Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Hyperkalemia and the Use of New Potassium Binders a Single Center Experience from Vestfold Norway (The PotBind Study)
Authors Bjune T , Bøe TB, Kjellevold SA, Heldal K, Abedini S
Received 22 December 2022
Accepted for publication 3 March 2023
Published 16 March 2023 Volume 2023:16 Pages 73—82
DOI https://doi.org/10.2147/IJNRD.S401623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Thea Bjune,1,* Thea Bjerkestrand Bøe,2,* Stig Arne Kjellevold,3,* Kristian Heldal,4,* Sadollah Abedini3,*
1Vear General Practitioner Group, Vear, Vestfold, Norway; 2Vestfold Hospital Trust, Medical Clinic, Toensberg, Vestfold, Norway; 3Vestfold Hospital Trust, Medical Clinic, Section for Kidney Disease, Toensberg, Vestfold, Norway; 4Department of Transplantation Medicine, Oslo University Hospital and Institute of Health and Society, University of Oslo, Oslo, Norway
*These authors contributed equally to this work
Correspondence: Thea Bjune, Vear General Practitioner group, Steinbruddveien 8, Vear, 3173, Norway, Tel +47 33362700, Email [email protected]
Purpose: Hyperkalemia is a common metabolic complication of chronic kidney disease (CKD) and is associated with several serious adverse events. We aimed to treat/prevent hyperkalemia using the new of potassium-binders, allowing maintained renin-angiotensin-aldosterone system inhibitors (RAASi) treatment in proteinuric CKD and/or congestive heart failure (CHF) patients.
Patients and Methods: We conducted a retrospective cohort study in long-term users of potassium binders for chronic hyperkalemia. Patients aged 18 years and older, treated with potassium-binders and who met the reimbursement criteria and indication for RAASi treatment were included.
Results: Fifty-seven percent of the patients were males and mean age was 65 years. During the study period, no patients were admitted to hospital due to hyperkalemia after initiation of potassium binders. Potassium maximum values were significantly lower after treatment. Few patients reported major side effects, and discontinuation was mostly due to normokalemia. We found no significant changes in bicarbonate, serum creatinine or GFR stage after starting potassium binder treatment. All patients on RAASi treatment before initiating potassium-binders were retained on RAASi treatment.
Conclusion: New potassium binders in clinical practice are an easy and safe treatment with few side effects and good tolerance, that significantly lowers the risk of hyperkalemia. Furthermore, and most importantly, patients can be maintained on RAASi treatment.
Keywords: chronic kidney disease, hyperkalemia, potassium-binder, RAASi, CKD, renin-angiotensin-aldosterone system inhibitors
Corrigendum for this paper has been published.
Introduction
Hyperkalemia is a common metabolic complication of chronic kidney disease (CKD) and occurs with deteriorating kidney function, especially among CKD patients’ stage 4–5.1,2 Hyperkalemia is defined as blood plasma potassium concentration > 5.0 mmol/l3 and is strongly correlated with the stages of CKD.4,5
The prevalence of hyperkalemia varies between studies depending on CKD stage, comorbidities, risk factors and concomitant medication.1,2,6–9 In a US study comprising 2270635 adults, annual hyperkalemia prevalence in 2014 was 1.57%7 For patients with CKD and/or congestive heart failure (CHF), 6.35% had hyperkalemia and patients with CKD and/or CHF counted for 48.43% of all patients with hyperkalemia.7 A Swedish population-based study showed that hyperkalemia occurred in 7% of the population with a 35.7% recurrence rate.6
The pathophysiology of hyperkalemia is complex and multifactorial. Most commonly, hyperkalemia is caused by increased potassium intake and/or impaired potassium secretion due to reduced glomerular filtration rate (GFR) in CKD, imbalance in potassium distribution between intracellular and extracellular space in metabolic acidosis and/or insulin deficiency.9
Hyperkalemia is associated with several serious adverse events such as cardiac arrhythmias, increased risk of hospitalization and associated mortality.9–15 Hyperkalemia is also associated with reduced quality of life among CKD patients.16
Disease-disease interaction such as co-existing CKD and CHF, and disease-drug interaction (especially in elderly patients), are important contributors to treatment-related complications including hyperkalemia.17,18
