Back to Journals » OncoTargets and Therapy » Volume 8
Ganetespib: research and clinical development
Received 18 April 2015
Accepted for publication 22 June 2015
Published 24 July 2015 Volume 2015:8 Pages 1849—1858
DOI https://doi.org/10.2147/OTT.S65804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Komal Jhaveri, Shanu Modi
Breast Medicine Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Abstract: Under stressful conditions, the heat shock protein 90 (HSP90) molecular chaperone protects cellular proteins (client proteins) from degradation via the ubiquitin-proteasome pathway. HSP90 expression is upregulated in cancers, and this contributes to the malignant phenotype of increased proliferation and decreased apoptosis and maintenance of metastatic potential via conservation of its client proteins, including human epidermal growth factor receptor 2, anaplastic lymphoma kinase, androgen receptor, estrogen receptor, Akt, Raf-1, cell cycle proteins, and B-cell lymphoma 2 among others. Hence, inhibition of HSP90 leads to the simultaneous degradation of its many clients, thereby disrupting multiple oncogenic signaling cascades. This has sparked tremendous interest in the development of HSP90 inhibitors as an innovative anticancer strategy. Based on the wealth of compelling data from preclinical studies, a number of HSP90 inhibitors have entered into clinical testing. However, despite enormous promise and anticancer activity reported to date, none of the HSP90 inhibitors in development has been approved for cancer therapy, and the full potential of this class of agents is yet to be realized. This article provides a review on ganetespib, a small molecule HSP90 inhibitor that is currently under evaluation in a broad range of cancer types in combination with other therapeutic agents with the hope of further enhancing its efficacy and overcoming drug resistance. Based on our current understanding of the complex HSP90 machinery combined with the emerging data from these key clinical trials, ganetespib has the potential to be the first-in-class HSP90 inhibitor to be approved as a new anticancer therapy.
Keywords: HSP90, lung cancer, breast cancer, colorectal cancer
Introduction
Heat shock protein 90 (HSP90), a 90 kDa ATP-dependent molecular chaperone, is the most abundant intracellular protein in mammalian cells and is essential for protein folding, assembly, and degradation processes.1,2 It is overexpressed in response to a variety of physiological and environmental insults, allowing cells to survive potentially lethal conditions due to its cytoprotective functions.3–5 In this regard, HSP90 has recently received much attention due to its overexpression in certain types of cancer,6–9 where it is associated with a poor prognosis and contributes to resistance to chemotherapy and radiation.10 Unlike other molecular chaperones that are involved in the primary folding of nascent polypeptides, HSP90 uses repeated cycles of client protein binding, hydrolysis of ATP, and interaction with its co-chaperones such as HSP70, Cdc37, HOP, p23, and Aha1 to control the stability and activity of hundreds of client proteins.11,12 Importantly, the majority of these client proteins are involved in critical signaling pathways necessary for cellular proliferation, cell cycle progression, and apoptosis, including steroid hormone receptors, kinases, transcriptions factors, human epidermal growth factor receptor 2 (HER2), Akt, mutant BRAF, and mutant p53 among others.12–16 Consistent with this, HSP90 inhibition results in the simultaneous degradation of many of these clients (via the ubiquitin-proteasome pathway), leading to cell-specific growth arrest, apoptosis of cancer cells, and antitumor activity in preclinical models.3,17 Thus, HSP90 has evolved as an important molecular target in cancer therapy, and 16 different first- and second-generation HSP90 inhibitors have entered clinical testing (Table 1). The prototype HSP90 inhibitor geldanamycin provided proof-of-concept for HSP90 inhibition; however, geldanamycin and its derivatives (17-allylamino-17-demethoxygeldanamycin [17-AAG] and 17-dimethylaminoethylamino-17-demethoxygeldanamycin [17-DMAG]) could not be fully developed due to a number of safety and pharmacological limitations. Consequent efforts using a variety of different chemical scaffolds have led to the development of highly potent, second-generation, small molecule HSP90 inhibitors with improved pharmacological properties and safety profiles.18 This review will summarize information on the pharmacology, preclinical activity, and current clinical development of ganetespib, a novel small molecule, second-generation HSP90 inhibitor developed by Synta Pharmaceuticals.
