Back to Journals » Research and Reports in Urology » Volume 15
High-Fat and High-Sucrose Diet Leads to Skeletal Muscle Loss and Bladder Dysfunction in Rat
Authors Wada N , Abe N, Miyauchi K, Makino S, Kakizaki H
Received 2 February 2023
Accepted for publication 29 April 2023
Published 3 July 2023 Volume 2023:15 Pages 305—313
DOI https://doi.org/10.2147/RRU.S406808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Naoki Wada, Noriyuki Abe, Kotona Miyauchi, Shogo Makino, Hidehiro Kakizaki
Department of Renal and Urologic Surgery, Asahikawa Medical University, Asahikawa, Japan
Correspondence: Naoki Wada, Department of Renal and Urologic Surgery, Asahikawa Medical University, Asahikawa, Japan, Tel +81-166-65-2533, Email [email protected]
Purpose: In this study, we investigated skeletal muscle loss and bladder dysfunction caused by high-fat/high-sucrose (HFS) diet.
Methods: Twelve-week-old Sprague–Dawley (SD) female rats were fed on normal (Group N) or HFS (Group HFS) diet for 12 weeks. We conducted urodynamic investigation and pharmacologic in vitro. In addition, we measured gastrocnemius and tibialis muscle weight and protein concentration. The hypoxia-inducible factor (HIF)-1α and 8-hydroxy-2’-deoxyguanosine (8-OHdG) in the bladder were assayed.
Results: The urodynamic investigations revealed the significantly shorter intercontraction intervals and lower maximal voiding pressure in Group HFS than in Group N. Furthermore, the absolute and relative weights of the gastrocnemius muscle were found to be significantly lower in Group HFS than in Group N. The protein concentration of the gastrocnemius muscle was also significantly lower in Group HFS than in Group N. The absolute and relative weights of the bladder were also significantly lower in Group HFS than in Group N. The contractile responses of the bladder strips to electrical field stimulation and carbachol were significantly lower in Group HFS than in Group N. The HIF1α and 8OHdG in the bladder muscle were significantly higher in Group HFS than in Group N. The HFS diet reduced bladder capacity and contractility along with the loss of the gastrocnemius muscle.
Conclusion: HFS diet promotes bladder dysfunction similar to detrusor hyperreflexia with impaired contractility.
Keywords: high-fat, high-sucrose, bladder dysfunction, rat, skeletal muscle
Introduction
Several countries, including Japan, have already become super-aged societies. Frailty is a highly prevalent condition among the elderly and has been increasingly considered a crucial public health issue. Undernutrition, inaction, chronic inflammation, and metabolic abnormalities, including insulin resistance, are the contributing factors to sarcopenia, a common pathological condition among older people with frailty.1 There are no established animal models representing fast muscle-dominated muscle loss, which is the characteristic change in sarcopenia. Animal models with insulin resistance or chronic inflammation can be used as substitutes for animals with aging-related sarcopenia.
Several previous studies in rats demonstrated that high-fat/high-sucrose (HFS) diet led to dynamic structural and inflammatory alterations of the skeletal muscle and accelerated the progression of sarcopenia by altering the postprandial stimulation of muscle protein synthesis.2,3 The animals fed on HFS diet exhibited local inflammatory changes and decreased insulin sensitivity, which are similar to the molecular changes in humans with sarcopenia.4 Changes in the lower urinary tract function of these animals are expected; however, this lacks information.
The structural and inflammatory alterations of the skeletal muscle may induce general or local oxidative stress reaction following ischemic change. We hypothesized that oxidative stress and ischemic change in the pelvis or the bladder in animals fed on HFS diet would induce bladder dysfunction. Thus, we investigated the change in the skeletal muscles and bladder function in rats fed on HFS diet.
Methods
We conducted all procedures, and this animal research was approved by the Institutional Animal Care and Use Committee in Asahikawa Medical University. All procedures were also conducted according to National Institutes of Health guideline (https://grants.nih.gov/grants/policy/air/index.htm).
