Back to Journals » Journal of Inflammation Research » Volume 17
Gastroprotective Effect of 2,3-Dimethylquinoxaline Against Indomethacin-Induced Gastric Ulcer in Rat
Authors Alfadil A
Received 18 December 2023
Accepted for publication 12 March 2024
Published 29 March 2024 Volume 2024:17 Pages 1983—1994
DOI https://doi.org/10.2147/JIR.S453425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Abdelbagi Alfadil1,2
1Department of Clinical Microbiology and Immunology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia; 2Center of Research Excellence for Drug Research and Pharmaceutical Industries, King Abdulaziz University, Jeddah, Saudi Arabia
Correspondence: Abdelbagi Alfadil, Department of Clinical Microbiology and Immunology, Faculty of Medicine, King Abdulaziz University, P.O. Box 80205, Jeddah, 21589, Saudi Arabia, Tel +96612 6952000 Ext:21062, Email [email protected]
Background: Gastric ulcers pose a significant health risk due to an imbalance between protective and aggressive factors on the mucous membrane. Nonsteroidal anti-inflammatory drug (NSAID)-induced gastric damage affects 25% of users. Quinoxaline compounds, known for their diverse biological properties, have potential applications in cancer therapy and as antimicrobial agents targeting various pathogens.
Objective: Our study aimed to investigate the impact of DMQ on gastroprotective mechanisms in an experimental model of indomethacin-induced gastric ulcer.
Methods: Thirty male Wistar rats were randomly assigned to five groups. Group 1 served as the control, while Group 2 received a single oral dose of IND (30 mg/kg). Groups 3 and 4 received oral DMQ (30 mg/kg and 60 mg/kg, respectively) for three days, with the final dose administered intragastrically one hour before IND administration. Group 5 received esomeprazole (30 mg/kg) orally for three days, with the final dose given one hour before IND administration. Rats were sacrificed four hours after IND induction.
Results: Indomethacin-induced ulcers were associated with epithelial damage and blood streaks on the gastric mucosa. However, DMQ significantly decreased levels of inflammatory biomarkers (TNF-α, IL-6, Cox-2, IFN-γ, and IL-β 1) while increasing gastroprotective mediator prostaglandin E2 (PGE2) and mucin levels. Histopathological analysis revealed a significant reduction in ulcer-induced pathological alterations and upregulation of tumor suppressor genes (NF-κB levels) following DMQ treatment. Rats treated with Indo+DMQ showed a significant decrease in ulcer index compared to the Indo group, with mild injuries observed.
Conclusion: DMQ demonstrated promising gastroprotective effects against IND-induced gastric ulcers, as evidenced by alterations in histopathological data and upregulation of gene expression.
Keywords: 2,3-dimethylquinoxaline, indomethacin, gastric ulcer, inflammatory biomarkers
Introduction
One of the most prevalent diseases affecting the gastrointestinal tract, Gastric Ulcers (GU) can have life-threatening implications and mortalities.1 As 10% of the global population is affected by GU, its control and earlier detection are regarded as major obstacles.2,3 Nearly 15 deaths per 15,000 challenges occur as a result of such events annually worldwide.1,4,5 An imbalance between gastrointestinal defensive factors and aggressive physical, pharmacological, or psychological variables on the epithelium of the mucous membrane is a common cause of benign lesions such as gastric ulcers.6 Helicobacter pylori infection, cigarettes, nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin, ethanol, stress, free radicals, bile acids, protease enzymes, physical activity, prominent tobacco consumption, caffeine, colds, hunger, and sedentary lifestyle are among the triggers for the development of ulcers in the gastrointestinal system.6,7 In particular, the annual incidence of gastrointestinal toxicity stemming from NSAID drugs can be as elevated as 4–8%, and the risks are further exacerbated, particularly for individuals with a previous history of ulcer disease.8 NSAIDs rank among the most frequently utilized medications globally.9 Gastric damage induced by NSAIDs is recognized as the predominant side effect among approximately 25% of users of these medications.9 In the past, it has been demonstrated that indomethacin (Indo) is more likely to cause gastric harm compared to standard NSAIDs.10
Previous studies on ulcers induced by Indomethacin have shown that it hinders the production of prostaglandin-E2 (PGE2) and angiogenesis, promotes the generation of free radicals, triggers the expression of cyclooxygenase-2 (COX-2), and induces the release of cytokines responsible for pro-inflammatory processes.11–13 Furthermore, the abnormal induction of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) leads to inflammatory tissue damage by causing an overproduction of pro-inflammatory agents, such as prostaglandins and nitric oxide.14 The primary mechanism driving its anti-inflammatory effects involves the suppression of COX-2, NF-κB, PGE2, and various cytokines, including TNF-α (Tumor Necrosis Factor-alpha), IL-6 (Interleukin-6), interleukin-1β (IL1β), and NO.15–17 A previous study reported that a decrease in mucin levels leads to gastrointestinal injury due to indomethacin.5 All of these characteristics hold the potential to be advantageous in averting the onset and progression of gastric ulcers. The combined attributes encompassing these properties may contribute to a preventative effect against the initiation and development of gastric ulceration. The primary mechanism driving the anti-inflammatory effects of 3-DMQ involves the suppression of COX-2, NF-κB, PGE2, and various cytokines, including TNF-α (Tumor Necrosis Factor-alpha), IL-6 (Interleukin-6), interleukin-1β (IL1β), and NO15–.17 Another study reported that level of mucin erosion has also been associated with gastrointestinal damage.5,18 The formation of stomach ulcers may be prevented by all of the above characteristics.
