Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
Incidence of Mortality and Its Predictors Among Adult Human Immune Virus Infected Patients on Antiretroviral Therapy in Wolaita Sodo University Comprehensive Specialized Hospital, Southern Ethiopia: A Retrospective Follow-Up Study
Authors Barata TY , Abiso G, Israel E , Molla S, Wolka E
Received 1 February 2023
Accepted for publication 21 May 2023
Published 19 June 2023 Volume 2023:15 Pages 361—375
DOI https://doi.org/10.2147/HIV.S401155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Tagese Yakob Barata,1 Girumneh Abiso,2 Eskinder Israel,3 Simegn Molla,4 Eskinder Wolka4
1Department of Public Health Emergency, Institute of Bele Awassaa Health Office, Wolaita, Ethiopia; 2Department of Infection Prevention and Health Promotion, Institute of Sodo Town Health Office, Wolaita, Ethiopia; 3Department of Reproductive Health, Institute of Public Health, Wolaita Sodo University, Wolaita, Ethiopia; 4Department of Epidemiology and Biostatistics, Institute of Public Health, Wolaita Sodo University, Wolaita, Ethiopia
Correspondence: Tagese Yakob Barata, Email [email protected]
Background: Although the goal of ART is to have better health, extend the life of the HIV-infected patient, and decrease HIV-related death, there is a continuation of HIV-related mortality with the use of ART. This study aimed to assess the incidence of mortality and its predictors among adult HIV/AIDS patients who were on ART follow-up at Wolaita Sodo Comprehensive specialized hospital in southern Ethiopia.
Methods: A retrospective follow-up study was conducted from May 1 to June 30, 2021 among adult HIV/AIDS patients with a total of 441 adult HIV/AIDS patients in this hospital included. Kaplan–Meier failure curve and Log rank test were fitted, and Cox-proportional hazards model was also used to identify the predictors of mortality. Both crude and adjusted hazard ratios (AHR) with their 95% confidence interval (CI) were calculated to show the strength of association. The proportional assumption was conducted by using a global test based on the Schoenfeld residuals.
Results: Incidence of the mortality rate was 5.61 (95% CI, 4.2– 7.3) per 100 person-years observation. In the multivariable analysis, HIV/AIDS patients were widowed (aHR; 10.9 (95% CI, 3.13– 37.99), poorly drug-adhered (aHR; 5.6 (95% CI, 2.4– 13.2) and fair adhered (aHR; 3.53 (95% CI, 1.58– 7.87), WHO clinical stage IV (aHR; 5.91, (95% CI, 1.41– 24.71), history of substance use (aHR; 2.02 (95% CI, 1.01– 4.06) and history of IV drug use (aHR; 2.26 (95% CI, 1.10– 4.74) independently predicted the mortality of patients.
Conclusion: In this study, incidence of mortality was relatively high. The rate of mortality may be minimized by paying particular attention to individuals with widowing, substance use at the baseline, advanced clinical stage IV, history of IV drug use at the baseline, and those with adherence problems.
Keywords: mortality, anti-retroviral therapy, HIV/AIDS, Wolaita Sodo town
Background
Human immunodeficiency virus continues to be an important public health problem. Death from HIV/AIDS is highly affecting the health of people and development-related problems in many countries around the world. It has taken more than 38.0 million lives so far and 32.7 million people have died of HIV cases worldwide after the start of outbreaks.1 According to the 2020 UNAIDS report, the number of adult new HIV infections increased tremendously up to 62% globally.2
According to the World Health Organization report, about 25.6 million people were infected with HIV in Africa, accounting for two-thirds of new infections globally in 2016.3 In sub-Saharan African countries, it has been a threatening health issue for more than the last 20 years.4 Ethiopia is one of the sub-Saharan African countries and nearly one million people are living with HIV, and more than 114,000 people died due to AIDS. As a result, many parents died of AIDS and many children became orphans, increasing the incidence of HIV-related death rates, which indirectly affects a country’s growth and development.4,5
Meanwhile, scale-up of early ART accesses and treatment in the last few decades and specially from 2010 onwards, nearly 12.1 million AIDS related mortalities were minimized through the escalating ART treatment rolled out by the program.6,7 Thus, in 2019, there was a decrement of 39% AIDS related mortality since 2010 and approximately 690,000 deaths happened worldwide.7–9
The primary goal of ART is the betterment of health, extending the life of the HIV-infected patients, and lowering HIV death. However, there is a continuation of HIV related mortality with the use of ART.10 Although a number of countries at a national level were working on track to achieve the 90-90-90 target by 2020, there is still 31% and 39% of unmet need for ART coverage and viral load suppression respectively in late 2017.11 Although a recent and institutional-based survey indicated several individuals were living with HIV, the estimated prevalence of HIV/AIDS in Sodo town was more than 0.84% in the last few years which is significant and the town was one of list high burden (geographical prioritized) among 100 districts in Ethiopia.9,12 Wolaita Sodo town is unique because of its seven main gets that cross Sodo town from other towns such as Hawassa and many mega projects including (GIII and IV) and NGO workers cross so as it is a destination for many Ethiopians and is collectively known as a town of diversity. Many studies were done in many parts of Ethiopia to come up with time to death and the predictors of mortality in the age group greater than 15 years among people living with HIV/AIDS who were on ART follow-up. Despite the estimated average death rate of HIV/AIDS among patients on following and treatment for ART was ranging from 8.5 to 12% during late 2016, more than half of the mortality happened in the first twelve months of treatment.13
