of the current study. Despite these limits, the encouraging results provided by our and other studies9,10 suggest that ICG-based laser angiography will soon become a useful adjunct for tissue perfusion evaluation and intraoperative decision-making during the surgical repair of infants with bladder exstrophy. We believe its use will reduce the incidence of intraoperative complications, including skin dehiscence, urinary fistulas, and penile loss.
Conclusion
ICG-based one-stage delayed bladder closure and radical soft-tissue mobilization (Kelly procedure) using the EleVisionTM IR platform (Medtronic Ltd) was safe and effective in intraoperatively evaluating the blood supply of critical vascular structures during surgery. We believe that FGS will soon become part of the armory of pediatric urologists, being a promising intraoperative adjunct to lower the risk of intra- and postoperative complications.
Consent Statement
Written parental consent for publishing this study was obtained.
Institutional Review Board Statement
Ethical review and approval were waived for this study. Indocyanine green (ICG) is the most adopted NIR fluorescent probe in clinical and surgical practice. ICG was developed for NIR photography by Kodak Research Laboratories in 1955 and approved for clinical use by the Food and Drug Administration (FDA) in 1956.
Acknowledgments
This work was supported by the “Associazione per il Bambino Nefropatico ABN Onlus”, Milan, Italy, and by the “Centro di riferimento per le malformazioni congenite del rene e delle vie urinarie: prevenzione e cura del danno renale dal feto al bambino Sergio Bonelli”.
This study was funded by the Italian Ministry of Health - Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy.
We thank Mr Francesco Avino from Medtronic, Italy who supported the pilot with the EleVisionTM IR platform, and Ms Penelope Anne Bristow for the voiceover recording.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Leclair MD, Villemagne T, Faraj S, Suply E. The radical soft-tissue mobilization (Kelly repair) for bladder exstrophy. J Pediatr Urol. 2015;11(6):364–365. doi:10.1016/j.jpurol.2015.08.007
2. Leclair MD, Faraj S, Sultan S, et al. One-stage combined delayed bladder closure with Kelly radical soft-tissue mobilization in bladder exstrophy: preliminary results. J Pediatr Urol. 2018;14(6):558–564. doi:10.1016/j.jpurol.2018.07.013
3. Purves JT, Gearhart JP. Complications of radical soft-tissue mobilization procedure as a primary closure of exstrophy. J Pediatr Urol. 2008;4(1):65–69. doi:10.1016/j.jpurol.2007.02.006
4. Paraboschi I, De Coppi P, Stoyanov D, Anderson J, Giuliani S. Fluorescence imaging in pediatric surgery: state-of-the-art and future perspectives. J Pediatr Surg. 2020;S0022346820305820. doi:10.1016/j.jpedsurg.2020.08.004
5. Paraboschi I, Farneti F, Jannello L, Manzoni G, Berrettini A, Mantica G. Narrative review on applications of fluorescence-guided surgery in adult and paediatric urology. AME Med J. 2021;7:87. doi:10.21037/amj-20-194
6. Paraboschi I, Mantica G, Minoli DG, et al. Fluorescence-Guided Surgery and Novel Innovative Technologies for Improved Visualization in Pediatric Urology. IJERPH. 2022;19(18):11194. doi:10.3390/ijerph191811194
7. Privitera L, Paraboschi I, Dixit D, Arthurs OJ, Giuliani S. Image-guided surgery and novel intraoperative devices for enhanced visualisation in general and paediatric surgery: a review. Innovative Surgical Sci. 2022;6:161. doi:10.1515/iss-2021-0028
8. Nagaya T, Nakamura YA, Choyke PL, Kobayashi H. Fluorescence-Guided Surgery. Front Oncol. 2017;7:314. doi:10.3389/fonc.2017.00314
9. Kaefer M, Saad K, Gargollo P, et al. Intraoperative laser angiography in bladder exstrophy closure: a simple technique to monitor penile perfusion. J Pediatr Urol. 2022;18(6):746.e1–746.e7. doi:10.1016/j.jpurol.2022.10.012
10. Raines A, Fernandez N, Ahn J, Shnorhavorian M, Silverii H, Merguerian P. V04-01 Novel utilization of indocyanine green to assess bladder plate and penile perfusion during bladder exstrophy closure. J Urol. 2023;209(Supplement 4). doi:10.1097/JU.0000000000003252.01
11. Kelly JH. Vesical exstrophy: repair using radical mobilisation of soft tissues. Pediatr Surg Int. 1995;10(5–6). doi:10.1007/BF00182207
12. Berrettini A, Castagnetti M, Rigamonti W. Radical soft tissue mobilization and reconstruction (Kelly procedure) for bladder exstrophy repair in males: initial experience with nine cases. Pediatr Surg Int. 2009;25(5):427–431. doi:10.1007/s00383-009-2356-4
13. Cuckow P, Desai D, Ryan K, Johal N. Long term Results of the Kelly soft tissue reconstruction for continence in classic bladder exstrophy. J Pediatr Urol. 2007;3:S82. doi:10.1016/j.jpurol.2007.01.150
14. Cervellione RM, Husmann DA, Bivalacqua TJ, Sponseller PD, Gearhart JP. Penile ischemic injury in the exstrophy/epispadias spectrum: new insights and possible mechanisms. J Pediatr Urol. 2010;6(5):450–456. doi:10.1016/j.jpurol.2010.04.007
15. Pippi Salle JL. Commentary to “Penile ischemic injury in the exstrophy/epispadias spectrum: new insights and possible mechanisms. J Pediatr Urol. 2010;6(5):457–458. doi:10.1016/j.jpurol.2010.05.005
16. Chua ME, Ming JM, Fernandez N, et al. Modified staged repair of bladder exstrophy: a strategy to prevent penile ischemia while maintaining advantage of the complete primary repair of bladder exstrophy. J Pediatr Urol. 2019;15(1):63.e1–63.e7. doi:10.1016/j.jpurol.2018.09.005
17. Paraboschi I, De Coppi P, Stoyanov D, Anderson J, Giuliani S. Fluorescence imaging in pediatric surgery: state-of-the-art and future perspectives. J Pediatr Surg. 2021;56(4):655–662. doi:10.1016/j.jpedsurg.2020.08.004
18. Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA. Indocyanine Green: historical Context, Current Applications, and Future Considerations. Surg Innov. 2016;23(2):166–175. doi:10.1177/1553350615604053
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.