Annually, a significant number of people, approximately 200,000, require hospitalization due to ulcer diagnosis, with an additional 3 million individuals seeking treatment at polyclinics. The economic burden of treating this condition reaches around 4 billion dollars.19 Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used worldwide, and gastric damage is recognized as their most prevalent and hazardous side effect.9 Indomethacin is a well-known NSAID, notorious for its high chance to induce gastric ulcers.11,20 Gastric ulcers occur when the equilibrium between defensive factors, such as mucus secretion, blood flow, cell renewal, prostaglandins (PGs), and nitric oxide (NO), is disrupted in favor of aggressive factors, including NSAIDs, acidity, oxidative stress, and ethanol.21,22 Indomethacin, a nonsteroidal anti-inflammatory drug, operates by inhibiting both COX and phosphodiesterase, resulting in the suppression of prostaglandin synthesis and release, ultimately reducing inflammation.
Indomethacin initiates a rise in lipid peroxidation and the creation of reactive oxygen species (ROS) within the gastric mucosa. The use of an Indomethacin-induced gastric ulcer model is a widely employed technique to induce gastric ulcers in rats.19 Currently, there are several synthetic antiulcer medications available, including drugs such as cimetidine, misoprostol, ranitidine, omeprazole, and esomeprazole, which are utilized for the management and treatment of NSAID-induced gastric ulcers. But, it is essential to note that each of these medications is associated with a spectrum of side effects, ranging from mild to severe. This has spurred the search for alternative antiulcer treatments that are non-toxic, readily accessible, and cost-effective.5,23,24
Quinoxaline compounds have a wide range of applications, possessing diverse biological properties and potential roles in cancer therapy, and the development of antimicrobial agents targeting bacteria, fungi, and viruses. The activities of these compounds are determined by the specific substituents and where they are located in the quinoxaline rings. Extensive research has focused on derivatives featuring a carbonyl group at the 2-position. The biological properties of many quinoxalines have been assessed in vitro, and some have found utility in veterinary medicine. For instance, 2,3-Dimethylquinoxaline presents itself in the form of yellow needle-shaped crystals.25
In this current study, the focus was on exploring the gastroprotective effects of DMQ (2,3-Dimethylquinoxaline) and investigating the potential underlying mechanisms using an experimental model of gastric ulcers induced by indomethacin.
Materials and Methods
Drugs and Chemicals
2,3-Dimethylquinoxaline, indomethacin, esomeprazole, and carboxymethyl cellulose sodium (CMC-Na) were acquired from Sigma-Aldrich in St. Louis, MO, USA. The research also used various ELISA kits, including Rat TNF-α ELISA Kit, Rat Interferon Alpha kit (Cat No. MBS267050), Rat Prostaglandin E2 (PGE2) ELISA Kit (Cat No. MBS262150), Rat Mucin ELISA (Cat No MBS1600651), Rat Interleukin 6 (IL-6) ELISA Kit (Cat No. MBS269892), Rat Inducible Nitric Oxide Synthase (iNOS) Elisa kit (Catalog Number: MBS723326), Rat Cyclooxygenase 2 (COX2) ELISA kit (Catalog Number: MBS725633), and Rat IL-1 beta ELISA Kit (Catalog # MBS825017), all of which were also obtained from Sigma-Aldrich in St. Louis, MO, USA.
The study utilized commercially available chemicals like formalin, phosphate buffer, and other necessary compounds, all chosen for their high purity grades. It’s important to note that all chemicals used in the research were of analytical grade.