  | Table 1 First- and second-generation HSP90 inhibitors |
Pharmacology and preclinical data
The HSP90 chaperone consists of an amino (N) terminal, a carboxy (C) terminal, and a middle (M) domain. The N-terminal contains the ATP-binding pocket, and both the N and C termini have a drug-binding site. The M domain mostly participates in forming active ATPase and acts as a docking site for clients and co-chaperones.19,20 Similar to many other HSP90 inhibitors, ganetespib also acts by binding to the N-domain ATP-binding pocket of HSP90.21
The first-generation inhibitors were a semi-synthetic derivative of the natural product, geldanamycin, and included 17-AAG (17-Allyl-17-Demethoxygeldanamycin [Tanespimycin]), 17-DMAG (17-desmethoxy-17-N,N-dimethylaminoethylaminogeldanamycin [alvespimycin]), IPI-504/17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (Retaspimycin), and IPI-493/17-desmethoxy-17-amino geldanamycin (Table 1).22 While the first-generation inhibitors provided proof-of-concept, they failed to advance further clinically due to various issues including poor pharmacological properties, adverse toxicity profiles, or suboptimal efficacy. The most clinically significant off-target toxicity with the geldanamycin derivatives was hepatotoxicity, which has largely been attributed to the presence of a benzoquinone moiety.22 Although 17-DMAG lacked the solubility issues observed with 17-AAG, the toxicities reported with 17-DMAG, including liver, ocular, and cardiac toxicities, ultimately led to the cessation of its further clinical development.23,24 IPI-504 was designed to be more water soluble than both 17-AAG and 17-DMAG. Although the clinical activity of IPI-504 was promising in both non-small cell lung cancer (NSCLC)25 and gastrointestinal stromal tumors (GIST),26 there were treatment-related deaths due to hepatotoxicity in the Phase III RING (Retaspimycin in GIST) trial of this HSP90 inhibitor.27 This prompted mandatory dose reductions of IPI-504 in the ongoing HER2-positive metastatic breast cancer trial, which ultimately may have led to the lack of efficacy seen with this inhibitor in that trial.27
In contrast, the second-generation HSP90 inhibitors generally encompass the resorcinol moiety of radicicol or are purine derivatives; the exception to this classification is the SNX-522 agent, which does not fit among these designations (Table 1).12,23 Notably, this group of inhibitors lack the hepatoxicity noted with the first-generation inhibitors due to the absence of the benzoquinone moiety. Ganetespib ([5-[2,4-Dihydroxy-5-(1-methylethyl)phenyl]-4-(1-methyl-1H-indol-5-yl)-2,4-dihydro-[1,2,4]triazol-3-one]) is a resorcinol-based, non-geldanamycin, synthetic small molecule second-generation HSP90 inhibitor (molecular weight =364.4), similar to NYP-AUY922, AT-13387, and KW-2478. In differentiation to the other HSP90 inhibitors within this subgroup, ganetespib contains a triazolone moiety (Figure 1, Table 1).28
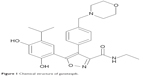  | Figure 1 Chemical structure of ganetespib. |
In vitro, ganetespib exhibits potent cytotoxicity in a wide variety of hematological and solid tumor cell lines, including those that express mutant kinases (including BCR-ABL, FLT3, c-KIT, EGFR, and B-RAF) that confer resistance to small molecule tyrosine kinase inhibitors. The half maximum inhibitory concentration values calculated for ganetespib are in the low nanomolar range, and ganetespib is at least 20-fold more potent than 17-AAG.28 Interestingly, ganetespib not only induces rapid degradation of known HSP90 client proteins but also exhibits sustained activity even with short exposure times.29 The cytotoxicity of ganetespib in these cell lines is predominantly mediated via an irreversible commitment to apoptosis, likely following growth arrest and effects on the cell cycle.29
Similarly, ganetespib has potent antitumor activity in vivo as demonstrated by significant growth inhibition and/or regression in solid tumor and hematological xenograft models.29 Evaluation of the microregional activity of ganetespib demonstrates efficient distribution throughout the tumor tissue, including the hypoxic regions >150 μm from the microvasculature, resulting in sustained inhibition of proliferation and induction of apoptosis throughout the tumors.28 Ganetespib also exhibits preferential tumor retention compared with normal tissues with a half-life of over 58 hours in NSCLC xenograft models vs 3 hours in the plasma and 5–6 hours in normal liver and lung tissues.30
With regards to its pharmacological profile, as discussed earlier, ganetespib lacks the dose-limiting hepatotoxicity reported with the geldanamycin analogs. Additionally, ganetespib lacks the ocular toxicity that has been reported with NVP-AUY922 and SNX-522. The mechanism of this ocular toxicity is hypothesized to be related to photoreceptor degeneration due to retinal drug distribution and retention, which has been seen with NVP-AUY922 exposure in rodent models. In contrast, ganetespib does not accumulate in the rat eye and is rapidly eliminated from the retinal tissues.31,32
Taken together, these promising results suggest that ganetespib may be more potent in its antitumor activity compared with first-generation inhibitors and has an optimal safety profile that predicts for a superior therapeutic index. This has provided a compelling rationale to further develop this agent clinically.
