Back to Journals » Research and Reports in Urology » Volume 15
Expanded Utilization of Intralesional Therapies for Treatment of Peyronie’s Disease
Authors Khooblall P, Bole R, Lundy SD , Bajic P
Received 23 March 2023
Accepted for publication 15 June 2023
Published 21 June 2023 Volume 2023:15 Pages 205—216
DOI https://doi.org/10.2147/RRU.S386340
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Prajit Khooblall, Raevti Bole, Scott D Lundy, Petar Bajic
Cleveland Clinic, Center for Men’s Health, Glickman Urological and Kidney Institute, Cleveland, OH, USA
Correspondence: Petar Bajic, Cleveland Clinic, Center for Men’s Health, Glickman Urological and Kidney Institute, 9500 Euclid Ave, Q10, Cleveland, OH, 44195, USA, Tel +1 216 379 6134, Fax +1 216 445 2267, Email [email protected]
Purpose: In this narrative review we explore additional indications for which intralesional collagenase Clostridium histolyticum (CCH) injection therapy may be used, in addition to those utilized in the IMPRESS trials. The goal is to provide updated assessment of available intralesional therapies and justify whether to expand clinical indications based on advancements over the last decade.
Results: Patients receiving CCH in the acute phase of PD have shown significant improvement in penile curvature - which may be even more significant than reported due to progressive curvature over the longitudinal course of injection therapy. Across studies, patients with ventral plaques achieved the greatest curvature improvement (~30°) compared to PD patients with dorsal or lateral plaques. Patients with curvature > 90° have been minimally documented. However, the concept of patients with higher degree of curvature achieving more significant degrees of improvement prevails across studies. Studies including PD patients with volume loss deformities or indentation(s) focus on curvature improvement and do not gauge improvement in these girth loss or indentation features specifically. PD patients with calcification may benefit from CCH, however, critical analysis of included study designs and results compared to placebo do not lend for strong support of CCH in PD at this time.
Conclusion: Based on the most recent research, the use of CCH in the acute phase of PD and patients with ventral penile plaques may be effective and safe. The limited available research on the efficacy of CCH on calcified plaque(s) and curvature greater than 90° is promising, however, more research is needed to ensure safety and success in this patient cohort. Finally, the current literature continues to show the use of CCH is not effective in PD patients with volume loss, indentation, or hourglass deformity. When expanding the use of CCH to patients not originally included in the IMPRESS trials, providers must prioritize minimizing chances of potential injury to urethral tissue. Finally, further investigation is required to determine whether CCH has utility for curvature greater than 90° or calcified plaques, although the limited available literature is promising.
Keywords: intralesional injection, Peyronie’s disease, collagenase, Clostridium histolyticum, penile curvature
Introduction
Peyronie’s disease (PD) is a common condition characterized by localized fibrosis of the tunica albuginea of the penis resulting in plaque, penile deformity, pain, and/or sexual dysfunction.1 PD begins to affect men in the 4th or 5th decade of life with prevalence rates reported between 0.5% to 10% in the general population. The plaques causing deformity in PD are thought to develop after traumatic injuries to the penis or repetitive microtraumas during sexual intercourse.1 However, only 30% of patients recall specific trauma preceding their PD, thus there exists many other potential risk factors and contributors to the development of this disease. In fact, incidental diagnosis of PD during workup for erectile dysfunction occurs in 16% of patients.2
PD is separated into two phases: The active phase has variable levels of penile pain and evolution of the penile deformity. The chronic phase is generally defined as resolution of pain and stabilization of penile deformity, although the duration of stability required to enter this phase has been disputed. Physical exam for patients with PD should include assessment of penile deformity such as curvature, the presence and number of palpable abnormalities, and general erectile dysfunction features.