Different studies were conducted to predict the death of HIV/AIDS patients who were on follow-up. Some studies suggested that ART patients who began follow-up with lower CD4 count were mostly predicted death of patients.9,13,14 However, the predictors of survival of HIV/AIDS-infected patients who were on ART follow-up were not identified in our study area so far. Hence, this study was designed to investigate and summarize the incidence of mortality and its predictors among adult HIV infected adults who were on ART follow-up in Wolaita Sodo Comprehensive specialized hospital in Southern Ethiopia.
Methods and Materials
Study Design and Setting
A retrospective follow up study was conducted in Wolaita Sodo comprehensive specialized hospital from May 1 to June 30, 2021 in Wolaita zone, Sodo town. It is found in Southern part of Ethiopia, located 380 Km away from Addis Ababa, the capital city of Ethiopia & 158 km from Hawassa city which is the administrative city of SNNPRS. In zonal level, Wolaita Sodo Comprehensive specialized hospital is the only public hospital giving service for greater than three million people in different outpatient and inpatient subdivisions with the depiction of different near districts and regions. The hospital was accepted as a clinic in Sodo town, Ottona village, in 1928 by missionaries and lived as a district general and secured referral hospital status on September 2013 G.C. The hospital has a total of 1563 ART follow up patients.12,15
Study Population
All adult HIV/AIDS patients who started ART in WSUCSH were the target population and all adult HIV/AIDS patients who started ART in Wolaita Sodo comprehensive specialized hospital from the year 2015–2020 G C was the source population but those all selected adult HIV/AIDS patients who started ART during the study period was this study population. All adults aged having HIV/AIDS who started ART and were on follow-up at the ART clinic during the study period at Wolaita Sodo Comprehensive specialized hospital were included in this study. But, patients with incomplete records and transferred in from other facilities were excluded from the study.
Sample Size and Sampling Technique
The sample size was calculated based on the double population formula by Epi info version 7.2.3.1 considering the following assumptions: 95% CI, power 80%, the ratio of unexposed to expose was 2:1, the anticipated proportion of mortality rate among patients with WHO clinical stage I and II (unexposed) was 3.5% and anticipated proportion of mortality rate among patient WHO clinical stage III and IV was 11.6%.16 Then, the final sample size after the adding of 10% of contingency for incompleteness; therefore, the sample size of WHO clinical stage I and II (unexposed group) was 294 and for WHO clinical stages III and IV (exposed group) was 147; which gives a final study population of 441.
After evaluation of all electronic records of patients who initiated ART in facility, those who fulfilled the inclusion criteria were selected and put in Excel spreadsheets by using a special ART code to prepare the sampling frame. To select study subjects, a simple random sampling (SRS) technique was used to select 441 records using computer-generated random number table. Finally, the selected especial ART numbers were helped to dig out data from the electronic sheet.
Data Collection Tools and Procedures
Data collection format was prepared in English version by reviewing from the national ART intake and follow-up forms and different similarly literatures.9,10,13,16 Socio demographic data (marital status, sex, age, occupation, residence, and educational status), baseline data, and laboratory data were collected by reviewing pre-ART registers (like socio-demographic data), ART intake forms, laboratory requests, individual patient’s folder and monthly follow-up charts by experienced data collectors and daily activities of data collection team evaluated by principal investigators. The most recent laboratory test before initiation of ART was used as a baseline data. If there was no pretreatment laboratory test, results obtained within two months of ART start were used as baseline. If two results were obtained within a month, their mean was used. Death from all AIDS related causes was collected by reviewing patients’ medical records of hospital or registrations and individual folders through the medical record numbers reported by trained data collectors. Data was collected by three trained data collectors who were having a diploma in nursing and one supervisor who held BSc in public health, who was trained on comprehensive HIV care and who has experience in the ART clinic were recruited.