Single-agent Phase I/II trials
An open-label dose-escalation study of single-agent ganetespib given intravenously in advanced solid tumors established the maximum tolerated dose at 216 mg/m2 given weekly for 3 out of 4 weeks. The recommended Phase 2 dose was hence established as 200 mg/m2. Dose-limiting toxicities (DLTs) included one grade 3 amylase elevation at 150 mg/m2, one grade 3 diarrhea, and two cases of asthenia (grade 3 and grade 4) at 259 mg/m2. Overall, the most common adverse events (AEs) were gastrointestinal in nature and fatigue, predominantly of grade 1 and 2 severity, and easily manageable.33 Partial response (PR) was noted in a patient with metastatic colorectal cancer, and disease stabilization (SD) was noted in 23 of the 44 evaluable patients, including 1 patient each with NSCLC and GIST, whose tumors harbored the BRAF G469A and PDGFRAD842V exon 18 mutations, respectively. The most common AEs in another Phase I trial of ganetespib in advanced solid tumors, administered twice weekly for 3 out of 4 weeks, were also diarrhea and fatigue. Two DLTs of elevated transaminases were noted at 10 mg/m2 and 144 mg/m2, respectively.34 In this trial, a durable PR was noted in a patient with metastatic melanoma, and durable SD was reported in two patients with NSCLC.
Two Phase I trials have also been conducted with single-agent ganetespib in patients with hematologic malignancies. These studies determined the recommended Phase 2 dose to be 200 mg/m2 for the once weekly and 90 mg/m2 for the twice weekly regimens, respectively. Again, the most common AEs in these studies were also diarrhea and fatigue. With regards to DLTs, there were elevated liver enzymes in one patient in the once weekly trial, and hyperbilirubinemia, hyponatremia, QTc prolongation, and transaminitis for the twice weekly trial.23,35,36 Notably, there were no reports of severe hepatotoxicity, ocular toxicity, or cardiac toxicity in these patients.
Since many HSP90 client proteins are mutated, overexpressed and/or chimeric kinases important for tumor growth and survival, including HER2 in breast cancer, mutant EGFR or anaplastic lymphoma kinase (ALK) in lung cancer, mutant KIT in GIST, and mutated BRAF in melanoma, a number of Phase II trials of single-agent ganetespib in selected tumor types that express such oncoproteins have been undertaken to exploit the concept of “oncogene addiction”.
Supported by preclinical evidence that ganetespib has potent antitumor activity in different breast cancer subtypes, a Phase II trial of single-agent ganetespib at 200 mg/m2 intravenously (IV) weekly for 3 out of 4 weeks was conducted in patients with unselected metastatic breast cancer.32 The most common toxicities in this trial were of grade 1 and 2 severity and included diarrhea, fatigue, nausea, and hypersensitivity reactions. While the study did not meet its prespecified criteria for overall response rate in this heavily pretreated group of patients, clinical activity was notable in patients with trastuzumab-refractory HER2+ and triple negative breast cancer (TNBC). Specifically, two patients achieved a PR, and four of the seven patients who achieved SD had estrogen receptor positive (ER+)/HER2+ disease.32 Unlike HER2+ breast cancer or hormone receptor-positive breast cancer, TNBC lacks a unique molecular alteration that can be therapeutically targeted. Yet, clinical activity has been noted with ganetespib in this subset of patients with evidence of tumor shrinkage, specifically in patients with lung metastases.32,37 This latter observation is in line with findings from syngeneic mouse models of spontaneous and experimental metastases, where ganetespib suppressed lung colonization and tumor growth.37 There are many molecular alterations for TNBC that have been identified but are not yet validated as therapeutic targets. Several of these abnormal proteins are known HSP90 client proteins, such as EGFR, hypoxia-inducible factor 1 (HIF-1α), and KIT among others, thus underscoring the therapeutic potential for ganetespib even in this aggressive subtype of breast cancer.38
Another Phase II trial evaluated the activity and tolerability of ganetespib administered at 200 mg/m2 IV for 3 out of 4 weeks in previously treated patients with NSCLC enrolled in three cohorts: cohort A (mutant EGFR), cohort B (mutant KRAS), and cohort C (no EGFR or KRAS mutations). Of the 66 patients enrolled to cohort C, four had PR with all four cases being crizotinib-naive and harboring the ALK gene rearrangement. As expected, the most common AEs were diarrhea, fatigue, nausea, and anorexia.39
A Phase II trial in patients with metastatic ocular melanoma is also evaluating progression-free survival with single-agent ganetespib administered at the same dosing schedule (ClinicalTrials.gov Identifier: NCT01200238). Another pilot window-of-opportunity study is evaluating changes in the expression of biomarkers due to ganetespib, which is administered twice weekly for 2 weeks prior to surgery in patients with head and neck cancers (ClinicalTrials.gov Identifier: NCT02334319).