Animals
Twelve-week-old female Sprague–Dawley (SD) rats (body weight (BW): 200–226 g) were used and fed on normal (Group N; n = 18) or HFS (Group HFS; n = 15) diet for 12 weeks. HFS diet includes 40% of total energy (357 Kcal/100 g) as fat, 45% as sucrose, and 15% as protein (custom Diet #AIN-76, CLEA Japan, Inc., Shizuoka, Japan). Contrarily, normal diet includes 12% of total energy (356 Kcal/100 g) as fat, 59% as nitrogen-free extract, and 29% as protein (#CE-2, CLEA Japan, Inc., Shizuoka, Japan). The rats were housed on a 12:12 h dark/light cycle. Some of the rats (n = 10 in Group N and n = 9 in Group HFS) were allocated to the cystometric analysis, whereas the others (n = 8 in Group N and n = 6 in Group HFS) were allocated to the organ bath assay. Each experiment was conducted simultaneously. The gastrocnemius and tibialis muscles of all rats were harvested after each procedure and preserved at −80°C until the protein assay. The animals were euthanized via inhaled analgesia (isoflurane). After the cystometric analysis, the bladder was removed and divided into the bladder mucosa and muscle layers using microscissors under a microscope. The remaining bladder tissues from the organ bath assay were also used for the protein assay.
Cystometric Analysis
We evaluated bladder function via continuous filling cystometrograms (CMGs) under a conscious condition. After the animals were anesthetized with 1.5–2.0% isoflurane, we incised the lower abdomen and inserted a PE-50 tube (Clay-Adams, Parsippany, NJ, USA) into the bladder. After recovering from anesthesia, the rats were gently restrained in a cage (Natsume Seisakusho, Yushima, Tokyo, Japan), and the catheter was hooked up via a T shape cock to both pressure transducer and syringe pump. After the rats completely recovered from anesthesia, cystometry was performed with intravesical saline infusion (0.1 mL/min) to monitor stable bladder contractions for 120 min. After CMG became stable, we emptied the bladder using the catheter, and the CMG evaluation started via saline reinfusion. The CMG parameters included (1) intercontraction interval (ICI), defined as intervals between bladder contractions; (2) maximal voiding pressure (MP); and (3) post-void residual (PVR) withdrawn through the cystostomy catheter gravitationally after the end of micturition. The voided volume (VV) and bladder capacity were calculated using the following equation: VV = infusion rate (0.1 mL/min) × mean ICI (min) and bladder capacity = VV + PVR. The voiding efficiency (VE) (VV/bladder capacity × 100) was also calculated. The average of ICI and MP during 30 min was taken. PVR was measured at the end of continuous CMG. These parameters were evaluated using LabChart from AD Instruments (Colorado Springs, CO, USA).
Organ Bath Assay
The bladder was excised and weighed, and two full-thickness longitudinal strips of 2×8 mm were taken from each bladder body. The strips were mounted in isolated muscle baths containing Tyrode’s solution (8.0 g/L NaCl, 0.05 g/L NaH2PO4-H2O, 0.2 g/L KCl, 1.0 g/L NaHCO3, 0.1 g/L MgCl2-6H2O, 1.0 g/L glucose, and 0.2 g/L CaCl2 adjusted to pH 7.4 at 37°C) and equilibrated over 1 h. The contractile responses of bladder strips to three levels of electrical field stimulation (EFS; 2, 8, and 32 Hz, 20 volts), carbachol (20 µM), and potassium chloride (KCl; 120mM) were measured in this order in each group. After organ bath assay, the weight of each bladder strip was measured. The responses were recorded isometrically, and the maximal tensions normalized to strip weight and the response to KCl were compared. The average value obtained from two strips of each bladder was adopted as the maximal tension of each rat. These parameters were evaluated using LabChart from AD Instruments (Colorado Springs, CO, USA).