Data Quality Managements
Pretest was done with 5% of study sample size of patient chart in Bale Primary Hospital to check data collection tools for necessary modification. The data collectors and the supervisor were trained on the data collection procedures for two days. The prober was a head substantially undertaken. All finalized data collection forms were examined for completeness and clarity before and during data management, storage, and analysis.
Definition and Operationalization of the Variable
Survival /Alive: No experience of any recorded death during ART follow up.14 Death: Any death recorded as HIV/AIDS-related, including deaths because of opportunistic infections, secondary to delayed ART start and inappropriate intake of both ART regimen and prophylactic antibiotics.14 Transferred out: A client who is transferred to another health facility for care after the start of treatment and follow up.14 Lost to follow-up: HIV/AIDS Patients who had not been seen at ART care and treatment center for ≥1.17 Censored: right censoring, are these cases like alive, transferred out, and lost to follow-up patients at the end of follow-up and coded as zero.17 Event: death; primary outcome variable and time variable is the time to occurrence of death measured from the date of initiation of ART to date event happen and coded as one.17 Incidence mortality rate: occurrence of death/outcome after initiation of ART. Survival time: the number of days from the date of enrollment of a patient in the HIV-care till one of the events death.17 Person time: a total time at risk that all participants contributed to study.17,18 Bedridden: Inability to attain self-care in daily living, ambulatory; clients able to perform activities of daily living and working; clients who are able to perform usual work in or out of the house.14 History of IV drug use: patients who admitted to inpatient and used IV medication for any illness within the first 2 months of follow-up after initiation of ART.
Data Process and Analysis
Data was entered into Epi-data software version 4.7.1.2 for clean and check for coding errors, control of missing values, and inconsistencies before exporting to STATA software version 14 for analysis. Descriptive analyses of the continuous and categorical data summarize socio-demographic data; baseline and laboratory data during follow-up were made. Patients were counted as a censor, if lost to follow-up, if transferred to another health facility, or if alive at the end of follow-up. The survival time was calculated in months and then changed to years using the time between the date of treatment initiation and the date of the event (death) or date of censoring. The outcome of each patient was dialectic into censor or dead. Kaplan–Meier failure curve together with Log rank test was fitted to compare failure curves between the different categories of explanatory variables. Mortality rate with respect to person-time at risk was calculated.19
Proportional hazard assumption was tested graphically and a global test based on Schoenfeld residuals of the global test at the level of p=0.05 was conducted. Multicollinearity regression coefficient based was checked by the help of variance inflation factor based on value of VIF <10 that updates no collinearity among independent predictors.20 Variable was checked for significant level at P< 0.2 in the bivariate analysis and selection for multivariable Cox-regression analysis to identify the independent predictors of mortality was done. Associated variable was tested at 95% of CI and summarized by using AHR and any variable with p< 0.05 in the final model was taken as statistically significant. Adequacy of the model was checked by Nelson–Aalen cumulative hazard function against Cox–Snell residual.19,20 Result was presented in a table, figure, and graph.
Ethical Considerations
Ethical approval was obtained from the institutional review board (IRB) of the ethical review committee of the College of Health Sciences and Medicine of Wolaita Sodo University with Ref no of CRCSD95/02/13 E.C. Permission letter to conduct the study was obtained from Wolaita Sodo Comprehensive Specialized Hospital with Ref no. WSUCSH/270/2013 E.C. Confidentiality of information was maintained through avoiding any personal identifiers such as a name of patients on the questionnaires during data collection. All methods were carried out in accordance with the principles of the Declaration of Helsinki. Informed consent from the subjects and or legal guardians was not sought as the study was fully retrospective review of the chart. Wolaita Sodo University IRB waived the informant consent with the Ref no of CRCSD95/02/13 E.C. Finally, recorded data was kept safely by locking it in the locker and key of the locker was accessed only by principal investigator.