The biology behind combination trials
Contrary to expectations based on the presence of mutant KIT, an HSP90 client, and therefore amenable to HSP90 inhibitor therapy, a Phase II trial of single-agent weekly ganetespib reported limited efficacy in patients with GIST (12/23 evaluable patients had SD: 4 SD ≥16 weeks, 8 SD ≥8 weeks).40 This negative result was considered to be related to a possible lack of a sufficient duration of inhibition of this client or its downstream pathways, which was observed both in preclinical models and in patient biopsies done as a part of this Phase II trial.40 The limited success of HSP90 inhibitors in GIST and other client-protein-driven patient populations remains poorly understood but has certainly led to the emergence of the concept of rationally designed combination studies to enhance efficacy. These strategies include combinations with cytotoxics, other targeted therapies, and radiation and are briefly discussed in the following.
HSP90 protects cells under conditions of stress, and, therefore, HSP90 inhibitors have the ability to sensitize cells to the toxic effects of chemotherapy and radiation therapy.22 In support of this, preclinical data from various cell lines and xenograft models suggest additive or synergistic antitumor activity when HSP90 inhibitors are combined with various systemic cytotoxics including anthracyclines and taxanes.22,41,42 The synergistic benefit observed by the addition of HSP90 inhibitors to taxanes is likely multifactorial, resulting from increased cytotoxicity and apoptosis, Akt inactivation and sensitization of the tumor cells to induction of apoptosis by a taxane, loss of pro-survival signaling, and exacerbation of mitotic catastrophe.12,41,43,44 Indeed, the combination of ganetespib with paclitaxel or docetaxel enhances antitumor growth and is synergistic in TNBC, NSCLC, and ovarian cancer models.37,45,46 Most importantly, this combination has a nonoverlapping toxicity profile.
Ganetespib can also potentiate the cytotoxic activity of doxorubicin via enhanced DNA damage and mitotic arrest and thus can confer superior efficacy to doxorubicin-containing regimens.37
Approximately 15%–25% of lung adenocarcinomas have tumor-associated KRAS mutations.47 These mutations are negative predictors of response to currently available EGFR tyrosine kinase inhibitors, and these patients have unfavorable clinical outcomes.48 Currently, there are no approved anti-KRAS-directed therapies. While KRAS by itself is not a known client protein, its downstream effector pathways, namely, the PI3K/AKT/mTOR and the RAF/MEK/ERK pathways, are shown to be sensitive to HSP90 inhibition. In fact, the combination of ganetespib with a dual PI3K/mTOR inhibitor demonstrated superior antitumor efficacy in a xenograft model, supporting further investigation of this dual-targeted approach.49 On the other hand, ALK is a sensitive client of HSP90 inhibition, and ganetespib can overcome acquired resistance to the ALK inhibitor, crizotinib in ALK + NSCLC, both in xenograft models and in patients.50 Furthermore, the L1196M mutant form of EML4-ALK, considered to be an acquired mutation conferring resistance to crizotinib, continues to be sensitive to other structurally different ALK tyrosine kinase inhibitors and HSP90 inhibitors like 17AAG and ganetespib in both cell lines and the clinical setting.50,51 Similarly, mutant BRAF is a very sensitive HSP90 client, and ganetespib was more potent than single-agent vemurafenib in BRAF-driven melanoma cell lines.52 Since BRAF mutations lead to dysregulation of the RAF/MEK/ERK signaling axis, a number of trials are evaluating the combination of BRAF inhibitors and MEK inhibitors not only to improve efficacy but also to overcome acquired resistance. Along the same lines, the combination of ganetespib with vemurafenib or selective MEK inhibitors has shown synergistic activity in xenograft models. In a preclinical study, ganetespib in combination with the MEK inhibitor TAK-733 also caused significant tumor regressions in vemurafenib-resistant xenograft tumors, thus providing a rationale for this combination to treat tumors that have developed acquired resistance to vemurafenib.52
HSP90 inhibitors can also enhance tumor cell sensitivity to radiation. HSP90 clients such as Akt and ErbB2 are thought to be associated with radioresponse, which in turn can protect against radiation-induced cell death. Degradation of these proteins by HSP90 inhibition therefore enhances tumor cell death in many cell lines and xenograft models.53 This combination can also increase apoptosis and enhance G2 arrest and hence is being evaluated in tumors such as rectal cancer.54
HSP90 inhibitors may also have a significant role to play in hormone-receptor resistant breast cancer based on the role of HSP90 in regulating the post-translational folding of the estrogen and progesterone receptors.55 In preclinical hormone receptor positive breast cancer models, ganetespib reverses endocrine resistance and reduces heterogeneity in the disease control achieved by hormonal therapies. This work has formed the basis for evaluating the combination of ganetespib with endocrine therapies such as fulvestrant, a complete estrogen receptor antagonist (Table 2).
