Back to Journals » Risk Management and Healthcare Policy » Volume 14
High Rate of Bacterial Contamination on Healthcare Worker’s Mobile Phone and Potential Role in Dissemination of Healthcare-Associated Infection at Debre Berhan Referral Hospital, North Shoa Zone, Ethiopia
Received 2 April 2021
Accepted for publication 3 June 2021
Published 21 June 2021 Volume 2021:14 Pages 2601—2608
DOI https://doi.org/10.2147/RMHP.S313387
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Marco Carotenuto
Tsegahun Asfaw, Deribew Genetu
Department of Medical Laboratory Science, Debre Berhan University, Debre Berhan, Ethiopia
Correspondence: Tsegahun Asfaw Email [email protected]
Background: Mobile Phone (MP) handling by healthcare workers (HCWs) in hospital is an open breeding place for the transmission of bacteria and healthcare-associated infection (HCAI). This HCAI is a huge problem to the healthcare system worldwide.
Methods: A laboratory-based cross-sectional study design was conducted from January 2020 to January 2021 in Debre Berhan Referral Hospital, North Shoa Zone, Ethiopia. A total of 65 swab samples were collected from HCWs mobiles phone. Other important data were collected using a self-administered questionnaire. The collected samples were processed for bacteriological identification and drug susceptibility testing. Data obtained were entered and cleaned into MS Excel spreadsheet and analyzed using SPSS version 21.
Results: From the total of 65 swab sample, 84 bacterial isolates were detected. Of these bacterial isolates, 46.4% were Gram-positive bacteria while 53.6% were Gram-negative bacteria. The overall MDR prevalence was found to be 42.9%. The proportion of MP contamination was higher in males (67.9%) and the age groups of 20– 35 years (50%). All the MP carried by HCWs was contaminated with at least one bacterial pathogen. The high rate of MP contamination was observed in the intensive care unit (ICU) (22.6%) followed by surgical ward (17.8) and laboratory rooms (17.8%). The rate of bacterial contamination of MP was higher among HCWs working in ICU, who did not disinfect or clean their phone regularly and who did not wash their hands regularly.
Conclusion: Healthcare worker’s MP was contaminated with pathogenic bacteria. Since MP could serve as a vehicle and a reservoir for pathogenic bacteria, HCWs should be aware of the public health risks of HCAI, and appropriate intervention mechanisms should be practiced to reduce the burden and cross-transmission.
Keywords: bacterial contamination, mobile phone, healthcare worker
Introduction
The mobile phone (MP) has become an important part of our lives, the use of which in different environments is rapidly growing. It is gradually becoming a significant means of communication globally. Mobile Phone is widely used in the health care setting and its role in the dissemination of HCAI is overlooked. Healthcare-associated infection is an increasing global concern for patient treatment outcome and safety. It affects more than 25% of the total healthcare admissions in developing countries.1,2
In the last decade, MP use has introduced the clinical practice, providing rapid access to medical information and allowing efficient communication with colleagues worldwide. However, the use of MP without regular disinfection, coupled with their portability, makes them a potential source of infection.3 Indication of MP contamination in health care settings has been observed; these may be implicated in outbreaks of HCAI. Therefore, MP probably represent a constant infection risk for patients, and developing countries are likely at a greater risk.4 The increased use of MP in hospital wards may have more serious hygiene consequences because, unlike fixed phone, MP could be close to the patients and these patients are more vulnerable to HCAI.4,5
Healthcare-associated infections and antimicrobial resistance are principal threats to the patients of ICU and are the major determining factors for patient outcome. Objects with frequent hand contact such as MP can serve as reservoirs from which infections can spread to the hands of healthcare providers and then to patients. These mobile devices are also carried out of the hospital and to the home of HCWs and hence to the community.6
Understanding the sources of these infections could help hospital authorities to improve services and information dissemination and to formulate full guidelines about restricting the use of MP in clinical environments, hand hygiene, and frequent decontamination of mobile devices.7 Therefore this study aims to determine bacterial contamination of MP of HCWs and potential role in the dissemination of HCAI. Also, it aims to examine the prevalence of antibiotic-resistant bacteria on MP of HCWs.