Results
Socio‑Demographic Characteristics of Patients
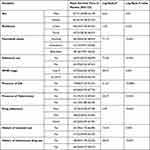
A total of 441 adult HIV-positive individual records were reviewed with the median age (IQR) of the respondents was 32 (28–40) years. From the total study participants, more than half (59.86%) were females and nearly two-thirds, 62.36%, of the patients lived in urban areas. About 28.34% were housewives and 38.32% were married. More than half (59.41%) of the participants were Protestant in religion and about 42.86% had primary education (Table 1).
 |
Table 1 Socio-Demographic Characteristics of the Study Participants of Adult HIV Patients on ART at Wolaita Sodo Comprehensive Specialized Hospital, Southern Ethiopia, 2021 |
Baseline Clinical and Laboratory Characteristics of Patients
Of the study participants, a mean weight (±SD) during ART start was 54.05±12.27kg. Around half of the patients (45.8%) had CD4 count rate ≤200 cells/μL; majority, 74.37%, had unimpaired functional status and greater than one-half of the patients (65.71%) were WHO clinical stage I and II in our study. Our results found from the study participants, 70 (15.87%) had a history of substance use and among them alcohol intake was commonly used 64 (91.42%); but only 22 (3.142%) used both chat and alcohol. Our results found the majority 361 (81.85%) of them had no TB comorbidity at the time of ART initiation, but 99 (22.44%) patients showed opportunistic infection at the time of enrolment. Our results revealed that greater than three-fourths of the study participants, 366 (82.99%), used a drug regimen of 1e combination (TDF-3TC-EFV) and 346(78.46%) patients disclosed their HIV status while 304 (68.93%) patients had a history of use of isoniazid at start of ART drugs. Great amount of patients, 315 (71.43%) initiated ART with no history of intravenous drug use and more than half, 226 (51.24%) had normal range of platelet count rate in their blood cells during the start of ART. Two thirds, 281 (63.7%), had a normal range of blood Creatinine level during the start of ART (Table 2).
 |
Table 2 Baseline Clinical and Laboratory Characteristics of the Study Participants of Adult HIV/AIDS Patients Receiving ART at Wolaita Sodo Comprehensive Specialized Hospital, Southern Ethiopia, 2021 |
Incidence of Death
A total of 441 patients were followed for a minimum of 1 month and a maximum of 71 with mean survival duration of follow-up was 25.66 months (95% CI, 1.09–23.51). In an investigation covering period, 53 (12.02%) with (95% CI, 9.28–15.41) patients died, 249 (56.46%) are alive and continuing their treatment in the health facility, 59 (13.38%) were lossed to follow-up and 80 (18.14%) were transferred to other facilities.
The cumulative proportion of survival at the end followed was 81.93% (95% CI, 76.38–8.63) (Figure 1). The cumulative proportion of survival at the end 6, 12, 24, 36 and 48 months were 92.2%, 90.64%, 87.09%, 85.58% and 83.66% respectively. Among patients who died during investigation covering period, 32 (60.37%), 5 (9.43%), 8 (15.09%), 3 (5.6%), 3 (5.6%) and 2 (2.77%) died during first 6 month, 6–12 month, 13–24month, 25–36month, 37–48 month and 49–60 months of follow up periods respectively and 31 (58.49%) of death happened among male patients.
 |
Figure 1 Overall survival probability of HIV/ADIS patients who started antiretroviral therapy at WSUCSH, Southern Ethiopia, 2021. |
The total risk time of the total participants was 943.2 person-years with an incidence of 5.61 (95% CI, 4.29–7.35) per 100 person-years of observation. Among the died patients during ART follow-up, 39 (73.58%) patients were WHO stage III and VI that gives a total risk time of 287.5 person-years with an incidence of 13.56 (95% of CI, 9.91–18.56) per 100 person-years of observation and the rest 14 (26.41%) with total death during follow up were WHO I and II that yield a total risk time of 655.7 person-years with an incidence of 2.13 (95% of CI, 1.26–3.60) per 100 person-years of observation. The graph showed that the survival time of STAGE III and IV is less than WHO stages I and II (Figure 2). At the end of 6, 12, 24, and 72 months of follow-up, the probability of survival among STAGE III and IV were 84.67%, 82%, 73.27%, and 62.12% and the probability of survival among STAGE I and II were 96.47%, 95.49%, 94.89% and 93.01% respectively.
 |
Figure 2 KM survival estimate for WHO stage among adult HIV patients receiving ART at WSUCSH, Southern Ethiopia, 2021. |
Comparison of the Survival Probability
From the Log rank test, there was a significant difference between sex (P=0.01), baseline functional status (p=0.001), baseline WHO stage (P=0.00), history of substance use (P<0.001), baseline drug adherence (p<0.001), baseline OI (p<0.0001), presence of Tuberculosis at baseline (p<0.001), history of use of ionized prophylaxis use at baseline (p=0.002), and history of intravenous drug use at baseline (p=0.001). From the Kaplan–Meier survival estimate, being female, the last known WHO stage III/IV (Figure 2), ambulatory and bedridden functional status, fair and poor drug adherence, having a history of substance use, having OI at baseline, having Tuberculosis at baseline, having a history of use of ionized prophylaxis use at baseline (p=0.002) and having a history of intravenous drug use at baseline had lower survival rate/were at higher risk of death (Table 3).