Bortezemib was the first therapeutic proteasome inhibitor that was approved in the United States by the Food and Drug Administration for the treatment of multiple myeloma and mantle cell lymphoma. Preclinical findings suggest that the combination of HSP90 inhibition with bortezemib can enhance bortezemib-triggered apoptosis and induce a prolonged intracellular accumulation of ubiquinated proteins credited to the synergistic suppression of chymotryptic activity of the 20S proteasome.56,57 This has been the basis for combination trials of various HSP90 inhibitors, (including the ongoing ganetespib studies) with bortezemib in patients with multiple myeloma.58,59
Together, these compelling preclinical studies have formed the rationale for many Phase I/II clinical trials in combinations with ganetespib as a way to optimize efficacy and delay or limit acquired resistance.
Combination Phase I/II trials
Table 2 lists several ongoing combination trials of ganetespib with cytotoxic agents such as taxanes and doxorubicin, radiation, fulvestrant, and other targeted agents such as sirolimus, crizotinib, Ziv-Aflibercept, and bortezemib for several tumor types. Some of these combination studies have reported results and are discussed in detail here.
The combination of docetaxel and ganetespib is in advanced clinical testing in NSCLC based on the results from the Phase IIb GALAXY-I (Study of Ganetespib + Docetaxel in Advanced NSCLC) trial.60 This Phase II trial randomized 252 stage III/IV NSCLC patients to docetaxel alone (75 mg/m2 on day 1 of a 3 week cycle; n=127) or the same dose of docetaxel and ganetespib (150 mg/m2 on days 1 and 15; n=125). There was a nonsignificant improvement in overall survival (OS) in the ganetespib arm vs the control arm (9.8 months vs 7.4 months; hazard ratio [HR] =0.82; P=0.082); there was also a trend toward improved PFS (4.5 months for ganetespib vs 3.2 months for the control group; HR =0.84; P=0.038). Importantly, in a prespecified group of patients enrolled in the trial more than 6 months after diagnosis (n=176), there was a significant 4.3 month OS advantage with ganetespib compared with the control of docetaxel alone (10.7 months vs 6.4 months; HR= 0.61; P=0.0093); PFS was also significantly improved in the treatment arm (5.4 months vs 3.4 months; HR= 0.61; P=0.0041). For the combination, the most common AE across all grades was diarrhea (48%), which was well managed with the use of over-the-counter antidiarrheals. The most frequently reported grade 3–4 AEs for ganetespib vs the control group were neutropenia (37% vs 38%), febrile neutropenia (11% vs 2%), and anemia (8% vs 2%).60
The Phase III GALAXY-2 trial will compare second-line ganetespib and docetaxel vs docetaxel alone in advanced adenocarcinoma of the lung of those who are more than 6 months past a diagnosis of advanced disease. The target enrollment is 500 patients, and the primary endpoint is OS. Key secondary endpoints include OS in three subpopulations (mKRAS, elevated lactate dehydrogenase, and lactate dehydrogenase-5), PFS, overall response rate, and duration of response. Tumor tissue and blood samples will be collected for planned correlative studies (ClinicalTrials.gov Identifier: NCT01798485).
