Back to Journals » OncoTargets and Therapy » Volume 8
Expression of Fascin-1 on human lung cancer and paracarcinoma tissue and its relation to clinicopathological characteristics in patients with lung cancer
Authors Zhao W, Gao J, Wu J, Liu Q, Wang Z, Li H, Xing L
Received 30 January 2015
Accepted for publication 25 March 2015
Published 15 September 2015 Volume 2015:8 Pages 2571—2576
DOI https://doi.org/10.2147/OTT.S81915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Daniele Santini
Wei Zhao, Jing Gao, Jing Wu, Qiu-hong Liu, Zhi-gang Wang, Hui-ling Li, Li-hua Xing
The Third Department of Respiratory Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, People’s Republic of China
Background: Lung cancer poses a severe threat to human life. Biomarkers of cancers are helpful in the diagnosis and treatment of patients with cancers. Biomarkers of lung cancers are rare, and thus deserve further research.
Objective: The objective of the present study was to explore the expression of Fascin-1 in human lung cancer and paracarcinoma tissue, its correlation with clinicopathological characteristics in patients with lung cancer, and study the possible relationship between Fascin-1 expression and clinical–biological behavior of lung cancer.
Method: This study used the MaxVision two-step immunohistochemical detection method to detect Fascin-1 expression in 84 of lung cancer and paracarcinoma tissues. This study set the expression of Fascin-1 in vascular endothelial cells as the positive control, and used phosphate buffered saline (replacing the primary antibodies) as negative control.
Result: Of all the 84 lung cancer tissues and paracarcinoma tissues, positive expression of the Fascin-1 protein were detected in 78 cases (92.9%) and 27 cases (32.1%), respectively, and the difference was statistically significant (P<0.05). Differences in Fascin-1 expression between different age groups, clinical stages, and lymph node metastases were statistically significant (P<0.05), while differences in Fascin-1 expression between sexes, tumor stages, and pathological types demonstrated no statistical significance (P>0.05). The survival times of the patients with different Fascin-1 protein-positive expressions in lung cancer tissues were statistically significant (P<0.05), while the survival times of the patients with different Fascin-1 protein-positive expressions in paracarcinoma tissues were not statistically significant (P>0.05).
Conclusion: In lung cancer, Fascin-1 expression was closely related to tumor invasion and metastasis, and the difference in expression of Fascin-1 had a significant effect on the survival time of the lung cancer patients. Therefore, Fascin-1 might be expected to serve as a possible potential biomarker of lung cancer.
Keywords: pulmonary carcinoma, Fascin-1 protein, immunohistochemistry, survival time
Introduction
Lung cancer severely threatens human life.1,2 Since there are few treatment methods that can cure cancers, it is important to diagnose and treat the disease in the early stage.2,3 Unfortunately, biomarkers to detect the occurrence and development of lung cancers are rare and deserve further research.
Fascin-1 is a kind of cytoskeletal protein, and it was found that the expression of Fascin-1 was increased in multiple human epithelial tumors.1 Therefore, Fascin-1 may be associated with clinical pathology and prognosis of cancers.1 Fascin-1 mainly interacts with F-actin, is then assembled into parallel actin bundles, and is one of the key molecules regulating cell movement.1
It has been demonstrated previously that increased level of Fascin-1 is involved in migration and invasiveness, but not proliferation, of non-small-cell lung cancer.1 Therefore, the underlying mechanism remains to be elucidated, and it is unclear whether Fascin-1 is related to all types of lung cancer or it is associated with the degree of development of lung cancers and survival of lung cancer patients.
This study adopted an immunohistochemical method (the MaxVision two-step method) to detect Fascin-1 expression in lung cancer and adjacent carcinoma tissue and analyzed its relationship with the clinical and pathological characteristics of lung cancers. Furthermore, we also investigated the possible relationship between Fascin-1 expression and clinical and biological behavior of lung cancer, and the possible correlation between level of Fascin-1 expression and the survival time of patients with lung cancers is also reported.