Historically, intralesional injections such as steroids, verapamil, IFN alpha-2b, Onabotulinumtoxin A, or Botox (AbbVie, Chicago, Illinois), among others and have proved effective when topical or oral medications fail for treatment of PD.3 Intralesional dexamethasone was reportedly used in the 1950s with reduction in plaque size and penile pain. While the significance of improvement remains unclear, this modality quickly fell out of favor as prolonged use resulted in local tissue atrophy over the penile skin.4 Verapamil, introduced in 1994, showed improvement in penile curvature and plaque volume, but has never had a definitive treatment regimen established for optimal improvement.5 Interferon alpha-2b was first used in 1995 and showed improved or stabilized PD in noncalcified plaques. Despite multiple studies showing good response to the use of interferon, it has always remained an off-label therapy that is no longer available in the United States. Over the past decade, the primary area of innovation in Peyronie’s disease has been through the introduction of intralesional Collagenase Clostridium histolyticum (CCH) - marketed as Xiaflex (Endo Pharmaceuticals, Malvern, PA, USA). Clostridium histolyticum is a bacterium that produces a purified collagenase capable of dissolving collagen inherent within penile plaques in PD. In 2013, CCH became the only form of intralesional injection therapy approved by the US Food and Drug Administration (FDA) for the treatment of Peyronie’s disease in men with dorsal or lateral penile curvature greater than 30° with or without a noncalcified plaque.3 CCH was also approved by the European Medicines Agency for the treatment of PD in 2014. Criteria for the Approval of CCH as defined by the FDA are found in Table 1.6 CCH is a local injection therapy that is widely accessible and has been shown to improve penile curvature and patient bother scores.7
 |
Table 1 FDA Criteria for Approval of CCH |
CCH is a purified mixture of two collagenase enzymes classes that downregulate cytokines, growth factors, and extracellular matrix-associated genes of type I and III collagen. Current standard dosages and incubation times of CCH do not impact any components of type IV collagen which is the primary structural component of connective tissue surrounding the vasculature and nerves within the penis. This ensures that the use of CCH is highly selective for only diseased tissue that causes PD.
Two large prospective, double-blinded, randomized, placebo-controlled trials (IMPRESS I and II) definitively established the efficacy and promoted the usage of CCH. These trials found a statistically significant improvement of 17° in penile curvature after a maximum of 4 treatment cycles separated by 6 weeks. While these trials are of high quality, the study design has not yet been replicated by additional investigators and similar studies typically have a follow-up time of 1 year. Further, the clinical significance of this improvement relative to surgical treatment remains a subject of debate among experts.8 Each treatment cycle consisted of two injections separated by 24–72 hours and a penile modeling procedure by the provider. One significant limitation of these trials is their exclusion of any PD patient presenting with features of ventral curvature, curvature outside the range of 30–90°, hourglass deformity, multiplanar curve, penile indentation, or plaque calcification. The inclusion and exclusion criteria of the IMPRESS trials are listed in Table 2 and Table 3. Notable differences between the FDA indications and IMPRESS trials indications are shown in Figure 1.
 |
Table 2 IMPRESS Trials Inclusion Criteria |
 |
Table 3 IMPRESS Trials Exclusion Criteria |
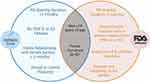 |
Figure 1 Venn Diagram representing similarities and differences between the FDA indications and IMPRESS trials indications for CCH. |
The objective of this narrative review is to provide updated comprehensive assessment of the available intralesional therapies and assess whether advancements over the last decade justify expansion of these therapies in acute phase PD, penile curvature >90°, ventral curvature, multiplanar curve, unilateral indentation, or calcified plaque. Research showing efficacy and safety of intralesional therapy in these patient cohorts may prompt subsequent updates to the current guidelines of treatment options for PD (Table 4).9–11
 |
Table 4 Guidelines for Intralesional Treatment of PD |
Therapies
The current AUA (2015) and EAU (2023) guidelines on Peyronie’s Disease recommend CCH use in alignment with the study population used in the IMPRESS trials. (Table 4) As a result, current guidelines do not support use of CCH in patients with curvature greater than 90°, ventral plaques, hourglass deformities, or during the acute phase. There is also a lack of research rigorously assessing outcomes in patients with these lesser studied features of PD.