Preliminary results from a Phase I trial of ganetespib plus paclitaxel and trastuzumab, a humanized monoclonal antibody against HER2, were recently presented at the San Antonio Breast Cancer Symposium.61 There were no DLTs or grade 3 toxicities attributable to ganetespib. In this group of patients heavily pretreated with prior pertuzumab and ado-trastuzumab emtansine, one of six evaluable patients achieved a PR and four others had SD for a CBR of 60%. This trial is ongoing.61
Given the exciting preclinical data of doxorubicin and ganetespib in xenograft models, this combination is being studied to determine a signal of efficacy in relapsed/refractory NSCLC (Table 2).
Limitations and future directions
As is the case with all targeted therapies, there is a pressing need to develop companion diagnostics or predictive biomarkers to better select patients who might derive the most benefit from ganetespib therapy. Due to the prolonged tumor retention of this inhibitor, it is evident that to optimize treatment, target modulation within the tumor itself is necessary. This will help expand the role of ganetespib and this class of agents beyond the tumors that are addicted to a sensitive client protein. Non-invasive [18F]-Fluorodeoxyglucose Positron Emission Tomography imaging has most recently been utilized as a means for measuring early response to ganetespib therapy; however, the utility of this approach has yet to be validated in large prospective trials.12,22,23 Ongoing studies such as the window of opportunity trial in head and neck cancer, a Phase I trial in rectal cancer, and the ongoing Phase III GALAXY-2 trial in NSCLC are collecting tumor tissues for biomarker analyses that may also yield valuable information and guide further development of this agent.
Conclusion
Altogether, our knowledge of the HSP90-complex machinery continues to grow. While as a class, HSP90 inhibitors have certainly emerged as an exciting multifaceted anticancer strategy, there are no currently approved HSP90 inhibitors for any cancer indication. The improved safety profile and superior efficacy of ganetespib compared with the other HSP90 inhibitors is attributed to its small molecular weight, increased lipophilicity, and absence of the benzoquinone moiety, allowing for a greater therapeutic index. Clinically, ganetespib has been shown to be very well tolerated with a notable lack of significant cardiac, liver, and ocular toxicity, which have been the limiting factors for further development of many of the other HSP90 inhibitors. While efficacy has been promising in NSCLC and HER2-positive metastatic breast cancer, many Phase I/II clinical trials are utilizing combinatorial approaches with other therapeutic agents to further improve the outcomes with ganetespib and also to expand its role in other disease types. Data from the ongoing Phase III trial in NSCLC along with the concurrent development of predictive biomarkers in other key studies have the potential to make a significant impact on the subsequent applications of ganetespib therapy and perhaps may lead to a path of regulatory approval for this promising HSP90 inhibitor.
Acknowledgments
Komal Jhaveri would like to thank the Terri Brodeur Breast Cancer Foundation and all the patients and their families who participated in the clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
Clare DK, Saibil HR. ATP-driven molecular chaperone machines. Biopolymers. 2013;99(11):846–859. | ||
Lai BT, Chin NW, Stanek AE, Keh W, Lanks KW. Quantitation and intracellular localization of the 85 K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984;4(12):2802–2810. | ||
Taldone T, Patel HJ, Bolaender A, Patel MR, Chiosis G. Protein chaperones: a composition of matter review (2008–2013). Expert Opin Ther Pat. 2014;24(5):501–518. | ||
Taldone T, Sun W, Chiosis G. Discovery and development of heat shock protein 90 inhibitors. Bioorg Med Chem. 2009;17(6):2225–2235. | ||
Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5(10):761–772. | ||
Nanbu K, Konishi I, Mandai M, et al. Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev. 1998;22(6):549–555. | ||
Uozaki H, Ishida T, Kakiuchi C, et al. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract. 2000;196(10):665–673. | ||
Chiosis G, Neckers L. Tumor selectivity of Hsp90 inhibitors: the explanation remains elusive. ACS Chem Biol. 2006;1(5):279–284. | ||