Materials and methods
Materials
This study collected 84 paraffin specimens of lung cancer (with complete pathological data) from patients who underwent surgery at the First Affiliated Hospital of Zhengzhou University from February 2007 to April 2008. The tissue parts surrounding the tumor tissues’ cut edge, which were not judged as cancer tissue, were taken as the reference controls. The average age of patients in the present study was 38–80 years (60.77±9.44). This study classified the patients according to the standard of the lung cancer staging of World Health Organization (WHO) (2012). According to the TNM staging, 37 cases were in stage I, 32 cases were in stage II, and 15 cases were in stage III. In March 2013, this study made a follow-up of the survival period; a total of 40 patients were followed up and 44 patients missed the follow-up. The specimens were fixed with 10% formalin solution and then embedded in paraffin. Slices with thickness of 4 μm were prepared and stained with hematoxylin and eosin. All the patients included in the study had no smoking habits. Ethics approval obtained from The Third Department of Respiratory Medicine, The First Affiliated Hospital of Zhengzhou University Ethics Committee. Informed patient consent was also obtained.
Reagents
The immunohistochemical reagent rat anti-human Fascin-1 monoclonal antibody (SPM133) was purchased from Santa Cruz Biotechnology Inc., Dallas, TX, USA. Immunohistochemical detection kits used were the ready-to-use, fast immunohistochemical MaxVision kit and the DAB chromogenic reagent kit, which were bought from Fuzhou Maxim Co., Ltd (Fuzhou, Fujian, People’s Republic of China).
Method
Immunohistochemical staining method was employed according to the manufacturer’s instructions, and the MaxVision two-step immunohistochemical staining was also used in the present study. Briefly, the following steps were conducted: first, the sodium citrate buffer solution was used for higher pressure antibody preparation, and then, the primary antibody against Fascin-1 was added (1:500) to the sample slices at 4°C and incubated overnight. The primary antibody was removed, and the secondary antibody was incubated with the samples (1:1,000). The kit solution was incubated with tissue samples at room temperature for 20 minutes, and then, DAB chromogenic reagent and hematoxylin staining was performed. The expression of Fascin-1 in vascular endothelial cells served as the positive control, and the primary antibody was replaced with phosphate buffered saline, which was used as the negative control.
Results detection
The tan or brown granules in the cytoplasm of cancer or paracarcinoma cells observed in the immunohistochemical test was regarded as positive staining. Immunohistochemical evaluation method was used according to the reference standards.2,3 Scoring of the stained samples was performed according to the dyeing strength: 0 point for colorless, 1 point for pale yellow, 2 points for tan color, and 3 points for brown tan color. According to the degree of dyeing score: no positive cell number represents 0 point, positive cells (1%–25%) represents 1 point, positive cells (26%–50%) represents 2 points, positive cells (51%–75%) represents 3 points, and positive cells (76%–100%) represents 4 points. The samples with a final point of ≥3 were taken as positive expression.
Statistical processing
This study used the SPSS 13.0 statistical software for analysis (SPSS Inc., Chicago, IL, USA). The comparison of sample rate was processed by χ2 tests, and the survival rate was calculated by Kaplan–Meier method. The comparison of survival rate was obtained by log-rank test.
Results
Expression and distribution of Fascin-1 protein in lung cancer and paracarcinoma tissue
Fascin-1 protein was diffused throughout the lung cancer tissues, and dyeing of the cancer cells was stronger than that near the cancer nests. Fascin-1 immunohistochemical dyeing was mainly localized in the cytoplasm and cell membrane of positive cells, with focal shape or diffused yellow granular shape. In all the 84 lung cancer tissues, there were 78 cases (92. 9%) with Fascin-1 protein-positive expression, as shown in Figure 1; in 84 cases of paracarcinoma tissues, there were 27 cases (32.1%) with Fascin-1 protein-positive expression, and the difference was statistically significant (χ2=66.06, P<0.05). In paracarcinoma tissues, Fascin-1 was mainly expressed in the glandular epithelial cells, with membranous type positive expression, as illustrated in Figure 2. Fascin-1 positive staining was visible in vascular endothelial cells of interstitial lung cancer. In the paracarcinoma tissues, capillary endothelial cells and dendritic cells were positive for the expression of Fascin-1, while the bronchial alveolar epithelial cells and pulmonary vascular endothelial cells were negative for the expression of Fascin-1.