Materials and Methods
Study Design, Period, and Setting
A laboratory-based cross-sectional study design was done at Debre Berhan referral hospital from January 2020 to January 2021. There are 3 obstetricians, 4 surgeons, 40 General Practitioners, 10 pharmacists, 25 druggists, 81 nurses, 20 laboratory technicians/technologist and other health professionals. This study included HCWs from intensive care unit (ICU), pediatric ward, surgical ward, gynecology ward, and laboratory rooms. We excluded HCWs who do not hold MP at the time of data collection and those who cleaned their phone once they heard about our study, and those their MP were contaminated by hands of sample collector at the time of sample collection.
Data and Sample Collection
Scio-demographic and MP-related data (hand hygiene practice, MP disinfection, and service year) were collected by pre-tested self-administered questionnaire (Supplementary Figure 3). Then, a total of 65 swab samples were collected aseptically by scrubbing over the entire surface of the MP by sterile swab moistened with sterile physiological saline. The swab sample is inoculated on Tryptic Soy Broth (TSB) medium for transportation.
Isolation and Identification of Bacteria
The TSB media was incubated aerobically at 35–37°C for 24 hours and then inoculated on blood agar (Himedia, Mumbai, India), MacConkey agar (SRL-sisco research laboratory), Salmonella-Shigella agar (Unichem, Mumbai, India), and Mannitol salt agar (Himedia, Mumbai, India) at 35–37°C for 18–24 hours. Further identification was done by Gram staining and biochemical tests like catalase test, coagulase test, oxidase test, carbohydrate fermentation, and H2S production, citrate utilization test, motility, indole test, lysine decarboxylase test, lysine deaminase test, urea test, and hemolysis on blood agar were used for identification.
Antimicrobial Susceptibility Testing
A standard Kirby-Bauer disk diffusion method was used on Muller-Hinton agar (Oxoid, Basingstoke, UK) to determine the antimicrobial susceptibility profiles of the isolates. Bacterial isolates were tested for susceptibility to the commonly prescribed antibiotics in Ethiopia. Those antibiotics are erythromycin (15μg), gentamycin (10μg), amikacin (30μg), amoxicillin-clavulunic acid (30μg), ceftriaxone (30μg), ciprofloxacin (5μg), tetracycline (30μg), cotrimoxazole (25μg), ampicillin (10μg), penicillin (10μg), chloramphenicol (30μg), doxycycline (30μg) and cefoxitin (30μg). Clinical Laboratory Standard Institute (CLSI) guideline was used to interpret the results.8
Multiple Drug-Resistance Isolates
After performing antimicrobial susceptibility test, the MDR isolates (acquired non-susceptibility to at least one agent in three or more antimicrobial categories) were determined according to suggested definition of international experts.9
Data Quality Assurance
All laboratory test procedures were done according to standard. Quality control strains: E. coli (ATCC 25922), S. aureus (ATCC 25923), and P. aeruginosa (ATCC 28753) were used to check the performance of each test according to the CLSI guideline.8
Data Management and Analysis
The data was analyzed by SPSS statistical software package version 21. Descriptive statistics were employed to report numerical summary of findings. A pattern of quantitative values (frequency and proportion) was presented using statistical tables and figures. All explanatory variables associated with outcome variable with P <0.25 were entered into multivariable logistic regression analysis. The significant association was identified by AOR, (95% CI) and P-value < 0.05.
Ethical Consideration
Ethical approval was gained from Debre Berhan University ethical review and research committee (Ethical code: ERC128/2020). Permission will be obtained from Debre Berhan referral hospital administration. All participants were informed about the purpose of the study, and that it was conducted in accordance with the Declaration of Helsinki (Supplementary Figure 1). Consent was obtained from all study participants (Supplementary Figure 2).