Moulick K, Ahn JH, Zong H, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7(11):818–826. | ||
Garcia-Carbonero R, Carnero A, Paz-Ares L. Inhibition of HSP90 molecular chaperones: moving into the clinic. Lancet Oncol. 2013;14(9):e358–e369. | ||
Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med (Berl). 2004;82(8):488–499. | ||
Jhaveri K, Modi S. HSP90 inhibitors for cancer therapy and overcoming drug resistance. Adv Pharmacol. 2012;65:471–517. | ||
Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188(3):281–290. | ||
Pearl LH, Prodromou C. Structure, function, and mechanism of the Hsp90 molecular chaperone. Adv Protein Chem. 2001;59:157–186. | ||
Maloney A, Workman P. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin Biol Ther. 2002;2(1):3–24. | ||
Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18(1):64–76. | ||
Mimnaugh EG, Chavany C, Neckers L. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J Biol Chem. 1996;271(37):22796–22801. | ||
Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. Heat shock protein 90: inhibitors in clinical trials. J Med Chem. 2010;53(1):3–17. | ||
Ali MM, Roe SM, Vaughan CK, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440(7087):1013–1017. | ||
Prodromou C, Pearl LH. Structure and functional relationships of Hsp90. Curr Cancer Drug Targets. 2003;3(5):301–323. | ||
Ehrlich ES, Wang T, Luo K, et al. Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc Natl Acad Sci U S A. 2009;106(48):20330–20335. | ||
Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823(3):742–755. | ||
Jhaveri K, Ochiana SO, Dunphy MP, et al. Heat shock protein 90 inhibitors in the treatment of cancer: current status and future directions. Expert Opin Investig Drugs. 2014;23(5):611–628. | ||
Jhaveri K, Miller K, Rosen L, et al. A phase I dose-escalation trial of trastuzumab and alvespimycin hydrochloride (KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin Cancer Res. 2012;18(18):5090–5098. | ||
Sequist LV, Gettinger S, Senzer NN, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28(33):4953–4960. | ||
Wagner AJ, Chugh R, Rosen LS, et al. A phase 1 study of the heat shock protein 90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft tissue sarcomas. Clin Cancer Res. 2013;19(21):6020–6029. | ||
Modi S, Saura C, Henderson C, et al. A multicenter trial evaluating retaspimycin HCL (IPI-504) plus trastuzumab in patients with advanced or metastatic HER2-positive breast cancer. Breast Cancer Res Treat. 2013;139(1):107–113. | ||
Ying W, Du Z, Sun L, et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol Cancer Ther. 2012;11(2):475–484. | ||
Proia DA, Foley KP, Korbut T, et al. Multifaceted intervention by the Hsp90 inhibitor ganetespib (STA-9090) in cancer cells with activated JAK/STAT signaling. PLoS One. 2011;6(4):e18552. | ||
Shimamura T, Perera SA, Foley KP, et al. Ganetespib (STA-9090), a non-geldanamycin HSP90 inhibitor, has potent antitumor activity in in vitro and in vivo models of non-small cell lung cancer. Clin Cancer Res. 2012;18(18):4973–4985. | ||
Zhou D, Liu Y, Ye J, et al. A critical role for the tissue distribution profile in heat shock protein (Hsp) 90 inhibitor-induced ocular toxicity in rats. Mol Cancer Ther. 2011;10(11 suppl):Abstr C212. | ||
Jhaveri K, Chandarlapaty S, Lake D, et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with metastatic breast cancer. Clin Breast Cancer. 2014;14(3):154–160. | ||
Goldman JW, Raju RN, Gordon GA, et al. A first in human, safety, pharmacokinetics, and clinical activity phase I study of once weekly administration of the Hsp90 inhibitor ganetespib (STA-9090) in patients with solid malignancies. BMC Cancer. 2013;13:152. | ||
Cho DC, Heath EI, Cleary JM, et al. A phase I dose-escalation study of the Hsp90 inhibitor ganetespib (STA-9090) administered twice weekly in patients with solid tumors: updated report. General Poster Session, Developmental Therapeutics – Experimental Therapeutics, 2011 ASCO Annual Meeting, Abstract 3051; 2011. Chicago. | ||
Lancet JE, Smith BD, Bradley R, Komrokji RS, Teofilovici F, Rizzieri DA. A phase 1/2 study of the potent Hsp90 inhibitor STA-9090 administered once weekly in subjects with hematologic malignancies. Blood (ASH Annual Meeting Abstracts). 2010;116:Abstract 3294. | ||
Padmanabhan S, Kelly K, Heaney M, et al. A phase I study of the potent Hsp90 inhibitor STA-9090 administered twice weekly in subjects with hematologic malignancies. Blood (ASH Annual Meeting Abstracts). 2010;116:Abstract 2898. | ||