Animals
The research involving animals adhered to approved procedures established by the Research Ethics Committee of the Faculty of Pharmacy at King Abdulaziz University, with reference number PH-1443-23. Male Wistar rats, aged 10 weeks and weighing between 200 and 230 grams, were sourced from the animal facility at the Faculty of Pharmacy, King Abdulaziz University. These rats were kept in a controlled environment with a temperature range of 20–24°C and a 12-hour light and 12-hour dark cycle. They had unrestricted access to a standard diet and water. Before commencing the experiments, the rats were given a one-week period to acclimate to the conditions of our experimental facility.
Experimental Design
Thirty rats were assigned randomly to five groups, each containing six rats. Here’s a breakdown of the groups and their treatments: Control Group 1: Rats in this group were administered the vehicle (0.5% w/v carboxymethyl cellulose sodium, 10 mL/kg) orally. IND Group 2: Rats in this group received a single oral dose of Indomethacin (30 mg/kg). Group 3 IND + 2,3-Dimethylquinoxaline 30 mg/kg, Group 4: Rats in this group were given 2,3-Dimethylquinoxaline orally at a dose of 30 mg/kg for three consecutive days. On the third day, they received Indomethacin (30 mg/kg) orally, followed by the last dose of 2,3-Dimethylquinoxaline one hour later, IND + 2,3-Dimethylquinoxaline 60 mg/kg Group: Similar to Group 3, but the rats in this group received 2,3-Dimethylquinoxaline at a dose of 60 mg/kg. Group 5. IND + esomeprazole Group: Rats in this group were orally administered esomeprazole (30 mg/kg) for three consecutive days. On the third day, they received indomethacin (30 mg/kg) orally, followed by the last dose of esomeprazole one hour later. After 4 hours of Indomethacin administration, the rats in the treatment groups were euthanized for further analysis.
Gastric Ulcer Induction
In line with earlier studies, it was observed that Indomethacin (IND) led to the development of gastric ulcers.20 On the 2nd day of the experiment, the rats underwent a 24-hour period of fasting, during which they were allowed access to water. On the third day, a dosage of 30 mg/kg of Indomethacin, suspended in a 0.5% carboxymethyl cellulose sodium (CMC-Na) solution, was administered via intragastric administration to all groups, except for the control group.
Mucin Protein Assessment
The sandwich technique was used to measure the mucin protein using a Rat MUC1 ELISA kit (Cat. No.# MBS1600651, Louis, MO, USA). Finely sliced stomach tissue was extensively rinsed in ice-cold phosphate-buffered saline (PBS) for removing any remaining blood. Following the measurement of the tissue pieces’ weight, homogenization was carried out in PBS using a glass homogenizer while the tissue weight (g) was w/v of PBS (mL) at a ratio of 1:9. Supernatant was obtained by centrifuging the homogenates for 5 minutes at 5000× g. At 37°C for 90 minutes, 100µL of both the standard and sample was added to each well. Following a one-hour incubation at 37°C and the discard the supernatant, 100 µL of detection antibody was aspirated and thoroughly cleaned three times. Following aspiration and five times of washing, 100 µL of horseradish peroxidase (HRP) conjugate was added to the solution. Following a 15-minute incubation period at 37°C, 90 µL of substrate solution was added and the optical density (OD) at 450 nm was determined promptly.13
Morphology and Histopathological Analysis
After the stomachs of animals were sliced, they were cleaned with normal saline (0.9% NaCl). The photos were taken using digital photography. The stomachs were then analyzed using a microscope to detect any hemorrhagic lesions present in the glandular mucus layers.13
As previously mentioned, the stomach samples were preserved in a solution containing 10% formalin and saline. Subsequently, the gastric tissues were washed, dehydrated using alcohol with progressively higher concentrations, subjected to xylene for clarification, and subsequently enclosed in paraffin wax for embedding. Thin sections, about 5 µm in thickness, were prepared and subjected to examination after staining with hematoxylin and eosin (H & E) to identify any structural alterations. The examination of the tissues was carried out using a light microscope. A scoring system was employed in the study, ranging from 0 to 4, to evaluate histopathological changes. This scoring system was used by a histopathologist who was unaware of the specific treatments and assessed factors such as edema in the gastric mucosa, infiltration of inflammatory cells, gastric hemorrhage, and necrosis.26,27
Determination of Analyses of Pro-Inflammatory Markers
The content of IL-6 (Cat No.# MBS269892) and TNF-α (Cat. No.# MBS2507393), Rat Interferon Alpha (IFN-α), (Cat No.# MBS267050), Rat IL-1 beta ELISA Kit (Catalog # MBS825017), TNF-α in the supernatant of stomach tissue homogenate, was quantified using enzyme-linked immunosorbent assay (ELISA) kits. These measurements were performed in accordance with the manufacturers provided protocol. All of them were gained from Sigma-Aldrich in St. Louis, MO, USA.