Result
Rate of Bacterial Contamination on MPs of HCWs
From the total of 65 swab sample, 84 bacterial isolates were detected. Of these bacterial isolates, 46.4% were Gram-positive bacteria while 53.6% were Gram-negative bacteria. Staphylococcus aureus, CoNS, Bacillus spp, P. aeruginosa, Klebsiella spp, E. coli, Citrobacter spp, Enterobacter spp, and Salmonella spp were among the isolated bacteria. The most frequently isolated bacteria were CoNS (14 isolates; 16.7%), S. aurous (13 isolates; 15.5%), and Bacillus spp (12 isolates; 14.3%), respectively [Table 1].
 |
Table 1 Frequency of Bacterial Isolates from MP of HCWs in Debre Berhan Referral Hospital, Ethiopia |
Factors Associated with MP Contamination
The proportion of MP contamination was higher in males (67.9%) and the age groups of 20–35 years (50%). All of the MP carried by HCWs was contaminated with at least one bacterial pathogen. A high rate of MP contamination was observed in the ICU (22.6%) followed by the surgical ward (17.8) and laboratory rooms (17.8%). From profession, the highest rate of MP contamination was obtained among the nurses (35.7%). A high rate of contamination was obtained among study participants with no frequent hand washing habit 36 (42.9) and undergo no regular MP disinfection 77 (91.7). Study participants with short service experience (1–5 years) were handed MP with a high rate of bacterial contamination [Table 2].
 |
Table 2 Rate of Bacterial Isolates with Socio-Demographic and Other Variables from MP of HCWs in Debre Berhan Referral Hospital, Ethiopia |
The rate of bacterial contamination of MP of HCWs working in ICU was higher than others (AOR: 0.452; 95% CI: 0.252–0.806). The rate of bacterial contamination of MP owed by those HCWs who did clean their phone regularly was also higher than those who cleaned their phones (AOR: 1.52; 95% CI: 1.246–2.117). And also the rate of bacterial contamination of MP owed by those HCWs who did not wash their hands regularly was higher than those who washed their hands (AOR; 0.512 95% CI: 0.212–1.233) [Table 2].
Antibiotic Susceptibility Profile of Bacterial Isolates
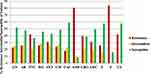
Bacterial isolates showed a higher resistance rate against penicillin (84%) followed by ampicillin (81%) and tetracycline (42%). However, lower resistance rate against ciprofloxacin (24%), gentamycin (23%), and chloramphenicol (18%). [Figure 1]
Multi-Drug Resistance (MDR) Profile of Isolates
The overall MDR prevalence was found to be (42.9%). Among isolates, (23.8%) were resistant to two antibiotics, (20.2%) were resistant to six and more antibiotics, and (16.7%) were resistant to one antibiotic, while (11.9%) were not resistant to any of the antibiotics tested [Table 3].
 |
Table 3 Multi-Drug Resistance Patterns of Bacterial Isolates from MP of HCWs in Debre Berhan Referral Hospital, Ethiopia |
The highest rate of resistance to many antibiotics (resistance for more than or equal to six antibiotics) was higher for CoNS (57.1%), E. coli and (27.3%), and Citrobacter spp (33.33%). In contrast, a lower rate of resistance to many antibiotics (resistance for more than or equal to four antibiotics) was observed among Bacillus spp isolates [Table 3].
Discussion
Mobile phones have become one of the important devices used for communication in daily life, and they are commonly used almost everywhere. HCWs use MP for communication within the hospital settings. And also many medical conditions like diabetes, asthma, and an increased rate of vaccination by travelers reminded by short message service (SMS) have been controlled by the aid of MP10–12 However, one of the most common concerns regarding the use of mobile devices in the hospital setting and outside is; they can act as a vehicle for transmitting pathogenic microorganisms particularly bacteria due to the adaptability of different environment.4,13,14 However, there are no institutional or manufacturer protocols that advise on strategies to reduce bacterial contamination on MP in Ethiopia.