Renin-angiotensin-aldosterone system inhibitors (RAASi) have been the cornerstone in treating patients with proteinuric kidney disease the last few decades, reducing the risk of deteriorating kidney function and hemodialysis and renal transplantation.19–21 RAASi therapy is also one of the most important therapies for patients with CHF. The positive effect of RAASi therapy in CHF has been demonstrated in several randomized placebo-controlled trials alone or in combination with digitalis, beta-blockers and mineralocorticoid receptor inhibitors (MRI), resulting in significant reduction of CHF related adverse events, e.g., hospitalization, burden of disease and most importantly reduced mortality.22–24
There is a growing number of observational and population-based studies which have demonstrated that treatment with angiotensin converting enzyme inhibitors (ACE-i), angiotensin II receptor blockers (AIIB) and MRI for CKD, hypertension, diabetes mellitus or CHF, are the most important risk factors for development of hyperkalemia.25,26 Consequently, hyperkalemia is considered the main reason for limiting the use of RAASi treatment.27
Until recently, treating hyperkalemia consisted of potassium reduced diet combined with fine-tuning medical treatment (i.e., reducing/stopping drugs that interfered with renal potassium secretion; prescribing diuretic, managing metabolic acidosis, therapy with low dose RAASi / MRIs and lastly stopping RAASi if potassium raised above 5.5 mmol /l).28
Sodium polystyrene sulfonate (Kayexalate) and calcium polystyrene sulfonate (Calcium Resonium) have both been used to treat hyperkalemia. However, long-term use has been limited due to side effects and adverse outcome.29,30 The evidence supporting the efficiency and tolerability of these agents has been very poor.31
Over the last years, new classes of potassium-binders for the treatment of hyperkalemia have been developed with promising efficacy and tolerability, allowing maintained RAASi treatment in proteinuric CKD and/or CHF patients.32–36
Materials and Methods
Study Objective
The primary objective of this study was to treat/prevent hyperkalemia using new class of potassium binders and maintain RAASi treatment in our CKD patients. The secondary aim was to reduce symptoms of decease and provide a better clinical outcome. Additionally, we aimed to assess the practical use of potassium binders with regard to initiation, dosage and the need for laboratory and clinical evaluation in the outpatient clinic. A quality assurance study on potassium binder’s treatment at our center.
The indication for starting potassium binders was hyperkalemia in adults treated with RAASi. It is important to emphasize that the study was designed to report our clinical experience in treating hyperkalemia, not to compare the efficacy and safety of the two drugs.
Material
We conducted a retrospective cohort study on long-term users of the new potassium binders patiromer or calcium zirconium cyclosilicate, for chronic hyperkalemia in the CKD and heart failure outpatient clinics at the Vestfold Hospital Trust, Toensberg, Norway. Patients received treatment if they met the reimbursement criteria defined by the Norwegian health authorities (P-K > 5mmol) and indication for RAASi treatment. The choice between patiromer or calcium zirconium cyclosilicate were primarily based on price agreements between the Norwegian health authorities and the manufacturing companies and varied during the study period. Patients on dialysis (hemodialysis and peritoneal dialysis) were also included.
Inclusion of patients started at the time of initiating potassium binder treatment (Figure 1). All long-term users of potassium binders who were 18 years or older were included. For the majority of patients, treatment was initiated at the outpatient clinic, although some started treatment during a hospital stay. Patients who received these agents as short-term treatment for acute hyperkalemia, were excluded. A senior nephrologist or resident in nephrology made the evaluation of each patient’s individual risk of worsening hyperkalemia and the risk of future discontinuation of RAASi treatment, and started potassium binder treatment based on individual assessment.
 |
Figure 1 PotBind study design. |
The number of outpatient visits and lab tests from treatment initiation until treatment were established, and were also individually assessed. After initiation of potassium binder treatment, each individual patient was followed in the outpatient clinic. Exposure time for potassium binder treatment was defined at initiation of potassium binder until the end of study (1 July 2021). All patients starting with potassium binder received individual follow up with blood tests for kidney function and relevant electrolytes (Figure 1).