Protein Assay
We used an HIF1 Alpha (sandwich ELISA) ELISA Kit (LS-F4225, LifeSpan BioSciences, Inc., Seattle, WA, USA) and a Highly Sensitive ELISA Kit for 8-OHdG (KOG-HS10/E, NIKKEN SEIL Co., Ltd., Shizuoka, Japan) to measure the HIF1α and 8OHdG concentrations in the bladder muscle and mucosa, respectively. The assayed HIF1α and 8OHdG values were standardized with the whole tissue protein concentration measured using BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The protein contents of the gastrocnemius and tibialis muscles were standardized with each muscle weight after measuring the protein levels using BCA Protein Assay Kit.
Statistics Analysis
The results were expressed as means ± SEM. The Mann–Whitney U-test was employed for the statistical comparison between the two groups. P < 0.05 was considered to indicate statistical significance.
Results
Body and Tissue Weight
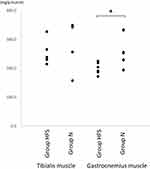
The BWs were similar in two groups at baseline (N: 202.0 ± 8.3 g; HFS: 204.8 ± 8.0 g) and 12 weeks after each diet (N: 305.0 ± 6.3 g; HFS: 309.1 ± 7.0 g) (Table 1). The tibialis muscle absolute weights and relative weight percent BW were 635.8 ± 14.5 mg and 0.22 ± 0.01 in Group N and 614.0 ± 9.2 mg (P = 0.214 vs Group N) and 0.21 ± 0.01 (P = 0.077 vs Group N) in Group HFS, respectively. The gastrocnemius muscle absolute weights and relative weight percent BW were 1071.1 ± 20.6 mg and 0.37 ± 0.01 in Group N and 1011.4 ± 9.4 mg (P = 0.014 vs Group N) and 0.34 ± 0.01 (P = 0.016 vs Group N) in Group HFS, respectively. The bladder absolute weights and relative weight percent BW were 85.1 ± 3.6 mg and 0.029 ± 0.001 in Group N and 69.9 ± 1.0 mg (P < 0.001 vs Group N) and 0.023 ± 0.001 (P < 0.001 vs Group N) in Group HFS, respectively (Table 1).
 |
Table 1 Body Weight, Muscle Weight and Bladder Weight in Rats Fed on Normal and HFS Diet |
Cystometric Analysis
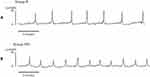
In Group HFS, the ICI was significantly shorter (176 ± 10 sec) than that in Group N (344 ± 51s) (mean differences 168 s [95% CI: 60, 276], P = 0.004) (Figures 1 and 2). The MP in Group HFS (15.7 ± 0.8 cmH2O) was significantly lower than that in Group N (21.5 ± 1.7 cmH2O) (mean differences 5.8 cmH2O [95% CI: 1.9, −9.7], P = 0.005) (Figures 1 and 2). The VV and bladder capacity in Group HFS (0.29 ± 0.02 mL and 0.32 ± 0.01 mL) were significantly smaller than those in Group N (0.57 ± 0.09 mL and 0.62 ± 0.09 mL) (VV; mean differences 0.28 mL [95% CI: 0.10, 0.46], P = 0.004, bladder capacity; mean differences 0.29 mL [95% CI: 0.10, 0.49], P = 0.004). The PVR (N: 0.04 ± 0.01 mL; HFS: 0.03 ± 0.01 mL) (mean differences 0.01 mL [95% CI: 0.00, 0.04], P = 0.253) and VE (N: 93.0% ± 1.0%; HFS: 90.7% ± 2.8%) (mean differences 2.3% [95% CI: −3.9, 8.5], P = 0.445) were similar between the two groups (Figures 1 and 2).