In this study, 84 bacterial isolates were detected from a total of 65 swab sample. All of the MP handled by HCWs was contaminated with at least one bacterial pathogen. This frequency is relatively high to those studies previously reported in Ethiopia and other countries.15–21 The observed variations might be also due to the difference in adherence to infection prevention or frequency of cleaning MP, hand washing practice, the policy of MP uses in the hospital, and awareness about the role of MP in microbial transmission.
The most frequently isolated bacteria were CoNS (16.7%), S. aureus (15.5%), Bacillus spp (14.3%), E. coli (13.1%), and P. aeruginosa (10.7%) respectively. A lot of studies conducted in Ethiopia,15,16,22,23 and outside Ethiopia21,24,25 reported similar bacterial isolates with different isolation rates. Some other organisms like Salmonella spp, Citrobacter spp, Enterobacter spp, Serratia spp, Proteus spp, and Acinetobacter spp were also detected in this study were not usually reported by other studies. This might be due to the type and the numbers of culture media and biochemical tests used.
In the present study, CoNS isolates were detected predominantly in line with other study.15,18,23,25,26 CoNS have comparatively low virulence and appears to be normal flora of the skin; however, it has become increasingly accepted as the most common cause of nosocomial bacteremia associated with indwelling devices. The S. aureus (15.5%) isolation rate was similar to the study done in Ethiopia23 and India.19,27 In this study, Bacillus spp (14.3%) was the third predominant isolates. It was also reported from a similar study in Ethiopia12 and Egypt.28 The isolation rate of Bacillus spp in this study confirms its ubiquitous nature as well as the abilities of its spores to resist environmental changes, and tolerate dry heat and chemical disinfectant for a certain time. The presence of P. aeruginosa, K. pneumonia, E. coli, Citrobacter spp, Enterobacter spp, Proteus spp, and Acinetobacter spp showed the high role of MP in transmission HCAI. The presence of E. coli, Salmonella spp, and Serratia spp indicates a low level of hand and MP hygienic practice as those organisms are part of the intestinal flora (first two) and Environment flora (last) and the leading cause of HCAI.
Different factors were associated with contamination of MP. In this study, sex difference is not associated with MP contamination. This is similar to the finding of Pal et al19 and Shooriabi et al.29 However, this was in contrast to a study conducted in Ethiopia,23 India,30 and Iran.31 The rate of bacterial contamination of MP of HCWs, working in ICU was higher than others. This is opposite to other similar studies done in Zambia.32 This difference might be the reflection of poor adherence to the infection prevention practice in ICU of the present study area. The rate of bacterial contamination of MP owed by those HCWs who did not disinfect or clean their phone was also higher than those who cleaned their phone. This was supported by other studies.3,16,23,33,34 Past studies indicate a significant decline of MP contamination after treating with disinfectant.35,36 The rate of bacterial contamination of MP owed by those HCWs who did not wash their hands was higher than those who washed their hands regularly. This might be because hand washing is the most important prevention method of communicable disease.
Antibiotic-resistant bacteria are the most serious health risk for patients.32 Most of the isolates (88.1%) in this study were resistant to at least one antibiotic tested. Bacterial isolates showed a higher resistance rate against penicillin (84%) followed by ampicillin (81%) and tetracycline (42%), Also, MRSA and methicillin-resistant CoNS were isolated. These findings were similar to other studies conducted in different regions.3,15,19 These may indicate that MP of HCWs are a habitat for antibiotic-resistant pathogens. And also many Gram-negative bacteria (eg, Klebsiella spp, E. coli, Citrobacter spp, and Enterobacter spp) isolates were found to be resistant to many antibiotics tested with similar antibiotic resistance profiles. This could suggest that they could have had a common source of contamination from patients, from the contaminated hospital environment, or any unknown source. In this study, relatively lower resistance was observed among bacterial isolates to Chloramphenicol, gentamycin, and ciprofloxacin whereas higher against tetracycline and ampicillin. This result was in line with other studies conducted elsewhere.16,17,19,37–39
MDR bacterial strains could be a result of unreasonable and excessive use of antibiotics.23 In the current study, high rate of MDR (42.9%) was reported. This is in contrast to other study.16,23,37 This difference might be due to the difference in the hospital environment, empirical treatment practice, use of antibiotics as prophylactic, easy availability of antibiotics without prescriptions, the dose of the antibiotics, and indiscriminate or prolonged use of antibiotics. The highest rate of MDR on MP proves the MP increase the burden of HCAI unless strict guidelines and measures are taken regarding the use and cleaning of MP in health care settings.