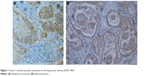  | Figure 1 Fascin-1 protein-positive expression in the lung cancer tissues (DAB ×400). |
  | Figure 2 Fascin-1 protein-positive expression in the paracarcinoma tissues (DAB ×400). |
Relationship between Fascin-1 protein expression in lung cancer and clinicopathological parameters
In order to study the relationship between Fascin-1 expression and clinicopathological features of lung cancer, this study classified all the 84 cases on the basis of age (≤61 years old, > 61 years old), sex (male, female), T staging (T1, T2, T3, and T4), N staging of regional lymph node metastasis (N0, N1, and N2), clinical pathological classification (adenocarcinoma, squa-mous carcinoma, large-cell carcinoma, and small-cell carcinoma), and TNM staging (stage I, stage II, and stage III). This study applied the χ2 detection method, and comparisons of differences in Fascin-1 expression between different groups were conducted. The results showed that differences in the Fascin-1 expression in different age groups, clinical stages, and lymph node metastases in the regions between different groups were statistically significant (P<0.05), while there was no statistically significant difference in Fascin-1 expression in the groups with different sexes, T staging, and pathological classifications (P>0.05) (Table 1).
  | Table 1 Relationship between Fascin-1 expression in lung cancer and clinical characteristics |
Relationship between Fascin-1 protein-positive expression in lung cancer and survival time
In the follow-up of 40 patients, until the finalization of follow-up on March 2013, there were 21 deaths, and 19 survivors. The overall survival rate was 47.5%, and the survival time was 8–70 months. Then, this study analyzed Fascin-1 protein-positive expression in lung cancer tissues and patient survival time, the median survival time of different patients, and the differences in intensity of Fascin-1 protein-positive expression in lung cancer tissue in different patients. The results demonstrated that there were significant differences in expression of Fascin-1 in different patients with different survival time (P<0.05); in paracarcinoma tissues, the median survival time of different patients and the difference in intensity of Fascin-1 protein-positive expression was not significantly different (P>0.05), as shown in Table 2 and Figure 3. Multivariable Cox regression analysis also showed that Fascin-1 protein-positive expression intensity in lung cancer tissue was closely related to the survival time of the patients (hazard ratio =4.505, 95% CI =1.615–12.569, P=0.004).
  | Table 2 Relation between Fascin-1 expression and median survival time |
  | Figure 3 Overall survival stairs of patients with different Fascin-1 protein-positive expression in lung cancer tissues. |
Discussion
The main reason of treatment failure and patient death in lung cancer was metastasis of lung cancer. Therefore, it was important to study the metastasis mechanism of lung cancer, which may be involved in assembly function of inhibiting Fascin-1 troponin and influence on formation of cell membrane surface pseudopodia and micro spine.4
The studies have reported that Fascin-1 showed an unusually high expression in many tumors, such as breast cancer, nasopharyngeal carcinoma cells, and its expression had a close correlation with tumor metastasis and invasion.5,6 However, there was little research that reported the expression of Fascin-1 in various lung cancers.