Predefined variables, which could affect or be affected by plasma potassium, were obtained from electronic patient records. Laboratory data from the hospital was obtained 4 weeks prior and 4 weeks after potassium binder initiation. The variables included kidney function by estimated glomerular filtration rate (eGFR) and serum creatinine, metabolic acidosis by plasma total CO2/bicarbonate, and the patient’s medication list. Especially, concomitant medication that could potentially influence potassium hemostasis was recorded.
We used IBM SPSS Statistics Version 26 for statistical analysis.
Results
Study-Population and Clinical Characteristics
Fifty-five patients received patiromer or calcium zirconium cyclosilicate. The majority of patients were males and the mean-age was 65 years. Patient characteristics are presented in Table 1.
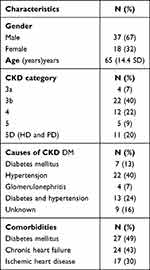 |
Table 1 Clinical Characteristics |
Mean treatment time with potassium binders was 58.4 (±45) weeks, minimum 2.7 weeks and maximum 167 weeks. The majority of the patients received calcium zirconium cyclosilicate (Table 2). Seven patients had been admitted due to hyperkalemia within 12 months prior to starting potassium binder; four of these with prior hyperkalemia were on maintenance hemodialysis. Hyperkalemia as a reason for hospital admission was 3.8 times higher in patients in maintenance hemodialysis compared with CKD patients not on dialysis. In comparison, no patients were admitted to hospital due to hyperkalemia after initiation of potassium binder.
 |
Table 2 Type of Potassium Binder Therapy |
Two patients on maintained hemodialysis had several unplanned acute dialysis sessions due to hyperkalemia before potassium binder treatment. In contrast, we observed no need for unplanned acute dialysis sessions in these patients under potassium binder treatment.
Most patients (n = 26, 47%) on calcium zirconium cyclosilicate started and were maintained during the study time with 5 grams once daily (mean dose 6.3 gram). Eight patients started with 10 grams once daily and only one patient started with 20 grams of calcium zirconium cyclosilicate. We observed the same trend in patients who received patiromer. The lowest dose of 8.4 gram once daily was used at the start and as a maintenance dose, during the study (mean dose 10.9 gram).
Forty-five (82%) patients were monitored with 0–6 lab tests before starting potassium binder and 47 (85%) with maximum six lab tests during 4 weeks after potassium binder (Table 3). In a smaller group of patients more lab tests were done in the period before and after potassium binder treatments. The majority of these patients were started on potassium binder during hospital stay and therefore lab test was done more frequently by routine.
 |
Table 3 Number of Laboratory Tests Before and After Potassium Binder Initiation |
Effect of Potassium Binders
Blood potassium values before and after potassium binder treatment are shown in Figure 2. The potassium maximum values were significantly lower after initiation of potassium binder (P<0.05), minimum and mean values were lower but not statistically significant (Figure 2).
 |
Figure 2 Plasma potassium levels before and after initiation of a potassium binder. |
There were no significant changes in the bicarbonate, serum creatinine or GFR stage after initiation of potassium binder treatment. Thirty-four (60%) of the patients received RAASi treatment before they started potassium binder (Table 4). All patients in the RAASi treatment were retained on RAASi treatment after starting potassium binder. The numbers of patients with MRI treatment increased significantly, with five patients (10%) after established potassium binder treatment (P < 0.05).
 |
Table 4 Concomitant Medications Before and After Potassium Binder |
Very little and non-significant changes were observed in concomitant medication with possible effect on serum potassium.