Organ Bath Assay
The mean bladder strip weights were similar in two groups (N: 1.6 ± 0.1 mg; HFS: 1.7 ± 0.3 mg, P = 0.745). The contractile responses normalized to strip weight to 8 Hz (144.7 ± 13.4 vs 219.6 ± 24.0 g tension/mg, mean differences 74.9 [95% CI: 18.0, 131.7], P = 0.012) and 32 Hz (158.4 ± 14.6 vs 256.1 ± 25.3 g tension/mg, mean differences 97.7 [95% CI: 37.3, 158.1], P = 0.003) of EFS and carbachol (211.0 ± 25.5 vs 295.8 ± 29.9 g tension/mg, mean differences 84.8 [95% CI: 3.9, 165.6], P = 0.041) in Group HFS were significantly decreased than those in Group N. The contractile responses to 2 Hz of EFS (118.1 ± 13.9 vs 148.3 ± 16.7 g tension/mg, mean differences 30.2 [95% CI: −14.5, 74.8], P = 0.177) and KCl (177.9 ± 23.0 vs 244.0 ± 28.9 g tension/mg, mean differences 66.1 [95% CI: −9.8, 142.0], P = 0.085) in Group HFS were decreased compared with those in Group N without a statistical significance (Figure 3A). The contractile responses normalized to the KCL response to 32 Hz (92.7 ± 12.0 vs 108.5 ± 11.7 ×102, mean differences 15.8 [95% CI: 5.4, 25.2], P = 0.002) in Group HFS was also significantly decreased than those in Group N (Figure 3B).
Protein Assay
The protein concentration of the tibialis muscle was similar in the two groups (HFS: 248.8 ± 11.8, N: 276.9 ± 29.4 mg/g muscle, mean differences 28.1 [95% CI: −43.3, 99.5], P = 0.397). The protein concentration of the gastrocnemius muscle in Group HFS (200.0 ± 5.5 mg/g muscle) was significantly lower than that in Group N (252.0 ± 19.2 mg/g muscle, mean differences 52.0 [95% CI: 6.0, 98.0], P = 0.031) (Figure 4).
In the bladder mucosa, HIF1α (N: 4.3 ± 1.2, HFS: 3.3 ± 0.8 ng/mg protein, mean differences 1.0 [95% CI: −2.0, 4.0], P = 0.484) and 8OHdG (N: 3.6 ± 0.6, HFS: 4.7 ± 0.8 ng/mg protein, mean differences −1.1 [95% CI: −3.1, 0.8], P = 0.253) were similar in the two groups. In the bladder muscle, HIF1α (N: 1.5 ± 0.2, HFS: 4.5 ± 1.1 ng/mg protein, mean differences −3.0 [95% CI: −5.5, −0.5], P = 0.022) and 8OHdG (N: 3.9 ± 0.6, HFS: 7.7 ± 0.7 ng/mg protein, mean differences −3.8 [95% CI: −5.7, −1.9], P < 0.001) were significantly higher in Group HFS than in Group N (Figure 5).
Discussion
This study demonstrated that HFS diet led to loss of the gastrocnemius muscle, voiding interval shortening, and bladder contractile force reduction in rats. Because the HIF1α and 8OHdG levels in the bladder muscle were increased, oxidative stress following bladder ischemia seemed to underlie these alterations of bladder function. HFS diet resulted in both skeletal muscle loss and bladder dysfunction similar to detrusor hyperreflexia with impaired contractility, which is most common in the elderly.
Aging is associated with a progressive loss of skeletal muscle mass and function, which is one of the causes of physical frailty in the elderly. Age-associated insulin resistance, low-grade inflammation, and oxidative stress reduce muscle mass.1 Several studies demonstrated that the load of sugars, including glucose, fructose, and sucrose, are responsible for the development of insulin resistance, inflammation, and oxidative stress.5,6 Gatineau et al reported that high-sucrose intake accelerated the progression of sarcopenia in rats by altering insulin sensitivity and muscle protein synthesis.2 Furthermore, increased intramuscular fat deposits are positively correlated with insulin resistance and inflammatory changes within the muscle in the development of sarcopenic obesity.