Determination of Prostaglandin E2 (PGE2) and Cyclooxygenase 2 (COX2)
Assessment of PGE2 activity in gastric tissue homogenates was conducted using the Rat Prostaglandin E2 (PGE2) ELISA Kit. (Cat No.# MBS262150 St. Louis, MO, USA), Rat Cyclooxygenase 2 (COX2) ELISA kit (Competitive ELISA) (Cat No.# MBS725633, St. Louis, MO, USA). Following a rinse in TBS, the samples underwent incubation with either biotinylated secondary antibodies, either anti-mouse or anti-rabbit, were utilized based on the reactivity of the primary antibody. These antibodies operate on a competitive inhibition enzyme immunoassay technique. Total RNA extraction and cDNA synthesis from stomach tissues.
The genomic DNA was extracted from the cell homogenate using the QIAmp DNA Mini kit from Qiagen, located in Valencia, CA. RNA isolation was carried out using the RNA assay kit, also from Qiagen, following the manufacturer’s provided protocols. To assess the concentration and quality of DNA and RNA, the ratio of optical density at 260/280 nm was measured using Nano-Drop equipment from Nano Drop Technologies in Wilmington, DE.
For the purpose of detecting gene mutations, the DNA extracted from the cells was subjected to amplification using the AmpliTaq Gold® Fast PCR Master Mix from Applied Biosystems, based in Carlsbad, CA. Subsequently, direct sequencing was performed using the designated primer sets.
Determination of Genes Expression Using Real Time Polymerase Chain Reaction (PCR)
Real-time PCR reactions were performed utilizing Strata Gene and following the SYBR® Green JumpStart™ Taq ReadyMix™ protocol from Sigma Aldrich, Germany. The reaction mixture for both genes comprised 0.5 μL of each primer NF-κB (Sense “5-GAAATTCCTGATCCAGACAAAAAC-3”, antisense 5’-ATCACTTCAATGGCCTCTGTGTAG-3’), GAPDH (Sense “5-GTCTCCTCTGACTTCAACAGCG-3”, antisense 5’- ACCACCCTGTTGCTGTAGCCAA). 7.5 μL SYBR-Green Ready Mix, 1 μL of cDNA and final volume of 16 μL was maintained up to 16ul. Samples were analyzed in sets of three replicates, and the average Ct (cycle threshold) values for both genes were computed. The NFKB and GAPDH gene reactions were executed in distinct tubes. For each experiment, a negative control was also included and tested three times. The thermal cycler settings were as follows: the protocol involved an initial denaturation step at 95°C for 10 minutes, succeeded by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 57°C for 60 seconds, elongation at 72°C for 60 seconds, and a final extension step at 72°C for 3 minutes. The ΔΔCq method was employed to ascertain the relative gene expression.12
Statistical Analysis
The data acquired in this study were analyzed using licensed software, specifically version 5.02 of Graph Pad Prism. The results were presented as the mean ± standard error of the mean (SEM). To assess the significance, we applied the one-way analysis of variance (ANOVA) method to compare different variables across each experimental group. Subsequently, a post hoc test (Tukey’s test) was conducted to further analyze the differences. In the context of statistical analysis, the p-value served as an indicator of significance, with a p-value of less than 0.05 signifying a statistically significant result.
Results
Effect of 2,3-Dimethylquinoxaline on Gastric Tissue Levels of Inflammatory Cytokines (Levels of TNF-α, IL-6, and IL-1β)
The administration of indomethacin orally resulted in a notable elevation in the gastric concentrations of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6. However, treated rats with DMQ 40% decreased gastric concentration of TNF-α, IL-1β, IL-6 as compared to animal treated with indomethacin. Pre-treated DMQ caused a remarkably reduced 45% in gastric level of TNF-α, IL-6, and IL-1β (Figure 1).
Effect of 2,3-Dimethylquinoxaline on s IFN-ɤ
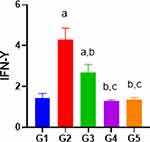
Oral induction of Indo exhibited a significant reduction (43%) in the gastroprotective level of IFN-ɤ as compared to control group (Figure 2). However, pretreatment with DMQ (30 mg and 60 mg/kg) or esomeprazole significantly decreased the IFN-ɤ level which is nearly equal to control group (Figure 3).