Adverse Events and Discontinuation
There were few associated side effects reported (n = 13, 13%) and consisted of hypomagnesemia or gastrointestinal symptoms. Discontinuation of treatment was mostly due to normokalaemia. Potassium binder associated side effects and causes of termination during the study period are presented in Table 5.
 |
Table 5 Adverse Events and Discontinuation of Potassium Binder Treatment |
Discussion
In this study of 55 consecutive patients who received potassium binder treatment, we were able to demonstrate that it was possible to retain all patients on RAASi or MRI treatment (proactive and pre-emptive approach), due to significant reduction in serum potassium. We were even able to start new patients on RAASi/MRI. We had no patient with serious hyperkalemia after initiating potassium binder treatment. Adverse events were few and only two patient discontinued treatment because of side effects.
Hyperkalemia is a serious clinical situation9,37,38 with potential life-threatening consequences. RAASi treatment is highly recommended in patient with heart failure and proteinuric CKD patients with and without diabetes.19,20,23,24 However, hyperkalemia limits the use of RAASi treatments in patients with CKD and heart failure due to increasing serious adverse events including hyperkalemia.6,8 The new potassium binders have enabled continuation of the RAASi treatment in patient and efficacy and safety of these drugs are already established in clinical trials.33,35,36,39
Our study was not designed to compare the two new potassium binders. Our intention was to conduct a quality assurance study on the new potassium binders and the possibility to retain our patients in the RAASi treatment and start RAASi treatment in patients who had to stop RAASi earlier due to hyperkalemia. There is accumulating real world evidence on the use of the new potassium binders.40,41 It is however important to conduct and publish new findings and experiences from observational studies in new treatments and methods.42 Norwegian health authorities encourage the conduction of observational and quality assurance studies.
Patiromer was introduced for treatment of hyperkalemia in Norway in April 2018, a month before calcium zirconium cyclosilicate. Initially it was necessary to apply for individual reimbursement for the patiromer from the Norwegian health authorities, but after a period, both potassium binders were reimbursed equally and automatically without the need for reimbursement application.
The majority of our patients received calcium zirconium cyclosilicate during the study and the reason for this was price-agreement in between the Norwegian health authority and calcium zirconium cyclosilicate manufacturing company (Astra Zeneca). Calcium zirconium cyclosilicate was cheaper and preferred potassium binder to prescribe. This changed during spring 2022 and the patiromer became the preferred agent for the same reason. This was after we had conducted our study.
The aim for potassium binder treatments in our clinic was to be able to retain patients on RAASi treatment and to avoid hyperkalemia and/or start RAASi treatment in patients who earlier had to stop RAASi due to hyperkalemia. Although several guidelines have been published recommending the use of the new potassium binders during the period we performed this study and after.24,43,44 There was however, no published detailed practical guidelines on how to use these hypokalemic agents. This was the reason for conducting this retrospective study in treatment quality assuring purpose. Our goal was to elaborate how our department practiced the use of these agents and how the results were especially regarding RAASi treatment in our CKD patients.
To avoid selection bias, we included all patients from the first patient starting on potassium binder for chronic use in consecutive manner (Figure 1). We experienced considerable differences between number of blood tests needed during the pre-initiation phase, and the initiation phase of potassium binder treatment. Around 50% of the patients had less than two blood tests, and around 27% had 3–5 blood tests 4 weeks before and prior to the potassium binder initiation. Differences in number of blood tests, depended on the patient characteristics. Hemodialysis patients were subject to blood testing almost under every dialysis session (3–4 times weekly), and patients who started potassium binder under hospital admission did almost daily blood tests during their hospital admission compared to patients in outpatient clinic, who had very few blood tests during potassium binder treatment initiation.