In this study, the relative weight and protein concentration of the gastrocnemius muscle in HFS diet rats were lower than those of normal diet rats, although the mean BW was similar between the two groups. The mean BW and protein concentration of the tibialis muscle were also decreased in HFS diet rats without a significant difference compared with normal diet rats. The reason why the tibialis muscle was not significantly changed might be insufficient feeding period with HFS diet compared with the previous study.2 Another explanation might be sex differences. In this study, we used female SD rats because the anatomy and function of the lower urinary tract are simpler than those of male rats to investigate the bladder function. Male rats were used in the previous research to investigate the change in the skeletal muscle after HFS diet.2,3 In a study on humans, adipose infiltration to the skeletal muscle is different between males and females.7 Furthermore, despite the HFS diet, a significant increase in BW was observed only in male rats, and female rats were resistant to enriched diets in some studies.8,9
Bladder ischemia is one of the key factors that induces bladder function deterioration. Previous studies demonstrated that bladder capacity and the contractile responses of bladder strips were decreased in atherosclerosis-induced chronic bladder ischemic model in rats and rabbits.10–12 In this study, the increased HIF1α and 8OHdG in the bladder muscle of HFS diet rats indicate the oxidative stress after ischemic change in the bladder. The oxidative stress in the bladder induces the sensitization of the afferent nerve pathway, resulting in bladder overactivity.13 The ischemia and oxidative stress affect the bladder nerves.14,15 In the bladder ischemic models, the number of the bladder nerves was found to decrease. The oxidative stress and ischemic change in the bladder lead to the shorter contraction intervals and lower voiding pressure on CMG analysis as well as reduced contractile responses of bladder strips. These changes in bladder function discussed in previous studies are consistent with the results of the present study. Low inflammatory change in the bladder urothelium might be happened to be correlated with shorter intervals of voiding. In future study, histological examination of the bladder is warranted. We do not have any firm explanation for the decrease in bladder weight in HFS diet rats. According to previous studies, HFS diet increased the weight of some organs, such as liver and pancreas,16 whereas it reduced the uterine and placental weights in pregnant rats.17 Reduction of the blood supply to the bladder might influence the bladder weight, although no significant change was observed in bladder weight in the atherosclerosis-induced chronic bladder ischemic animal model.10–12
The greatest question is whether there is a direct association between skeletal muscle loss and bladder dysfunction following bladder ischemia in HFS diet rats. We speculated that the blood supply is decreased in the pelvis because of relative reduction of the skeletal muscle. However, there are not any data or literature to support this speculation. One reason might be that HFS diet is only the common factor to induce skeletal muscle loss and bladder dysfunctions. To date, there is no literature on the changes in bladder function of HFS diet animals. However, in other studies, frequent voiding and the decrease in detrusor contractile response to the cholinergic stimulation were observed in hypercholesterolemia rats.18 Rats fed on fructose, but not sucrose, showed decreased detrusor contractile response to high KCl concentration and bladder overactivity and urinary frequency on CMG analysis.19–21 Thus, HFS diet might induce bladder ischemia due to change in arterial sclerosis and bladder dysfunction, including bladder overactivity, urinary frequency, and reduced detrusor contraction independent of skeletal muscle loss.
A clinical study showed that psoas muscle volume was positively associated with bladder contractility in male patients.22 The exact mechanism of this association remains to be elucidated. During the past couple of decades, it has been apparent that the skeletal muscle works as an endocrine organ, which can produce and secrete hundreds of myokines that exert their effects in either autocrine, paracrine, or endocrine manners.23 Recent literature demonstrated that the skeletal muscle produces myokines in response to exercise, which allow for crosstalk between the muscle and other organs, including brain, adipose tissue, bone, liver, gut, pancreas, vascular endothelium, and skin as well as the muscle itself.23–26 Furthermore, some myokines such as myostatin negatively regulate myogenesis.27 It is tempting to speculate that an increase or decrease in any myokines due to skeletal muscle loss might have any impact on bladder function. The effects of myokines secreted from the skeletal muscle on bladder function might be an interesting target in future research.