Effect of 2,3-Dimethylquinoxaline on Plasma PGE2
Oral induction of Indo exhibited a remarkable reduction in the gastroprotective activity of PGE2 as comparison to control. However, pretreated DMQ (30 mg and 60 mg/kg) or esomeprazole significantly increased the PGE2 level which is nearly equal to control group (Figure 4).
Effect of 2,3-Dimethylquinoxaline on Mucin Content
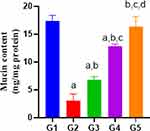
Our results presented that the Indo group significantly markedly reduced mucin level as compared to the control, whereas the decline was markedly reversed by DMQ (30 or 60 mg/kg) and esomeprazole (30 mg/kg) treatments (Figure 5).
Effect of 2,3-Dimethylquinoxaline on mRNA Expression Levels of NF-κB Marker Genes
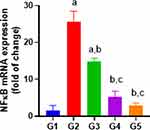
In Indo group, NF-κB expression activity were significantly higher in comparison with the control group while pretreatment with DMQ (30 or 60 mg/kg) caused significant decrease ie, 40% in NF-κB expression activity in comparison to indomethacin-treated group, which is nearly equivalent to control or standard group (Figure 6). Furthermore, esomeprazole (30 mg/kg) exhibited decreases expression of NF-κB corresponding to control group.
Effect of DMQ Pretreatment on Stomach Morphology
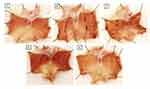
Figure 7A indicates that there was no damage and the stomach mucous membrane in the control group remained in a healthy state. In spite of this, the Indo group showed 0.5–5 mm bloody streaking wounds (Figure 7B), as indicated by a significant rise in the index of ulcers (Figure 7F). In contrast to the Indo group, the Indo+ DMQ (30 mg/kg) group showed a lower occurrence of bloody streaking (Figure 7C). After receiving the Indo+ DMQ treatment (60 mg/kg), the rats showed a notable reduction in their ulcer index in comparison to the Indo group. Additionally, there was evidence of mild injuries, as indicated by the obstruction of superficial blood vessels (Figure 7D). Having no noticeable reduction in ulcer index in comparison to the control group, esomeprazole treatment protected the layer of the gastric mucosa (Figure 7E and F).
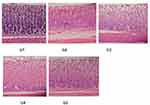
Effect of DMQ on Histopathological Analysis
Examinations of photomicrographs of excised stomach tissues showed that DMQ had a protective effect against ulcers induced by Indo. Hematoxylin and eosin staining was employed for this purpose. Additionally, in the group treated as the normal control (Figure 8G1), the epithelial tissue appeared normal, and there were very few inflammatory cells present. Histopathological studies revealed reduction in the total number of inflammatory cells and hemorrhage in the 30 mg/kg treatment group III as compared to indomethacin treated group II. However, restoration of normal epithelial tissue and negligible inflammatory cells were seen in 60 mg/kg treated group IV which was similar to control group (group I).
Discussion
Gastric ulcers are a prevalent ailment influenced by numerous mucosal factors.13,28,29 The inhibitory effects of indomethacin on prostaglandin synthesis and the generation of free radicals are considered crucial biochemical events in the development of gastric ulcers.5,18,30 An understanding of these processes is crucial for the development of new antiulcer medications. Given the side effects and high costs associated with synthetic drugs, it is worth exploring natural plant-based products, considered to be safe, efficacious, and reasonably priced for treating gastric ulcers.5
Indomethacin is known to have a higher propensity to induce ulcers compared to other NSAIDs, making it a preferred choice for experimental induction of gastric ulcers.11 In our study, we hypothesize that oral administration of indomethacin (30 mg/kg) to rats leads to elevated gastric acidity and ulceration compared to the control group. These findings align with previous reports that demonstrated how indomethacin elevates gastric acidity, leading to ulcer formation.18,31 The effect of 2,3-Dimethylquinoxaline (DMQ) on the induced gastric ulcer model was effectively assessed. The efficacy of DMQ in treating ulcers was determined through in vivo pharmacokinetics. In our study, ulcer symptoms were alleviated in rat groups pre-treated with either esomeprazole or DMQ.