Among isolates, (23.8%) were resistant to two antibiotics, (20.2%) were resistant to six and more antibiotics, and (16.7%) were resistant to one antibiotic, while (11.9%) were not resistant to any of the antibiotics tested. The highest rate of resistance to many antibiotics (resistance for more than or equal to six antibiotics) was higher for CoNS (57.1%), E. coli and (27.3%), and Citrobacter spp (33.33%). In contrast, a lower rate of resistance to many antibiotics (resistance for more than or equal to four antibiotics) was observed among Bacillus spp isolates. The rate of contamination with MDR bacteria of MP of HCWs showed that Coagulase-negative staphylococci were the most frequently isolated species among potential pathogenic bacteria identified. This might be due to the ability to survive for long periods in aerosolized states and harsh conditions like drying as compared to others.40
Limitation of the Study
This study did not address the effect of period variations since it is a cross-sectional study design. Due to lack of budget, time and laboratory facility, this study did not include other important bacterial pathogens responsible for HCAI like anaerobes. The small sample size makes it difficult to understand the actual practice of health professionals and to perform further multivariable analysis to identify the effect of specific factors on MP contamination with MDR bacteria.
Conclusion
In this study, the result showed that MP of HCWs was contaminated with a variety of pathogenic bacteria that have been implicated in HCAI. Most bacterial isolates were resistant to at least one antibiotic and the majority of isolates were MDR. Working in ICU, the lack of regular hand washing and deficiency in disinfection of MP were the significantly associated factors of bacterial contamination of MP of HCWs in the current study. Based on these findings, HCWs should clean their MP after use and wash their hands before and after handling patients in the health care settings. It is better to develop and implement the mobile use guideline in health care settings.
Data Sharing Statement
All raw data are available upon request from the primary author.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Center for Disease Control and Prevention. HAI data and statistics; 2016 Available from: http://1.usa.gov/1CWnvuu.
2. Tagoe DNA, Baidoo SE, Dadzie I, Tengey D, Agede C. Potential sources of transmission of hospital acquired infections in the volta regional hospital in Ghana. Ghana Med J. 2011;45(1):22–26. doi:10.4314/gmj.v45i1.68918
3. Heyba M, Ismaiel M, Alotaibi A, et al. Microbiological contamination of mobile phones of clinicians in intensive care units and neonatal care units in public hospitals in Kuwait. BMC Infect Dis. 2015;15:434. doi:10.1186/s12879-015-1172-9
4. Zakai S, Mashat A, Abumohssin A, et al. Bacterial contamination of cell phones of medical students at King Abdulaziz University, Jeddah, Saudi Arabia. J Microsc Ultrastruct. 2016;4:143–146. doi:10.1016/j.jmau.2015.12.004
5. Sepehri G, Talebizadeh N, Mirzazadeh A, Mir-shekari T, Sepehri E. Bacterial contamination and resistance to commonly used antimicrobials of healthcare workers’ mobile phones in teaching hospitals, Kerman, Iran. Am J Appl Sci. 2009;6(5):806–810. doi:10.3844/ajassp.2009.806.810
6. Kilic IH, Ozaslan M, Karagoz ID, Zer Y, Davutoglu V. The microbial colonization of mobile phone used by healthcare staffs. Pak J Biol Sci. 2009;12(11):882–884. doi:10.3923/pjbs.2009.882.884
7. Angadi KM, Misra R, Gupta U, Jadhav S, Sardar M. Study of the role of mobile phones in the transmission of Hospital acquired infections. Med J DY Patil Univ. 2014;7(4):435–438. doi:10.4103/0975-2870.135256
8. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
9. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
10. Vilella A, Bayas J, Diaz MT, et al. The role of mobile phones in improving vaccination rates in travelers. PrevMed. 2004;38:503–509.