In HFS diet rats, the PVR and voiding efficiency were similar to those in normal diet rats despite the reduced voiding pressure and bladder contractility. Thus, the bladder function in HFS diet rats is compensated for by an unknown mechanism. One possible explanation might be related to the urethral function. Some species, including rats, have a pumping activity of the urethra to eliminate urine.28 Detailed analysis using external urethral sphincter (EUS)–electromyogram (EMG) recordings in rats revealed rhythmic contractions and relaxations of EUS during voiding, referred to as EUS bursting.28 Some research showed that in rats with spinal cord injury, the voiding efficiency was recovered and equal to normal rats through the EUS bursting activity.29 Further evaluation of the urethral function of HFS diet rats is warranted.
Weight loss, but not sarcopenic obesity, is one of the key features of frailty after sarcopenia, inactivity, and undernutrition in the elderly. Sarcopenic emaciation animal models will be needed to investigate the pathophysiology of frailty in the elderly. The link between skeletal muscle loss and bladder dysfunction is poorly understood. We have a lot of problems to resolve to understand the underlying mechanisms. This study is preliminary but is the first report to describe the relationship between skeletal muscle loss and bladder dysfunction in HFS diet rats. In the future, it should be warranted to examine whether exercise and nutrition therapy might have therapeutic benefits to improve skeletal muscle mass and bladder function.
Conclusion
This is a preliminary but first attempt to elucidate the alterations of bladder function in HFS diet rats. HFS diet reduced bladder capacity and contractility with gastrocnemius muscle loss in rats. In addition, it led to the development of lower urinary tract dysfunction that is similar, at least in part, to detrusor hyperreflexia with impaired contractility.
Acknowledgment
This work was supported by JSPS KAKENHI Grant Number JP-19K18602.
The authors would like to thank Enago (www.enago.jp) for the English language review.
Disclosure
All authors do not have any conflict of interest to disclose.
References
1. Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. 2017;33:17–26. doi:10.1016/j.cger.2016.08.002
2. Gatineau E, Savary-Auzeloux I, Migné C, Polakof S, Dardevet D, Mosoni L. Chronic intake of sucrose accelerates sarcopenia in older male rats through alterations in insulin sensitivity and muscle protein synthesis. J Nutr. 2015;145:923–930. doi:10.3945/jn.114.205583
3. Collins KH, Hart DA, Reimer RA, et al. High-fat high-sucrose diet leads to dynamic structural and inflammatory alterations in the rat vastus lateralis muscle. J Orthop Res. 2016;34:2069–2078. doi:10.1002/jor.23230
4. Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic syndrome and sarcopenia. Nutrients. 2021;13:3519. doi:10.3390/nu13103519
5. Sivaraman KS, Senthilkumar GP, Sankar P, Bobby Z. Attenuation of oxidative stress, inflammation and insulin resistance by allium sativum in fructose-fed male rats. J Clin Diagn Res. 2013;7:1860–1862. doi:10.7860/JCDR/2013/6924.3334
6. Tappy L, Leˆ K-A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi:10.1152/physrev.00019.2009
7. Merrigan JJ, White JB, Hu YE, Stone JD, Oliver JM, Jones MT. Differences in elbow extensor muscle characteristics between resistance-trained men and women. Eur J Appl Physiol. 2018;118:2359–2366. doi:10.1007/s00421-018-3962-4
8. Fourny N, Lan C, Bernard M, Desrois M. Male and female rats have different physiological response to high-fat high-sucrose diet but similar myocardial sensitivity to ischemia-reperfusion injury. Nutrients. 2021;13:2914. doi:10.3390/nu13092914
9. Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp. Anim. 2007;56:263–272.