This study observed the expression of Fascin-1 in lung cancer surgery pathological specimens. The results showed that Fascin-1 was mainly located in the cytoplasm or cell membrane of the lung cancer cells, with features of focus or diffused yellow granular. There was no obvious relationship between the expression of Fascin-1 and sex, T staging, and pathological type of tumor. In a relevant report, domestic authors Yu et al7 analyzed Fascin-1 expression in 51 cases of lung cancer, showing the positive Fascin-1 expression rate of 66.7% and 43.5% in patients with lung squamous carcinoma and lung adenocarcinoma, respectively. However, in poorly differentiated lung cancer, the expression of Fascin-1 was increased, and there were differences based on the sex of the patient, with higher expression rate in males than females. The findings were not consistent with our results, and the reason might be that different patients were included in these studies. In addition, the limited number of patients also might be a cause for the discrepancies.
In the results of the study by Gao and Wu8 there was no significant difference between Fascin-1 protein expression in lung cancer tissues and normal tissues. This experimental result showed that Fascin-1 protein expression in lung cancer tissue was relatively higher than that in lung cancer adjacent tissues. Therefore, in Gao and Wu’s8 study, there might be epithelial–mesenchymal transformation occurring in these patients, the specific reason of which needs to be further explored. Furthermore, this study had found that the patients with Fascin-1 protein-positive expression in lung cancer tissue had lower overall survival rate and less overall survival time, compared with patients with negative expression of Fascin-1, indicating that the positive expression of Fascin-1 might be used to forecast a poor prognosis and low survival rate in patients with lung cancers. This result is also consistent with that of other studies.9–11
The study by Pelosi et al’s12 overseas research group reported 220 cases of Fascin-1 protein expression in stage I non-small-cell lung cancer. They showed that 98% of lung squamous carcinoma patients and 78% of lung adenocarcinoma patients had Fascin-1 positive expression, and that Fascin-1 was generally expressed in cancer screening. In addition, Fascin-1 expression was diffused and expressed in patients with contralateral malignant pleural effusion or adenocarcinoma distant metastasis. However, when compared with that of keratin pearl, the immune response strength of Fascin-1 in paracarcinoma tissues was weak, and there was poor expression of Fascin-1 in lung squamous carcinoma patients. From the follow-up results, it was revealed that the patients with diffused Fascin-1 or strong positive expression had poor prognosis. For the first time, this study proved that Fascin-1 protein expression was increased in the invasive lung cancer, and Fascin-1 protein had the possibility of being an independent prognostic factor in patients with lung cancer, considering the clinical aspects. Later, this study continued to evaluate the expression analysis of Fascin-1 protein in 128 cases of resected pulmonary neuroendocrine tumor and its relationship with lymph node metastasis. The results from another study in typical and atypical lung carcinoid cancer showed that Fascin-1 protein expression was closely related to the regional lymph node metastasis.13 Fascin-1 expression difference was statistically significant in regional lymph node metastasis and in patients in different clinical stages of the disease. The wider the regional lymph node metastasis range, the later the clinical stage of the disease in patients and the higher Fascin-1 expression in specimens. Multiple factor analysis proved that Fascin-1 might be an independent marker to predict lymph node metastasis in patients with lung cancers.14