Proportional Hazard Assumption and Model Fitness
The p-value of the Schoenfeld residual test was insignificant (p>0.05) and there was no evidence for violation of the null-hypothesis. Global test of proportionality of hazards derived from Schoenfeld residuals of significant variables was not significant (df = 14.10, ch2 = 11, p = 0.2278) that shows the model was fit. The model fitness was checked by plotting Nelson-Aalen cumulative hazard with Cox-Snell residual and the Cox-Snell residual is almost in line with the Nelson-Aalen cumulative hazard (the hazard function follows the 45° line very closely showing the model was fit) (Figure 3).
 |
Figure 3 Nelson-Aalen cumulative hazard graph with Cox-Snell residual in adult ART patients at WSUCSH, Southern Ethiopia, 2021. |
Predictors of Mortality
Assessment of the proportional hazard assumption although the graphical (-ln (-ln) survival probabilities that parallel to each other and statistically scaled Schoenfeld residual global test for which was non-significant; (p>0.05) for each covariate indicated that the proportional hazard assumption was not violated and it indicated supporting proportional hazard modeling. Multi-collinearity regression coefficient based mean VIF was 1.37 which is a less <10 cutoff point that updates no collinearity among independent predictors and no variable dropped in case of collinearity.
To select independent predictors of mortality, multivariate regression was done for all predictors that recorded as significantly associated in the bivariate analysis at p<0.2. Based on the bivariate analysis of Cox survival regression model, sex of the patient, marital status, CD4 count, body mass index (BMI), functional status, CLINICAL stage, substance use, opportunistic infection, disclosure status, TB comorbidity, drug adherence, history of use of isoniazid, anemia, history of intravenous drug use, creatinine level and platelets count rate were associated with mortality of the patient and selected for multivariate analysis but only marital status, WHO clinical stage, substance use, history of intravenous drug use and drug adherence were found to be strong predictors of mortality in the multivariate analysis.
The hazard of death among widowed patients was 10.9 times higher than other marital status with a confidence interval of 95% (CI, 3.13–37.99), the hazard of death among poorly drug-adhered patients was 5.61 (95% CI, 2.4–13.2) times higher and faired adhered was 3.53 (95% CI, 1.58–7.87) times higher when compared to good drug adhered patients. The hazard of death was 5.91 times (95% CI, 1.41–24.71) higher in patients with WHO clinical stage IV disease compared to the rest STAGES. The hazard of death among history of substance use was 2.02 times (95% CI, 1.01–4.06) more likely than their counter parts. The hazard of death was 2.26 times (95% CI, 1.10–4.74) times greater than in the patient with intravenous drug users when compared to counter parts that were statistically significant (Table 4).
Discussion
This study sought to assess the incidence of HIV/AIDS patients who were on ART follow-up and determine the risk factors associated among HIV/AIDS patients. The study demonstrated various issues that contribute to the knowledge base in understanding the root cause as to why a patient with HIV/AIDS on ART survival deceased despite being on follow-up and treatment.
In our study, the overall survival probability of HIV/AIDS patients who were on ART was found to be 81.93% (95% CI, 76.3–86.30) at a total of 72 months follow-up. This result was consistent with a study conducted at Debre-berhan Referral Hospital, Ethiopia, in which the survival probability was 81.7%21 and in South Gondar, Northwest Ethiopia, in which the survival probability was 86.33%.22 Similarly, our study result also concedes with a study carried out across six Asia-Pacific countries, that the survival probability of 84%23 and India was indicated 78.3%.24 However, our study finding is higher than the study conducted in Jinka (South Omo), Ethiopia, where the mean survival probability was 64% (95% CI, 61.85 to 66.21%).13 In contrast, it is low when compared to a study carried out in Illubabor and Buno-bedele Zones, Ethiopia which was 94.5%.8 This might be due to differences in care, awareness, and support of patients in Ilibabur and Buno-bedele area health facilities.
Our study also indicated that the mean survival following time of a patient under ART at WSUTRH was 25.66 months (95% of CI, 23.51–27.82). This result was low when compared with the finding done at Attat Referral Hospital of Gurage Zone which was 46 months10 and 34.9 months in Southeastern Ethiopia.14 This could be due to the large number of patients in this study showed that early death and the effect of transfer to other facilities and a number patient lossed to follow-up before the end of the study highly appreciated during our study.