10. Nomiya M, Burmeister DM, Sawada N, et al. Prophylactic effect of tadalafil on bladder function in a rat model of chronic bladder ischemia. J Urol. 2013;189:754–761. doi:10.1016/j.juro.2012.07.141
11. Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999;162:1768–1778. doi:10.1016/S0022-5347(05)68236-5
12. Nomiya M, Yamaguchi O, Akaihata H, et al. Progressive vascular damage may lead to bladder underactivity in rats. J Urol. 2014;191:1462–1469. doi:10.1016/j.juro.2013.10.097
13. Masuda H, Kihara K, Saito K, et al. Reactive oxygen species mediate detrusor overactivity via sensitization of afferent pathway in the bladder of anaesthetized rats. BJU Int. 2008;101:775–780. doi:10.1111/j.1464-410X.2007.07310.x
14. Coban YK, Ciralik H, Kurutas EB. Ischemic preconditioning reduces the severity of ischemia-reperfusion injury of peripheral nerve in rats. J Brachial Plex Peripher Nerve Inj. 2006;1:2. doi:10.1186/1749-7221-1-2
15. Azadzoi KM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J Urol. 2007;178:710–715. doi:10.1016/j.juro.2007.03.096
16. Rospond B, Krakowska A, Krośniak M, Muszyńska B, Opoka W. The influence of high-fat and high-sucrose feeding regimes on organ weight, body weight, and serum concentration of bioelements in rats. J Trace Elem Med Biol. 2022;73:127020. doi:10.1016/j.jtemb.2022.127020
17. Gáspár R, Hajagos-Tóth J, Schaffer A, et al. High fat high sucrose diet modifies uterine contractility and cervical resistance in pregnant rats: the roles of sex hormones, adipokines and cytokines. Life. 2022;12:794. doi:10.3390/life12060794
18. Son H, Lee SL, Park WH, et al. New unstable bladder model in hypercholesterolemia rats. Urology. 2007;69:186–190. doi:10.1016/j.urology.2006.09.062
19. Lee WC, Chien CT, Yu HJ, Lee SW. Bladder dysfunction in rats with metabolic syndrome induced by long-term fructose feeding. J Urol. 2008;179:2470–2476. doi:10.1016/j.juro.2008.01.086
20. Lee WC, Leu S, Wu KL, Tain YL, Chuang YC, Chan JYH. Tadalafil ameliorates bladder overactivity by restoring insulin-activated detrusor relaxation via the bladder mucosal IRS/PI3K/AKT/eNOS pathway in fructose-fed rats. Sci Rep. 2021;11:8202. doi:10.1038/s41598-021-87505-3
21. Chen IH, Cheng JT, Tong YC. Metabolic syndrome induced bladder cannabinoid receptor changes in the fructose-fed rats. Low Urin Tract Symptoms. 2018;10:198–203. doi:10.1111/luts.12156
22. Majima T, Funahashi Y, Matsukawa Y, et al. Investigation of the relationship between bladder function and sarcopenia using pressure flow studies in elderly male patients. Neurourol Urodyn. 2019;38:1417–1422. doi:10.1002/nau.24001
23. Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41:594–609. doi:10.1210/endrev/bnaa016
24. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi:10.1038/nrendo.2012.49
25. Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. doi:10.1038/nrrheum.2014.193
26. Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15:383–392. doi:10.1038/s41574-019-0174-x
27. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi:10.1038/387083a0
28. Kadekawa K, Yoshimura N, Majima T, et al. Characterization of bladder and external urethral activity in mice with or without spinal cord injury--a comparison study with rats. Am J Physiol Regul Integr Comp Physiol. 2016;310:R752–8. doi:10.1152/ajpregu.00450.2015
29. Wada N, Karnup S, Kadekawa K, et al. Current knowledge and novel frontiers in lower urinary tract dysfunction after spinal cord injury: basic research perspectives. Urol Sci. 2022;33:101–113. doi:10.4103/UROS.UROS_31_22
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.