For a protein such as Fascin-1, which has some degree of potential to be considered as a biomarker for lung cancer, more quantitative experiments such as Western blotting or RT-PCR should be further performed to solidify the conclusion. However, we conclude that there is possible correlation between Fascin-1 and lung cancer and that Fascin-1 might serve as a possible biomarker. Furthermore, we also reported the possible link between level of Fascin-1 and survival time of the patients, which is our novel finding in this study. Although it has been demonstrated that upregulated Fascin-1 protein promotes migration and invasiveness in non-small-cell lung cancer, we show that there is correlation between expression of Fascin-1 and the clinicopathological characteristics of patients with lung cancer, by using human lung cancer and paracarcinoma tissues. It was also shown that Fascin-1 is possibly associated with the survival times of the patients.15–20 Furthermore, because Fascin-1 is associated with various types of lung cancer, we propose that Fascin-1 might serve as a possible biomarker of lung cancer. The mechanisms of Fascin-1 in regulation of metastasis and invasion might be that it enhances actin cytoskeleton structure and cell movement. However, it remains to be investigated.15
Therefore, the comprehensive analysis of this study and previous research related to this topic, illustrated that Fascin-1 protein was closely correlated with lymph node metastasis of human lung cancer cells, and the different expression of Fascin-1 in cancer tissue had significant effect on the survival times of the patients. Therefore, Fascin-1 may be expected to serve as a possible potential biomarker of lung cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
Adams JC. Fascin protrusions in cell interactions. Trends Cardiovasc Med. 2004;14(6):221–226. | ||
Soumaoro LT, Uetake H, Higuchi T, et al. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10(24):8465–8471. | ||
Masunaga R, Kohno H, Dhar DK, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6(10):4064–4068. | ||
Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18(3):276–284. | ||
Ang L, Wu Z-S, Wang X, et al. Expression of breast cancer FSCN1 and its effect on cancer cell proliferation activity. Chin J Clin Exp Pathol. 2013;29(3):240–243. | ||
Liu L, An S-l, Guan J, et al. The expression and clinical significance of Fascin-1 on nasopharyngeal carcinoma. Guangdong Med J. 2010;31(8):976–978. | ||
Yu W-x, Xiong S-D, Lu Z-h, et al. Expression of Fascin and Ki-67 in poorly differentiated non-small cell lung cancer. Chin J Pract Internal Med. 2005;25(8):705–707. | ||
Gao X, Wu D-h. Expression and clinical significance of FSCNI protein in human epithelial tumor tissue. J South Med Univ. 2008;28(6):953–955. | ||
Wu D, Chen L, Liao W, et al. Fascinl expression predicts poor prognosis in patients with nasopharyngeal carcinoma and correlates with tumor invasion. Ann Oncol. 2010;21(3):589–596. | ||
Oh SY, Kim YB, Suh KW, et al. Prognostic impact of fascin-1 expression is more significant in advanced colorectal cancer. J Surg Res. 2012;172(1):102–108. | ||
Yoder BJ, Tso E, Skacel M, et al. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin Cancer Res. 2005;11(1):186–192. | ||
Pelosi G, Pastorino U, Pasini F, et al. Independent prognostic value of fascin immunoreactivity in stage I nonsmall cell lung cancer. Br J Cancer. 2003;88(4):537–547. | ||
Pelosi G, Pasini F, Fraggetta F, et al. Independent value of fascin immunoreactivity for predicting lymphy node metastases in typical and atypical pulmonary carcinoids. Lung Cancer. 2003;42(2):203–213. | ||
Zhang FR, Tao LH, Shen ZY, et al. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 2008;56(2):193–199. | ||
Zhao J, Zhou Y, Zhang Z, et al. Upregulated fascin1 in non-small cell lung cancer promotes the migration and invasiveness, but not proliferation. Cancer Lett. 2010;290(2):238–247. | ||
Ma Y, Faller WJ, Sansom OJ, et al. Fascin expression is increased in metastatic lesions but does not correlate with progression nor outcome in melanoma. Melanoma Res. 2015;25(2):169–172. | ||
Adams JC. Fascin-1 as a biomarker and prospective therapeutic target in colorectal cancer. Expert Rev Mol Diagn. 2015;15(1):41–48. | ||
Schoumacher M, El-Marjou F, Laé M, et al. Conditional expression of fascin increases tumor progression in a mouse model of intestinal cancer. Eur J Cell Biol. 2014;93(10–12):388–395. | ||
Snyder M, Huang J, Huang XY, Zhang JJ. A signal transducer and activator of transcription 3·Nuclear Factor κB (Stat3·NFκB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-α. J Biol Chem. 2014;289(43):30082–30089. | ||
Ghebeh H, Al-Khaldi S, Olabi S, et al. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway. Br J Cancer. 2014;111(8):1552–1561. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