11. Gibson DG, Ochieng B, Kagucia EW, et al. Mobile phone delivered reminders and incentives to improve childhood immunization coverage and timeliness in Kenya (M-SIMU): a cluster randomized controlled trial. Lancet Glob Health. 2017;5:e428–38. doi:10.1016/S2214-109X(17)30072-4
12. Ferrer-Roca O, Cardenas A, Diaz-Cardama A, Pulido P. Mobile phone text messaging in the management of diabetes. J Telemed Telecare. 2004;10:282–286. doi:10.1258/1357633042026341
13. Girma G. Potential health risks with microbial contamination of mobile phones. GRJE. 2015;3(1):246–254.
14. Ustun C, Cihangiroglu M. Health care workers’ mobile phones: a potential cause of microbial cross-contamination between hospitals and community. J Occup Environ Hyg. 2012;9:538–542. doi:10.1080/15459624.2012.697419
15. Misgana MG, Abdissa K, Abebe G. Bacterial contamination of mobile phones of healthcare workers at Jimma University Specialized Hospital, Jimma, South West Ethiopia. 2014;1:2–8.
16. Gashaw M, Abtew D, Addis Z. Prevalence and antimicrobial susceptibility pattern of bacteria isolated from mobile phones of health care professionals working in gondar town health centers. ISRN Public Health. 2014;2014(1):1–6. doi:10.1155/2014/205074
17. Daka D, Yihdego D, Tadesse E. Level of contamination and antibiotic resistance of bacterial isolates from mobile phone of HCW’s in Hawassa Referral Hospital. Asian J Med Sci. 2015;7(3):30–35. doi:10.19026/ajms.7.5177
18. Sedighi I, Alikhani MY, Ramezani S, Nazari M, Nejad ASM. Bacterial contamination of mobile phones of health care providers in a teaching hospital in Hamadan Province, Iran. Arch Clin Infect Dis. 2015;10(2):365. doi:10.5812/archcid.10(2)2015.22104
19. Pal K, Chatterjee M, Sen P, Adhya S. Cell phones of health care professionals: a silent source of bacteria. Natl J Lab Med. 2015;4(4):33–38.
20. Jagadeesan Y, Deepa M, Kannagi M. Mobile phones as fomites in miocrobial dissemination. Int J Curr Sci. 2013;5:6–14.
21. Chaman R, Nargeseyan S, Jannesar R, Ravangard S, Nikbakht G. Survey of prevalence and types of bacterial contamination of mobile phones of personnel employed in major wards of educational hospitals in Yasuj. J Fundam Appl Sci. 2018;10(2).
22. Verma DK, Barasa A, Dara D, et al. Isolation and characterization of bacteria from mobile phones of students and employees at University of Gondar, Ethiopia. Bull Pharm Res. 2015;5(3):96–100.
23. Bodena D, Teklemariam Z, Balakrishnan S, Tesfa T. Bacterial contamination of mobile phones of health professionals in Eastern Ethiopia: antimicrobial susceptibility and associated factors. Trop Med Health. 2019;47:15. doi:10.1186/s41182-019-0144-y
24. Amala S, Ejikema I. Bacteria associated with the mobile phones of medical personnel. Am J Biomed Sci. 2015;7(1):36.
25. Bahmani T, Moradi J, Aliabadi M, Sohrabi N, Amiri Z, Babaei S. The bacterial contamination of nurses’ mobile phones in a general Hospital in Kermanshah, west of Iran. Pharmacophore. 2017;8(6S):5.