CCH Combination Therapy with Penile Traction and/or Manual Modeling
Patients with PD may notice penile shortening most commonly during the active phase of disease. A prospective trial found patients in the active phase of PD who received no intervention developed a mean 2.4 cm loss of penile length, causing significant distress in these patients.12 Penile traction therapy (PTT) is the application of force by an external mechanical device to stretch the penis and stop or prevent the progression of scarring, recover penile length and girth, and reduce curvature by favorably reorganizing the disordered collagen fibrils.13
Ziegelmann et al conducted a randomized, single-blind control trial on the efficacy of CCH with or without PTT in a study including 51 men.14 These patients all had penile curvature between 30 and 90°, adequate erectile function, and palpable plaques of minimal calcification on exam. Men who used combination CCH + PTT did not have significant percentage improvement of curvature (42.2% vs 31.4%, p =0.42) nor stretched penile length (+0.4 cm vs −0.35 cm, p= 0.21) compared to men using CCH alone. A subsequent study with 2nd generation PTT and CCH was conducted in 113 men.15 This 2nd generation device changed the traction clamping mechanism, increased traction, allowed lengthening of traction without removal of the device, and allowed for counterbending in 4 primary directions. This 2nd generation PTT device, termed RestoreX (PathRight Medical Inc, Plymouth, Minnesota), conferred a 49% improvement in penile curvature in the CCH + RestoreX PTT group vs 30% with a PTT device other than RestoreX (p< 0.01), and 31% with CCH alone (p<0.04).15 Unlike the initial study on 2nd generation PTT,15 Ziegelmann et al investigated the improvement in curvature and length with or without RestoreX and focused on patients with lesser studied features of PD at a mean duration of 49.7 months.16 39.9% of subjects presented with hourglass deformity, 40% with indentation, and 67.9% had prior PD therapy. The use of RestoreX improved curvature and length improvement in 77% of men. The mean curvature improvement was −18.3° in patients using PTT compared to worsening curvature of 3.9° in the control group with no intervention (p<0.01). Further, there was a mean 1.5 cm length improvement in the PTT group vs 0.0 cm improvement in the control group (p<0.05). These studies align with the results of the IMPRESS trials where CCH alone did not significantly impact penile length.17 However, the use of penile traction therapy alone or in combination with CCH or verapamil has consistently shown to improve or restore penile length in PD by up to 1.3 cm during the active phase.18,19
Manual modeling is the process of bending the penis in the direction opposite the curvature to stretch and elongate the plaque to reduce the severity of penile curvature. Physician performed penile modeling was done in combination with CCH therapy during the IMPRESS trials 24–72 hours following the second injection of each treatment cycle – with subsequent home modeling recommended to patients of three times per day during the 6-week period between treatment cycles.8 Exact quantification of the effect from modeling in these trials was not done, however, the maneuver was hypothesized to improve outcomes. A study conducted after the IMPRESS trials assessed whether CCH + vacuum therapy + clinician manual modeling provided additional benefit to patients who receive just CCH + vacuum therapy.20 The results showed the degree of curvature decreased by 23° between both groups, suggesting modeling provides no additional benefit.