Proia DA, Zhang C, Sequeira M, et al. Preclinical activity profile and therapeutic efficacy of the HSP90 inhibitor ganetespib in triple-negative breast cancer. Clin Cancer Res. 2014;20(2):413–424. | ||
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. | ||
Socinski MA, Goldman J, El-Hariry I, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res. 2013;19(11):3068–3077. | ||
Demetri GD, Heinrich MC, Chmielowski B, et al. An open-label phase II study of the Hsp90 inhibitor ganetespib (STA-9090) in patients (pts) with metastatic and/or unresectable GIST. ASCO Annual Meeting, 2011 (Vol 29, No 15_suppl (May 20 Supplement); 2011. Chicago. | ||
Nguyen DM, Lorang D, Chen GA, Stewart JH 4th, Tabibi E, Schrump DS. Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg. 2001;72(2):371–378; discussion 378–379. | ||
Munster PN, Basso A, Solit D, Norton L, Rosen N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. See: E. A. Sausville, Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters, Clin Cancer Res. 7:2155–2158, 2001. Clin Cancer Res. 2001;7(8):2228–2236. | ||
Proia DA, Bates RC. Ganetespib and HSP90: translating preclinical hypotheses into clinical promise. Cancer Res. 2014;74(5):1294–1300. | ||
Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63(9):2139–2144. | ||
Proia DA, Sang J, He S, et al. Synergistic activity of the Hsp90 inhibitor ganetespib with taxanes in non-small cell lung cancer models. Invest New Drugs. 2012;30(6):2201–2209. | ||
Liu H, Xiao F, Serebriiskii IG, et al. Network analysis identifies an HSP90-central hub susceptible in ovarian cancer. Clin Cancer Res. 2013;19(18):5053–5067. | ||
Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62(23):6997–7000. | ||
Riely GJ, Ladanyi M. KRAS mutations: an old oncogene becomes a new predictive biomarker. J Mol Diagn. 2008;10(6):493–495. | ||
Acquaviva J, Smith DL, Sang J, et al. Targeting KRAS mutant non-small cell lung cancer with the Hsp90 inhibitor ganetespib. Mol Cancer Ther. 2012;25:2012. | ||
Sang J, Acquaviva J, Friedland JC, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;3(4):430–443. | ||
Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108(18):7535–7540. | ||
Acquaviva J, Smith DL, Jimenez JP, et al. Overcoming acquired BRAF inhibitor resistance in melanoma via targeted inhibition of Hsp90 with ganetespib. Mol Cancer Ther. 2014;13(2):353–363. | ||
Yin X, Zhang H, Lundgren K, Wilson L, Burrows F, Shores CG. BIIB021, a novel Hsp90 inhibitor, sensitizes head and neck squamous cell carcinoma to radiotherapy. Int J Cancer. 2010;126(5):1216–1225. | ||
Russell JS, Burgan W, Oswald KA, Camphausen K, Tofilon PJ. Enhanced cell killing induced by the combination of radiation and the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin: a multitarget approach to radiosensitization. Clin Cancer Res. 2003;9(10 pt 1):3749–3755. | ||
Scaltriti M, Dawood S, Cortes J. Molecular pathways: targeting hsp90 – who benefits and who does not. Clin Cancer Res. 2012;18(17):4508–4513. | ||
Mitsiades N, Mitsiades CS, Poulaki V, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99(22):14374–14379. | ||
Mitsiades CS, Mitsiades NS, McMullan CJ, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107(3):1092–1100. | ||
Richardson PG, Badros AZ, Jagannath S, et al. Tanespimycin with bortezomib: activity in relapsed/refractory patients with multiple myeloma. Br J Haematol. 2010;150(4):428–437. | ||
Richardson PG, Chanan-Khan AA, Lonial S, et al. Tanespimycin and bortezomib combination treatment in patients with relapsed or relapsed and refractory multiple myeloma: results of a phase 1/2 study. Br J Haematol. 2011;153(6):729–740. | ||
Ramalingam S, Goss G, Rosell R, et al. A randomized study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel versus docetaxel alone for second-line therapy of lung adenocarcinoma (GALAXY-1). J Clin Oncol. 2013;31(suppl):abstr CRA8007. | ||
Jhaveri K, Chandarlapaty S, Lake D, et al. A phase I clinical trial of ganetespib (heat shock protein 90 inhibitor) in combination with paclitaxel and trastuzumab in human epidermal growth factor receptor-2 positive (HER2+) metastatic breast cancer. Abstract # 1403. Presented at the San Antonio Breast Cancer Symposium, December 2014; 2014. San Antonio. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