The severity of hyperkalemia and CKD stage was another important reason for doing blood test more often for evaluating changes in kidney function and electrolyte balance during potassium binder treatment. In outpatient-settings, variation in numbers of blood tests can be influenced by how close or far a patient residence is from the hospital lab center, in other words is taking an extra blood test burdensome for the patient? After potassium binder treatment was established individually, normal outpatient routine was followed for all patients CKD stage and co-morbidity.
Our policy and goal regarding potassium binder treatment in patients who need to continue RAASi treatment is proactive and pre-emptive. We start potassium binder treatment in case of mild to moderate hyperkalemia on RAASi treatment and before we have to stop RAASi treatment due to serious hyperkalemia. This is reflected in our results. Another possible approach, is to start potassium binders in severe hyperkalemia, but the risk of hyperkalemia related adverse events and the risk of discontinuation of the RAASi treatment is much higher compared to treatment with potassium binder at mild to moderate hyperkalemia.
We measured plasma bicarbonate, kidney function (by eGFR and creatinine) and registered all concomitant medication with potential effect on plasma potassium during initiation and follow up. In this way, we ensured that only potassium binder treatment could explain the significant reduction in serum potassium, allowing patients retained on RAASi or start RAASi treatment. Our results also reflect what has already been reported from large clinical trials.
Our data from a single-center experience shows, in accordance with other real-world observational studies and clinical trials, that new potassium binder binders are effective in treating hyperkalemia and allows continuation of the RAASi treatment in CKD. After we performed this study, we continued the treatment of hyperkalemia in same manner at our center.
Our study has several strengths and of course is subject to different limitations and biases due to study size and retrospective study design. Our data is however robust since all patients are treated and followed by one hospital, serving a county with approximately 280,000 inhabitants for kidney diseases and in particular for CKD and CKD related complications including renal replacement therapy (dialysis and transplantation). We have no patients lost to follow up, and all patients received same standard of treatment, regardless of socioeconomic differences due to general Norwegian health insurance. The most important limitation, is that the majority of CKD patients are not diagnosed and not registered in hospital records. However, this will not influence our study significantly since our patients predominately were in CKD stage 4–5 or dialysis treatment, and more likely to be registered in hospital records.
Conclusion
Hyperkalemia is an important and frequent complication of CKD, and often the reason for discontinuation of the RAASi treatment. We have demonstrated that the use of the new potassium binders in clinical practice is an easy and safe treatment, with few side effects and good tolerability in the majority of CKD patients, including dialysis with significant lower risk for hyperkalemia. Most important, more patients can be maintained on RAASi treatment on low-dose of the new potassium binder. It is important to conduct and report data from real world settings to ensure the best treatment for our patients.
Ethics Approval and Informed Consent
Informed consent did not apply for this study due to observational design, and the ethic committee waived the requirement for informed consent. Furthermore, it was not a requirement for this type of study under institutional guidelines and not from national laws. This study was registered at The Norwegian Center for Research Data (NSD) with NSD study reference nr: 549818. (The list for approved studies is officially available at https://minside.nsd.no/r/ms-549818). The study was registered at the hospital register for research studies. The study was conducted with respect to local and national GDPR. Vestfold Hospital Trust authority and local ethic committee approved this study.
Author Contributions
All authors made a significant contribution to the work reported, including conception, study design, execution, acquisition of data, analysis and interpretation, drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Sadollah Abedini has received honorarium for advisory board meetings from Vifor Pharma and AstraZeneca and limited research grant from Astra Zeneca Norway and Vifor pharma Norway. The other authors have not reported any conflict of interests regarding this work.