Use of Intralesional CHH in Acute Phase Disease
Two studies evaluated patients in the active phase of PD and had promising results for the use of intralesional therapy in this subset of patients. One single-provider study gave the first injection of CCH during the active phase and found a significant improvement in curvature when compared to CCH being given to patients with stable disease (−20° in acute phase versus −13° in stable, p<0.001), there was no difference in the rate or severity of complications.21 Despite a sample size of 49 patients, this suggests there may be a possible role for CCH during the acute phase of disease, however, this is confounded by lack of a uniform definition and duration of disease for “acute phase”. A larger single-site retrospective study included 162 patients, with 32 in the active phase (defined as penile pain and duration of PD ≤12 months) for a median of 8.5 months. Curvature among these patients was reduced by a mean of 16.7° (p<0.001), but there was no statistically significant difference in the curvature improvement (p= 0.654) or complications between groups (p = 0.356).22
Given that the definition “acute phase” varies within the literature, a retrospective study from 2022 was the first to conduct separate analyses on patients based on two options for the definition of acute phase PD.23 Definition 1 included 65 patients with symptom duration and changes in penile morphology occurring for ≤6 months; definition 2 included 37 patients with penile pain for a duration ≤12 months – some patients fit both definitions. There was no difference in initial curvature of patients regardless of the definition used for patients with acute phase PD. After a total of 8 injections, patients of Definition 1 had a 7.3° (16.0%) improvement in overall curvature compared to 8.1° (16.6%) improvement in patients with chronic phase PD (p= 0.89). Using Definition 2, acute phase PD patients had a decrease in overall curvature of 7.8° (19.9%) compared to 7.8° (15.7%) in the chronic phase PD subgroup (p= 0.43). When using patients fitting either definition of acute phase PD, 76 patients were included and showed a post-treatment improvement in overall curvature by 7.3° (16.5%) compared to 8.1° (16.3%) in all chronic phase PD patients (p= 0.96).23 There was no statistically significant difference in the number of treatment related side effects between acute and chronic phase PD patients.
These promising results show no difference in safety or efficacy for CCH treatment in the acute and chronic phases (Table 5). It is possible that the benefit of therapy in this subset of patients may be even more significant but confounded by the progressive curvature of PD in the active phase over the longitudinal course of injection therapy. Accordingly, recent preliminary data from recent studies suggest that treatment in the acute phase of PD can be effective and safe.
 |
Table 5 Efficacy of Intralesional CCH on Decreasing Penile Curvature in Acute Phase PD |
Use of Intralesional CCH in Ventral Curvature
Despite 10% of patients with PD presenting with ventral plaques, this patient population has commonly been excluded from studies due to concerns of urethral injury with intralesional therapies; thus, current guidelines do not support the use of intralesional therapies for these patients.24 Unlike the other non-CCH injection therapies that have shown positive results for ventral plaques, the use of CCH has been hypothesized to cause structural changes of the urethra and surrounding tissues due to its impact on collagen type I and III.25 However, a preliminary study using rat models investigated the use of CCH to treat urethral fibrosis, a fibrotic proliferative condition similar to PD, in collagen type and found injections to reduce urethral fibrosis and expression of collagen type I and III without any adverse effect to the urethral tissues.26 Cocci et al conducted a retrospective study in 2020 on the use of intralesional CCH therapy with 53 of the total 65 patients having ventral plaques. They found significant improvements in penile curvature of a median −20° (p<0.01).27 Another study from 2019 investigated the success of CCH for ventral plaques with patients in the active phase of PD and found significant improvement in penile curvature with a mean change of −29.5° (p< 0.01).25 This study found the greatest improvement in curvature to be with patients having ventral plaques and not dorsal (−15.0°) or lateral plaques (−11.4°), with no difference in complication rates between groups.
These promising results show no difference in safety or efficacy of intralesional therapy for PD patients having ventral plaques compared to dorsal or lateral plaques. Based on these available studies, there appears to be favorable data to support the use of intralesional therapy in this patient population by experienced providers to minimize any potential injury to urethral tissue.