Here, the histopathology data on gastric tissue in ulcer model revealed evident ulcer injury. Additionally, these alterations were consistent with the increased gastric level of the inflammatory marker TNF-α quantified. It has been documented that TNF-α plays an important role in the pathogenesis of indomethacin-induced gastric ulcers, particularly by activation of neutrophil infiltration.11 It is worth noting that TNF-α production, typically inhibited by PGE2, increases in this context.32,33 TNF-α is a significant mediator in NSAIDs-induced gastropathy, and it may also activate pro-apoptosis caspases that regulate apoptosis of gastric epithelial cells in NSAID-treated rats.34,35 A rat TNF-α kit was used to determine TNF-α levels in plasma, revealing a notable decrease in IL-6 and TNF-α levels between the group treated with indomethacin and DMQ (60 mg/kg body weight) (group IV). This suggests the potential of 2,3-dimethylquinoxaline in reducing the inflammatory response in ulcers, while slight differences were observed between group II and group III due to the higher mucosal damage caused by indomethacin. Biomarker analysis indicated that a 60 mg/kg dose of DMQ is needed for maximum anti-ulcer activity. As other studies showed, to suppresses pro-inflammatory markers (TNF-α, IL-6 IL-β1).13
Clinical biomarkers like COX-2, iNOS, TNF-α, IL-1, IL-6, and mucin were used to assess the pharmacodynamic activity of DMQ in ulcer treatment. Rats were divided into five groups, and in this context, histopathological studies showed a decreased in the total number of inflammatory cells and hemorrhage in the 30 mg/kg (DMQ) treatment group (group III) compared to the indomethacin-treated group (group II). However, the 60 mg/kg (DMQ) treated group (group IV) showed the restoration of normal epithelial tissue and minimal inflammatory cells, similar to the control group (group I). Omeprazole also displayed an anti-inflammatory effect, evident in the histopathological results and the reduction of TNF-α levels in stomach tissue, consistent with a previous study showing that omeprazole administration reduced serum TNF-α in an ethanol-induced ulcer model.36 Our findings corroborate previous studies, indicating that administering tetramethylpyrazine beforehand resulted in increased mucin levels and decreased TNF-α and IL-6 levels. Additionally, their research suggested that the group treated with tetramethylpyrazine showed enhancements in both morphological and histopathological aspects compared to the peptic ulcer group induced by indomethacin.37
It is also believed that NSAID-mediated gastropathy may be facilitated by a reduction in mucin levels in the stomach mucosa. As a result of maintaining mucus formation, reactive oxygen metabolites may be partially but significantly protected from causing damage.9,38 Based on our findings, the amount of gastric mucus was decreased by stomach ulcers. As a result, the mucosal membrane may be less likely to prevent the mucosa from harm and hydrogen ion back diffusion, which could impede epithelial healing.
The measurement of gastroprotective mediator PGE2 suggested a huge decrease in the indomethacin group, while rats pre-treated with omeprazole or DMQ showed a huge improvement in gastric PGE2 levels.11,13,18 Reduced PGE2 synthesis plays a role in NSAID-induced gastric ulcers, as PGE2 is responsible for protecting the gastric mucosa by enhancing mucus secretion, keeping blood flow, and reducing acid secretion.10,11,39 PGE2 has also been reported to inhibit TNF-α production, consistent with our findings.36 Several key cytokines, including TNF-α, IL-6, IL-10, PGE2, and IL-1β, which are released by macrophages during inflammation, contribute to the understanding of the study.40 Based on these findings, a 60 mg/kg dose of DMQ proved to be significant in all the parameters mentioned above. Pretreatment with esomeprazole increased gastric PGE2 levels, aligning with other reports that documented esomeprazole’s ability to raise PGE2 levels in rats with IND-induced ulcers.36 Omeprazole has been found to possess anti-ulcerative activity through the alpha-2 adrenergic receptor, which is directly related to gastroprotective cyclooxygenase-1 (COX-1) and PGE2.41,42 Additionally, omeprazole upregulates cyclooxygenase-2 (COX-2) and PGE2, contributing to ulcer healing through re-epithelization.38
Institutional Review Board Statement
The animal study protocol was approved by the King Abdulaziz university, Jeddah, Saudi Arabia (Reference No “PH-1444-55`) for studies involving animals.
Funding
Grant number IFPIP: 1272-140-1443-260499 from the Instructional Improvement Fund supported this study. The authors would like to express their appreciation to the Saudi Arabian MOE and King Abdulaziz University, DSR in Jeddah for their technical and financial aid.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Yekta RF, Amiri-Dashatan N, Koushki M, et al. A metabolomic study to identify potential tissue biomarkers for indomethacin-induced gastric ulcer in rats. Avicenna J Med Biotechnol. 2019;11(4):1.