References
1. Caravaca-Fontán F, Valladares J, Díaz-Campillejo R, Barroso S, Luna E, Caravaca F. Renal potassium management in chronic kidney disease: differences between patients with or without hyperkalemia. Nefrología. 2020;40(2):152–159. doi:10.1016/j.nefroe.2020.03.006
2. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156–1162. doi:10.1001/archinternmed.2009.132
3. McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi:10.1093/eurheartj/ehs104
4. Ahn SY, Ryu J, Baek SH, et al. Incident chronic kidney disease and newly developed complications related to renal dysfunction in an elderly population during 5 years: a community-based elderly population cohort study. Research Support, Non-U.S. Gov’t. PLoS One. 2013;8(12):e84467. doi:10.1371/journal.pone.0084467
5. Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20(1):164–171. doi:10.1681/asn.2008020159
6. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277–284. doi:10.1016/j.ijcard.2017.07.035
7. Betts KA, Woolley JM, Mu F, McDonald E, Tang W, Wu EQ. The prevalence of hyperkalemia in the United States. Curr Med Res Opin. 2018;34(6):971–978. doi:10.1080/03007995.2018.1433141
8. Savarese G, Xu H, Trevisan M, et al. Incidence, predictors, and outcome associations of dyskalemia in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2019;7(1):65–76. doi:10.1016/j.jchf.2018.10.003
9. Karaboyas A, Robinson BM, James G, et al. Hyperkalemia excursions are associated with an increased risk of mortality and hospitalizations in hemodialysis patients. Clin Kidney J. 2021;14(7):1760–1769. doi:10.1093/ckj/sfaa208
10. Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120(1):c8–16. doi:10.1159/000329511
11. Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB. Serum potassium levels and mortality in hemodialysis patients: a retrospective cohort study. Am J Nephrol. 2016;44(3):179–186. doi:10.1159/000448341
12. Gasparini A, Evans M, Barany P, et al. Plasma potassium ranges associated with mortality across stages of chronic kidney disease: the Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial Transplant. 2019;34(9):1534–1541. doi:10.1093/ndt/gfy249
13. Kovesdy CP, Matsushita K, Sang Y, et al. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J. 2018;39(17):1535–1542. doi:10.1093/eurheartj/ehy100
14. An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16(6):R225. doi:10.1186/cc11872
15. Núñez J, Bayés-Genís A, Zannad F, et al. Long-term potassium monitoring and dynamics in heart failure and risk of mortality. Circulation. 2018;137(13):1320–1330. doi:10.1161/circulationaha.117.030576
16. Grandy S, Jackson J, Moon R, Bluff D, Palaka E. Health-related quality of life and lifestyle changes in patients with chronic kidney disease and hyperkalaemia: real-world data from the US, five European countries and China. Int J Clin Pract. 2021;75(8):e14326. doi:10.1111/ijcp.14326
17. Schneider J, Algharably EAE, Budnick A, Wenzel A, Dräger D, Kreutz R. High prevalence of multimorbidity and polypharmacy in elderly patients with chronic pain receiving home care are associated with multiple medication-related problems. Front Pharmacol. 2021;12:686990. doi:10.3389/fphar.2021.686990
18. Afshar S, Roderick PJ, Kowal P, Dimitrov BD, Hill AG. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health. 2015;15:776. doi:10.1186/s12889-015-2008-7
19. Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–878. doi:10.1056/NEJMoa011489
20. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi:10.1056/NEJMoa011161
21. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi:10.1056/NEJMoa011303
22. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
23. Heidenreich PA, Fonarow GC, Breathett K, et al. 2020 ACC/AHA clinical performance and quality measures for adults with heart failure: a report of the American College of Cardiology/American heart association task force on performance measures. Practice GuidelineReview. Circ Cardiovasc Qual Outcomes. 2020;13(11):e000099. doi:10.1161/HCQ.0000000000000099
24. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2021. doi:10.1093/eurheartj/ehab368
25. Belmar Vega L, Galabia ER, Bada da Silva J, et al. Epidemiology of hyperkalemia in chronic kidney disease. Nefrología. 2019;39(3):277–286. doi:10.1016/j.nefroe.2018.11.014
26. Hunter RW, Bailey MA. Hyperkalemia: pathophysiology, risk factors and consequences. Nephrol Dial Transplant. 2019;34(Suppl3):iii2–iii11. doi:10.1093/ndt/gfz206
27. Vijayakumar S, Butler J, Bakris GL. Barriers to guideline mandated renin–angiotensin inhibitor use: focus on hyperkalaemia. Eur Heart J. 2019;21(Supplement_A):A20–A27. doi:10.1093/eurheartj/suy030
28. Palmer BF. Managing hyperkalemia caused by inhibitors of the renin–angiotensin–aldosterone system. N Engl J Med. 2004;351(6):585–592. doi:10.1056/NEJMra035279
29. Dardik A, Moesinger RC, Efron G, Barbul A, Harrison MG. Acute abdomen with colonic necrosis induced by Kayexalate-sorbitol. South Med J. 2000;93(5):511–513. doi:10.1097/00007611-200093050-00016
30. Abraham SC, Bhagavan BS, Lee LA, Rashid A, Wu TT. Upper gastrointestinal tract injury in patients receiving kayexalate (sodium polystyrene sulfonate) in sorbitol: clinical, endoscopic, and histopathologic findings. Am J Surg Pathol. 2001;25(5):637–644. doi:10.1097/00000478-200105000-00011
31. Natale P, Palmer SC, Ruospo M, Saglimbene VM, Strippoli GF. Potassium binders for chronic hyperkalaemia in people with chronic kidney disease. Cochrane Database Syst Rev. 2020;6(6):Cd013165. doi:10.1002/14651858.CD013165.pub2
32. Tamargo J, Caballero R, Delpón E. New therapeutic approaches for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors. Cardiovasc Drugs Ther. 2018;32(1):99–119. doi:10.1007/s10557-017-6767-5
33. Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. doi:10.1056/NEJMoa1410853
34. Weir MR, Bakris GL, Gross C, et al. Treatment with patiromer decreases aldosterone in patients with chronic kidney disease and hyperkalemia on renin-angiotensin system inhibitors. Kidney Int. 2016;90(3):696–704. doi:10.1016/j.kint.2016.04.019
35. Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–2233. doi:10.1001/jama.2014.15688
36. Zhang Y, Xu R, Wang F, et al. Effects and safety of a novel oral potassium-lowering drug-sodium zirconium cyclosilicate for the treatment of hyperkalemia: a systematic review and meta-analysis. Cardiovasc Drugs Ther. 2021;35:1057–1066. doi:10.1007/s10557-020-07134-2
37. Sarwar CM, Papadimitriou L, Pitt B, et al. Hyperkalemia in Heart Failure. J Am Coll Cardiol. 2016;68(14):1575–1589. doi:10.1016/j.jacc.2016.06.060
38. Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46(3):213–221. doi:10.1159/000479802
39. Haller H, Bianchi S, McCafferty K, et al. Safety and efficacy of patiromer in hyperkalemic patients with CKD: a pooled analysis of three randomized trials. Kidney. 2022;3(12):2019–2026. doi:10.34067/KID.0001562022
40. Kovesdy CP, Rowan CG, Conrad A, et al. Real-world evaluation of patiromer for the treatment of hyperkalemia in hemodialysis patients. Kidney Int Rep. 2019;4(2):301–309. doi:10.1016/j.ekir.2018.10.020
41. Kovesdy CP, Gosmanova EO, Woods SD, et al. Real-world management of hyperkalemia with patiromer among United States Veterans. Observational Study. Postgrad Med. 2020;132(2):176–183. doi:10.1080/00325481.2019.1706920
42. Yang W, Zilov A, Soewondo P, Bech OM, Sekkal F, Home PD. Observational studies: going beyond the boundaries of randomized controlled trials. Diabetes Res Clin Pract. 2010;88(Suppl 1):S3–9. doi:10.1016/S0168-8227(10)70002-4
43. National Institute for Health and Care Excellence. Sodium zirconium cyclosilicate for treating hyperkalaemia. Available from: https://www.nice.org.uk/guidance/ta599.
44. Clase CM, Carrero JJ, Ellison DH, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020;97(1):42–61. doi:10.1016/j.kint.2019.09.018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