Our study revealed that an overall proportion of 53 (12.02% (95% CI, 9.28–15.41)) patients died while they were on ART follow-up for the last six years. Among the total deaths, thirty-two (63.9%) died within the first 6 months of starting ART. This is comparable with the study conducted in Jinka, South Omo (10.0%),13 Aksum (8.85%)25 and at Hiwot Fana Specialized University Hospital, Eastern Ethiopia (11.9%).26 Regarding death, our study result agrees with a collaborative study conducted in North America that reported 15.3% death rate.27 In contrast to this, our study finding was higher than the study conducted from six Asia-Pacific countries that reported lower death rate of 5.3%,23 in India of 2%28 and in Europe of 4.7%.27 However, our study finding is lower than a collaborative follow-up study conducted in South Africa which revealed 16.6%27 and 29.7% death rate was reported at Attat Referral Hospitals, Gurage Zone, Ethiopia10 as well as 16% in Illubabor and Buno-bedele zone.8 This might be due to an improved care of opportunistic infections at facilities and the way of lifestyle that directly or indirectly affect the health seeking behavior of the patients.
Our study showed that the incidence of mortality rate was 5.61 (95% CI, 4.29–7.35) per 100 person-years. This study finding is higher than studies conducted in different parts of Ethiopia such as; Axum (2.03/100PYs),25 Zewditu Memorial Hospital (3.8 deaths per 100PYs),29 Jinka South Omo (1.75 deaths per 100PYs),13 Southern Ethiopia (4.2/100PYs),16 Goba (Oromiya) (2.5/100 PYs),14 Debre-berhan Referral Hospital (4.18 per 100 person years),21 HiwotFana specialized University Hospital, Eastern Ethiopia (2.8 per 100 PYs)26 and South Gondar, Northwest Ethiopia (2.59 per 100 PYs).22 It was also higher than the study reported from India 0.56/100 PYs28 and Uganda (2.5 deaths per 100PYs).30 This marked variation in death rates might be due to the inequality in a number of participants, time extent of the study period, and the period of each cohort supported.
In our study, widowing was independently predicted mortality compared to the other marital status which agrees with the study conducted at Debre-berhan Referral Hospital, Ethiopia21 and contrary to this, being married could reduce 10% risk of death among HIV/AIDS patients who were on ART follow-up (aHR: 0.90; 95% of CI, 0.31–2.55) that was not statistically significant. This might be due to married individuals having better social assistance, more titanic family affairs, and minimize suffering and worry. Correspondingly, widowed individuals may have damaging health impacts related with worry and painful in the different situation. This might lead to a great number risk of mortality in widows than in those who have married ones.
According to substance use, substance use and mortality were significantly associated. This study finding is consistent with conducted in Jinka South Omo, Ethiopia.13 This could be due to the missing of ART drugs due to substance use as they stay longer with the use of substance intake may alter the decision making capacity to have daily drug intake which may also further expose them to many other chronic co-morbidities.
According to the World Health Organization clinical stage, stage IV patients were more likely to die 5.91 times (95% CI, 1.41–24.71, P=0.015) higher than the rest WHO clinical stages. This is supported by the findings from different studies conducted in different parts of Ethiopia. One study from southern Ethiopia indicated that the risk of death in advanced STAGE IV was 7.36 times higher when compared to the rest of WHO clinical stages.31 However, another similar report from Systematic Review and Meta-Analysis conducted in Ethiopia revealed that an advanced stage of the disease (stage IV) was strongly associated with ART follow up patients mortality.32 The study from Jinka (South Omo, Ethiopia) and elsewhere indicated that independent predict death were in line with that of WHO clinical stage IV.25,29,31,33
Mortality of HIV/AIDS patients who were on ART follow-up was highly predicted with patients having a history of intravenous drug use during the start of ART. This statement is in line with similar study conducted in Asia.28 This might be due to patients with history of intravenous drug use, who had decreased immune system and patients might be exposed to too many disease causing agents.
In our study, fair drug adherence and poor drug adherent patients had higher risk of death than patients with good drug adherence. This study is consistent with studies conducted in Jinka (South Omo), Ethiopia, and with the study done in Southern Ethiopia where poor ART drug adherence is a high risk of death than good adherence13,16 and comparable with report on Systematic Review conducted in Ethiopia where poor treatment adherence was a risk factor for mortality than good adherence.32 Likewise a study conducted in Southern Ethiopia public health facility-based study revealed that poor ART drug adherence was a higher risk of death than good adherence.13,16 This could be due to the lack of good adherence to ART, mostly related with clinical failure and failure to suppress viral duplication, and thus could increase the possibility of advancement of HIV alteration leading to the unfolding of drug-resistant viral strains. Adherence to ART is essential to the durability of HIV/AIDS infected people because lack of good adherence is the fundamental cause for poor treatment outcome among those who receiving antiretroviral therapy.34,35 Even though we pulled secondary data of patients to assess the status of adherence, it is better that HIV patient’s need great attention during follow-up, advice and counseling during each follow-up on how they properly and appropriately take their drugs.