2. Zheng YF, Xie J-H, Xu Y-F, et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem Biol Interact. 2014;222:27–36. doi:10.1016/j.cbi.2014.08.008
3. Boligon AA, de Freitas RB, de Brum TF, et al. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm Sin B. 2014;4(5):358–367. doi:10.1016/j.apsb.2014.05.001
4. Taher MA, Nyeem MA, Ahammed MM, et al. Moringa oleifera (Shajna): the wonderful indigenous medicinal plant. Asian J Med Biol Res. 2017;3(1). doi:10.3329/ajmbr.v3i1.32032
5. Sabiu S, Garuba T, Sunmonu T, et al. Indomethacin-induced gastric ulceration in rats: protective roles of Spondias mombin and Ficus exasperata. Toxicol Reports. 2015;2. doi:10.1016/j.toxrep.2015.01.002
6. Tripathi K. Essentials of Medical Pharmacology; 2013.
7. Mousa AM, El-Sammad NM, Hassan SK, et al. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complement Altern Med. 2019;19(1). doi:10.1186/s12906-019-2760-9
8. Griffin MR, Scheiman JM. Prospects for changing the burden of nonsteroidal anti-inflammatory drug toxicity. Am J Med. 2001;110(1 SUPPL. 1):S33–S37. doi:10.1016/s0002-9343(00)00634-3
9. Chattopadhyay S, Adhikary B, Yadav SK, Roy K, Bandyopadhyay SK. Black tea and theaflavins assist healing of indomethacin-induced gastric ulceration in mice by antioxidative action. Evid Based Complement Altern Med. 2011;2011. doi:10.1155/2011/546560
10. Musumba C, Pritchard DM, Pirmohamed M. Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther. 2009;30(6):517–531. doi:10.1111/j.1365-2036.2009.04086.x
11. Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33(4):224–234. doi:10.1007/s10753-009-9176-5
12. Yadav SK, Adhikary B, Chand S, Maity B, Bandyopadhyay SK, Chattopadhyay S. Molecular mechanism of indomethacin-induced gastropathy. Free Radic Biol Med. 2012;52(7):1175–1187. doi:10.1016/j.freeradbiomed.2011.12.023
13. Shaik RA, Eid BG. Piceatannol affects gastric ulcers induced by indomethacin: association of antioxidant, anti-inflammatory, and angiogenesis mechanisms in rats. Life. 2022;12(3):356. doi:10.3390/life12030356
14. Liu L, Li J, Kundu JK, Surh YJ. Piceatannol inhibits phorbol ester-induced expression of COX-2 and iNOS in HR-1 hairless mouse skin by blocking the activation of NF-κB and AP-1. Inflamm Res. 2014;63(12):1013–1021. doi:10.1007/s00011-014-0777-6
15. Jeong SO, Son Y, Lee JH, et al. Resveratrol analog piceatannol restores the palmitic acid-induced impairment of insulin signaling and production of endothelial nitric oxide via activation of anti-inflammatory and antioxidative heme oxygenase-1 in human endothelial cells. Mol Med Rep. 2015;12(1):937–944. doi:10.3892/mmr.2015.3553
16. Molnar V, Matišić V, Kodvanj I, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. 2021;22(17):9208. doi:10.3390/ijms22179208
17. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008. doi:10.3390/ijms20236008
18. Abdallah IZA, Khattaba HAH, Heebab GH. Gastroprotective effect of Cordia Myxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci J. 2011;8(3):1.
19. Eraslan E, Tanyeli A, Güler MC, Kurt N, Yetim Z. Agomelatine prevents indomethacin-induced gastric ulcer in rats. Pharmacological Reports. 2020;72(4):984–991. doi:10.1007/s43440-019-00049-2
20. Abbas AM, Sakr HF. Effect of selenium and grape seed extract on indomethacin-induced gastric ulcers in rats. J Physiol Biochem. 2013;69(3):527–537. doi:10.1007/s13105-013-0241-z
21. Kang JW, Yun N, Han HJ, et al. Protective effect of flos lonicerae against experimental gastric ulcers in rats: mechanisms of antioxidant and anti-inflammatory action. Evid Based Complement Altern Med. 2014;2014:1–11. doi:10.1155/2014/596920
22. El-Ashmawy NE, Khedr EG, El-Bahrawy HA, Selim HM. Gastroprotective effect of garlic in indomethacin induced gastric ulcer in rats. Nutrition. 2016;32(7–8):849–854. doi:10.1016/j.nut.2016.01.010
23. Akah PA, Orisakwe OE, Gamaniel KS, Shittu A. Evaluation of Nigerian traditional medicines: II. Effects of some Nigerian folk remedies on peptic ulcer. J Ethnopharmacol. 1998;62(2):123–127. doi:10.1016/S0378-8741(98)00060-9
24. Hawkins C, Hanks GW. The gastroduodenal toxicity of nonsteroidal anti-inflammatory drugs. A review of the literature. J Pain Sympt Manage. 2000;20(2):140–151. doi:10.1016/S0885-3924(00)00175-5
25. Peralta-Cruz J, Díaz-Fernández M, Ávila-Castro A, Ortegón-Reyna D, Ariza-Castolo A. An experimental and theoretical study of intramolecular regioselective oxidations of 6-substituted 2,3-dimethylquinoxaline derivatives. New J Chem. 2016;40(6):5501–5515. doi:10.1039/c5nj03282b
26. Kaleem M, Haque SE. Evaluation of cardioprotective role of vinpocetine in isoproterenol induced myocardial infarction in rats. J Pharm Res. 2015;9(7):408–414.