26. Balapriya P, Padmakumari J, Vijayalakshmi A. Screening for nosocomial pathogens in stethoscopes, sphygmomanometers and mobile phones of health care providers in a tertiary care hospital. Int J Curr Microbiol App Sci. 2016;5(10):91–98. doi:10.20546/ijcmas.2016.510.011
27. Tankhiwale N, Gupta V, Chavan S, Tawade V. Nosocomial hazards of doctor’s mobile phones. J Med Sci. 2012:283–285.
28. Selim HS, Abaza AF. Microbial contamination of mobile phones in a health care setting in Alexandria, Egypt. GMS Hyg Infect Control. 2015;10. ISSN 2196-5226. doi:10.3205/dgkh000246
29. Shooriabi M, Chabi A, Satvati SAR, et al. Investigating the ratio and type of bacterial contamination of dentists’ mobile phones in dentistry unit of Sina Hospital in Ahvaz in 2014. Health Sci. 2016;5(8):317–325.
30. Kokate SB, More SR, Gujar V, Mundhe S, Zahiruddin QS. Microbiological flora of mobile phones of resident doctors. J Biomed Sci Eng. 2012;5(11):696. doi:10.4236/jbise.2012.511086
31. Jalalmanesh S, Darvishi M, Rahimi M, Akhlaghdoust M. Contamination of senior medical students’ cell phones by nosocomial infections: a survey in a university-affiliated Hospital in Tehran. Shiraz E-Medical J. 2017;18(4). doi:10.5812/semj.43920
32. Mushabati NA, Samutela MT, Yamba K, et al. Bacterial contamination of mobile phones of healthcare workers at the University Teaching Hospital, Lusaka, Zambia. Infect Prev Pract. 2021;3:100126. doi:10.1016/j.infpip.2021.100126
33. Tiwari A, Ankola AV, Mishra H, Kakkar M. Assessment of bacterial contamination in cellular phones of dental professionals working in a dental institution in Belgium city–a cross sectional study. Med Res Chron. 2016;3(3):266–273.
34. Shakir IA, Patel NH, Chamberland RR, Kaar SG. Investigation of cell phones as a potential source of bacterial contamination in the operating room. JBJS. 2015;97(3):225–231. doi:10.2106/JBJS.N.00523
35. Arora U, Devi P, Chadha A, Malhotra S. Cellphones: a modern stayhouse for bacterial pathogens. JK Sci. 2009;11(3):127.
36. Koscova J, Hurnikova Z, Pistl J. Degree of bacterial contamination of mobile phone and computer keyboard surfaces and efficacy of disinfection with chlorhexidine digluconate and triclosan to its reduction. Int J Environ Res Public Health. 2018;15:2238. doi:10.3390/ijerph15102238
37. Khadka S, Nshimiyimana JB, Thapa A, Akayezu V, Mwizerwa EM, Woldetsadik AG. Bacterial profile of mobile phones used by college students in Kigali, Rwanda. Int J Appl Microbiol Biotechnol Res. 2018;6:87–94.
38. Alemu A, Misganaw D, Wondimeneh Y. Bacterial profile and their antimicrobial susceptibility patterns of computer keyboards and mice at Gondar University Hospital, Northwest Ethiopia. Biomed Biotechnol. 2015;3(1):1–7.
39. El kady H. Microbial contamination of mobile phones in the medical laboratory technology department of a private university in Alexandria, Egypt. Int J Curr Microbiol App Sci. 2017;6(6):200–211. doi:10.20546/ijcmas.2017.606.024
40. Sudharsanam S, Srikanth P, Sheela M, Steinberg R. Study of the indoor air quality in hospitals in South Chennai, India–Microbial Profile. Indoor Built Environ. 2008;17:435–441. doi:10.1177/1420326X08095568
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.