Limitation of Study
Even though this study used double-checking of electronic database excel data with different official medical folders and registration book reports of each patient at the time of data collection for not clear and incomplete data, this study has some limitations. Firstly, due to its retrospectiveness, some variables were missed in the record which could predict the death of the patient. Secondly, deadliness might be underrated due to patients that lost to follow up and transfer out to other facilities before the end of the study; supposable to include furthermore individuals dying at home and other facilities without being revealed. Furthermore, there might be selection bias introduced due to the removal of incomplete records and unknown follow-up status.
Conclusion
In this study incidence mortality rate was relatively high. The independent predictors of mortality were widowing, substance use, advanced clinical stage IV, history of intravenous drug use, and fair and poor drug adherence. Policymakers, as well as governmental and non-governmental organizations, should pay more attention to patients widowing, substance use, advanced clinical stage IV, history of intravenous drug use at baseline, and adherence issues to decrease the incidence of mortality.
Abbreviations
3TC, Lamivudine; AIDS, Acquired Deficiency Immune Syndrome; ART, Antiretroviral Therapy; AZT, Zidovudine; COVID-19, Corona Virus Disease in 2019; D4T, Stavudine; DTG, Dolutegravir; EFV, Efavirenz; G-III/VI, Gilgile/saline project III/IV; HIV, Human Immune virus; HPACO, HIV/ADIS Prevention and Control Office; NVP, Nevirapine; MoH, Minister of Health; PLHIV, Patient Living with Human Immune Virus; PYs, Person Years; TDF, Tenofovir Disoproxil Fumarate; UNAIDS, Joint United Nation program on HIV/ADIS; WCA, Western and Central Africa; WSUCSH, Wolaita Sodo comprehensive specialized hospital.
Acknowledgments
The Authors would like to forward our gratitude to Wolaita Sodo University, College of Health Sciences and Medicine. We also thank the Head of Health institutions and data collectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. UNAIDS. Global HIV & AIDS Statistics Fact Sheet United Nations Political Declaration on HIV and AIDS. Geneva: UNAIDS; 2020.
2. UNAIDS. Global AIDS Monitoring: Indicators for Monitoring the 2016 United Nations Political Declaration on HIV and AIDS. Geneva: UNAIDS; 2017.
3. World health organization. HIV Drug Resistance Report. World health organization; 2017.
4. HAPCO. HIV/AIDS Estimates and Projections in Ethiopia 2011–2016 Addis Ababa. HAPCO; 2012.
5. world health organization fact sheet; 2012. Available from; http://www.who.int/mediacentre/factsheets/fs360/en/index.html.
6. The Joint United Nations Programme on HIV and AIDS. Global statistics-2016; 2014. Available from: http://aidsinfo.unaids.org/#.
7. UNAIDS. UNAIDS data received upon request by MSF; 2015.
8. Endalkachew M, Nezif H, Teka F. Reasons and predictors for antiretroviral therapy change among HIV‑infected adults at South West Ethiopia. BMC Res Notes. 2018;11:351. doi:10.1186/s13104-018-3470-y
9. Tadege M. Time to death predictors of HIV/AIDS infected patients on antiretroviral therapy in Ethiopia. BMC Res Notes. 2018;11(1). doi:10.1186/s13104-018-3863-y
10. Gebeyehu A. Survival analysis of HIV/AIDS patients under ART follow up in attat referral hospital. Sci J Appl Mathem Stat. 2020;8:42–46. doi:10.11648/j.sjams.20200803.11
11. UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic; 2017.
12. Beyamo A, Wolde F, Moshago T. Depression and associated factors among adult HIV/AIDS patients attending antiretroviral therapy at Wolaita Sodo University Teaching and Referral Hospital, Southern Ethiopia. HIV/AIDS Res Palliat Care. 2021;2021:707–715.
13. Erdaw Tachbele GA. Survival and predictors of mortality among human immunodeficiency virus patients on anti-retroviral treatment at Jinka Hospital, South Omo, Ethiopia: a six years retrospective cohort study. Epidemiol Health. 2016;38:e2016049.