27. Al KhalafA, AbdulrahmanAO, KaleemM, et al. Comparative analysis of the impact of urolithins on the composition of the gut microbiota in normal-diet fed rats. Nutr. 2021;13:3885. doi:10.3390/NU13113885
28. Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135(1):41–60. doi:10.1053/j.gastro.2008.05.030
29. Shaker E, Mahmoud H, Mnaa S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camel thorn). Food Chem Toxicol. 2010;48(10):2785–2790. doi:10.1016/j.fct.2010.07.007
30. Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Ann Rev Physiol. 1995;57(1):565–583. doi:10.1146/annurev.ph.57.030195.003025
31. Oluwabunmi I, Abiola T. Gastroprotective effect of methanolic extract of Gomphrena celosioides on indomethacin induced gastric ulcer in Wistar albino rats. Int J Appl Basic Med Res. 2015;5(1):41. doi:10.4103/2229-516x.149238
32. Werawatganon D, Rakananurak N, Sallapant S, et al. Aloe vera attenuated gastric injury on indomethacin-induced gastropathy in rats. World J Gastroenterol. 2014;20(48):18330. doi:10.3748/wjg.v20.i48.18330
33. Konturek PC, Duda A, Brzozowski T, et al. Activation of genes for superoxide dismutase, interleukin-1β, tumor necrosis factor-α, and intercellular adhesion molecule-1 during healing of ischemia-reperfusion-induced gastric injury. Scand J Gastroenterol. 2000;35(5). doi:10.1080/003655200750023697
34. Lazzaroni M, Bianchi Porro G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment Pharmacol Ther. 2004;20(SUPPL.2):48–58. doi:10.1111/j.1365-2036.2004.02037.x
35. Thong-Ngam D, Choochuai S, Patumraj S, Chayanupatkul M, Klaikeaw N. Curcumin prevents indomethacin-induced gastropathy in rats. World J Gastroenterol. 2012;18(13):1479. doi:10.3748/wjg.v18.i13.1479
36. Abood WN, Abdulla MA, Ismail S. Involvement of inflammatory mediators in the gastroprotective action of Phaleria macrocarpa against ethanol-induced gastric ulcer. World Appl Sci J. 2014;30(30 A). doi:10.5829/idosi.wasj.2014.30.icmrp.48
37. AlKreathy HM, Alghamdi MK, Esmat A. Tetramethylpyrazine ameliorates indomethacin-induced gastric ulcer in rats: impact on oxidative, inflammatory, and angiogenic machineries. Saudi Pharm J. 2020;28(8):916–926. doi:10.1016/j.jsps.2020.06.012
38. Sun J, Shen X, Li Y, et al. Therapeutic potential to modify the mucus barrier in inflammatory bowel disease. Nutrients. 2016;8(1):44. doi:10.3390/nu8010044
39. Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88(4). doi:10.1152/physrev.00004.2008
40. Gilani SJ, Bin-Jumah MN, Al-Abbasi FA, et al. Protective effect of fustin against ethanol-activated gastric ulcer via downregulation of biochemical parameters in rats. ACS Omega. 2022;7(27):23245–23254. doi:10.1021/acsomega.2c01341
41. Suleyman H. The role of alpha-2 adrenergic receptors in anti-ulcer activity. Eurasian J Medi. 2012;44(1):43–45. doi:10.5152/eajm.2012.09
42. Kisaoglu A, Ozogul B, Cetyn N, et al. The role of alpha-2 adrenergic receptors in the anti-ulcerative activity of famotidine and omeprazole in rats and its relationship with oxidant-antioxidant parameters. Int J Pharmacol. 2011;7(6):682–689. doi:10.3923/ijp.2011.682.689
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.