14. Tesfaye AT, Gonzalez R, Coffer JL, Djenizian T. Predictors of Mortality among Adult Antiretroviral Therapy Users in Southeastern Ethiopia: retrospective Cohort Study. ACS Appl Mater Interfaces. 2015;7(37):20495–20498. doi:10.1021/acsami.5b05705
15. Woreda base plan. Wolaita Sodo town administration Health office; 2020.
16. FT AK, Gadisa B, Zerihun K, Hailu M, Merga H. Epidemiology of survival pattern and its predictors among HIV positive patients on highly active antiretroviral therapy in Southern Ethiopia public health facilities: a retrospective cohort study. AIDS Res Ther. 2020;17:49. doi:10.1186/s12981-020-00307-x
17. Tegiste Assefa EW. Survival analysis of patients under chronic HIV-care and antiretroviral treatment at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. EthiopJ Health Dev. 2012;26(1):22–29.
18. Lorraine K, Alexander D, Brettania Lopes MPH. Calculating Person-Time, UNC CH Department of Epidemiology; ERIC NOTEBOOK.
19. Vittinghoff GD, Shiboski S, McCulloch CE. Regression Methods in Biostatistics_ Linear, Logistic, Survival, and Repeated Measures Models. Springer Science+Business Media, Inc; 2005.
20. Hosmer LS, May S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data.
21. Nigussie F, Alamer A, Mengistu Z, Tachbele E. Survival and predictors of mortality among adult HIV/AIDS patients initiating highly active antiretroviral therapy in Debre-Berhan Referral Hospital, Amhara, Ethiopia: a retrospective study. HIV/AIDS Res Palliat Care. 2020;12:757–768. doi:10.2147/HIV.S274747
22. GD W. Survival time and associated factors among adults living with HIV after initiation of HAART in South Gondar, Northwest Ethiopia: a Retrospective Cohort. J Multidiscip Healthc. 2021;14:1463–1474. doi:10.2147/JMDH.S314004
23. Mata NL, Kumarasamy N, Khol V, et al. Improved survival in HIV treatment programs in Asia. PMC. 2017;21(6):517–527.
24. Dandona R, Rewari BB, Kumar GA, et al. Survival outcomes for first-line antiretroviral therapy in India’s ART program. BMC Infect Dis. 2016;16:555. doi:10.1186/s12879-016-1887-2
25. Kidane FH, Neway H. Predictors of mortality among patients enrolled on antiretroviral therapy in Aksum Hospital, Northern Ethiopia: a Retrospective Cohort Study. PLoS One. 2014;9(1):e87392.
26. Gemeda AB. Predictors of mortality among adult patients enrolled on Antiretroviral Therapy in Hiwotfana specialized University Hospital, Eastern Ethiopia: retrospective Cohort study. J HIV Clin Scient Res. 2018;5(1):007–0011.
27. Andrew MS, Margaret TM, Robert SH, et al. Mortality in Patients with HIV-1 Infection Starting Antiretroviral Therapy in South Africa, Europe, or North America: a Collaborative Analysis of Prospective Studies. PLoS Med. 2014;11:e1001718.
28. Rimke B, Nagalingeswaran K, Sanjay P, et al. Survival after long-term ART exposure: findings from an Asian patient population retained in care beyond five years on ART. Antivir Ther. 2021;25(3):132.
29. Shibre AK. Predictors of Survival in HIV-Infected Patient After Initiation of HAART in Zewditu Memorial Hospital, Addis Ababa. Ethiopia: Hindawi Publishing Corporation; 2014.
30. Victor EN, Racheal A, Thomas CQ, Frank C, Anja van H, Steven JR. Temporal trends of early mortality and its risk factors in HIV-infected adults initiating antiretroviral therapy in Uganda. EClinicalMedicine. 2020;28:100600.
31. Armel AH, Jacques Z, Nongodo Firmin K, et al. Mortality of HIV-infected patients on antiretroviral therapy in a large public Cohort in West Africa, Burkina Faso: frequency and Associated Factors. Adv Infect Dis. 2013;3:284.
32. Ayalew MB. Mortality and its predictors among HIV infected patients taking antiretroviral treatment in Ethiopia: a systematic review. AIDS Res Treat. 2017;30. doi:10.1155/2017/5415298
33. Laxmi EK, Keshab D, Rachana S, Deepak Kumar K, Anna Mia E, Luai Awad A. Survival on antiretroviral treatment among adult HIV-infected patients in Nepal: a retrospective cohort study in far-western Region, 2006–2011. BMC Infect Dis. 2013;13:1–9.
34. World Health Organization. Consolidated guidelines on the us for the of Antiretroviral drugs for the treating and preventing HIV infection; 2013. Available from: https://www.who.int/hiv/pub/guide.lines/arv2013/en/.
35. Federal Minstry of Health. National Guidelines for Comprehensive HIV Prevention, Care and Treatment. Addis Ababa: Springer; 2017:1–226.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.