Intralesional CCH Use in Curvature >90°
Very few studies have included patients with penile curvature greater than 90° and of these, subgroup analysis of patients with curvature greater than 90° is not included. Yang and Bennett studied 49 patients with a curvature range from 30–100°, but overall mean curvature of 49.3°.21 They reported a mean curvature decrease of 15.4° with at least 1 cycle of CCH, similar to results of other studies that utilized more stringent inclusion criteria.21 It is unclear how many patients had a baseline curvature greater than 90°, thus definitive conclusions can not be extrapolated to specific patients of curvature greater than 90°. A recent study from 2022 found more than double the percentage of curvature improvement in patients with >60° of baseline curvature compared to patients <30° (60% vs 29%).28 Similarly, another study demonstrated men with baseline curvatures > 60° are 2.5x more likely to have >20% improvement in degree of curvature with CCH compared to patients with lesser degrees of curvature.29 Hu et al compared efficacy of CCH in acute versus chronic phase PD patients found that baseline curvature was an effect modifier on the success of CCH treatment.23 This study noted that for every 1° of curvature, there was a 0.38% increase in penile curvature improvement after treatment (p= 0.002). Although these studies did not include patients with curvature greater than 90°, their conclusions suggest that further investigation is warranted for patients with more severe curvature.29
Given the limited literature available on the use of intralesional therapy in patients that are explicitly stated to have curvature greater than 90°, there is insufficient evidence to support its use in this population.
Use of Intralesional Injection of CCH in Girth Loss Deformity: Indentation and Hourglass
While penile length loss in PD occurs due to involvement of the longitudinal fibers of the tunica albuginea, girth loss deformities are the result of restricted expansion of circumferential fibers. These deformities exist on a spectrum from unilateral indentation to complete hourglass deformity or narrowing. There is a lack of standardized definition as to what constitutes mild, moderate, or severe girth loss, making comparison of results across studies difficult. The resultant volume loss of these conditions can lead to a “hinge effect” which is what primarily interferes with sexual activity independent of degree of curvature. The largest study of patients with unilateral indentation included 50 patients and found indentation at the distal end of the penis resulted in 88% of patients having decreased sexual activity.30 Men with combined volume-loss deformity and penile curvature were 5x more likely to report psychological distress related to their condition compared to men with the same degree of curvature but no volume loss.30 Given the clinical significance, office examinations should make every effort to induce a sufficient erection to accurately characterize the deformity.
In 2020, El-Khatib et al studied the use of CCH injections in 17 patients with penile indentation and a baseline curvature of 51.6°. They found a statistically significant −20.3 ± 8.1° (p= 0.0001) improvement of curvature in this population.24 They also assessed treatment outcomes in 7 patients with multiplanar penile deformities with a baseline curvature of 36.7° and found a statistically significant 15.8 ± 8.0° (p=0.0001) improvement in this population.24 Caution must be used in interpretation of these results as the degree of improvement in some cases was self- assessed by patients, which has been proven to significantly differ from in-office measurements.31 Several other studies of unilateral indentation have been conducted, however, they lack subgroup analysis on the effects of intralesional CCH therapy in these cohorts. These studies focus on unilateral indentation or multiplanar curves, however, when looking at studies that evaluate both unilateral indentation and hourglass deformity patients, the data to support the use of CCH to improve the deformities is less substantial.
El-Khatib et al used CCH on 17 men with penile indentation and 6 with hourglass deformity.24 Mean change in penile curvature for indentation deformity was −20.3° and −17.5° for hourglass deformities (p<0.001). In the subcategory of men with indentation or hourglass deformity, 64% were satisfied and reported subjective improvement in the narrowing/indentation after CCH injections. Yang and Bennett et al utilized CCH therapy in a patient population of broad inclusion criteria: ventral deformities, hourglass configurations, active disease, and no maximum degree of curvature limit.21 The entire cohort had a 32.4% mean improvement in degree of penile curvature when using CCH, or a mean curvature decrease of 15.4° (p < 0.0001), but no outcome measurements of indentation improvement were reported. Cocci et al studied 7 patients with hourglass deformity, finding significant improvements in penile curvature by a median of −20° (p<0.01) with CCH in this cohort, but again no mention of improved hourglass deformity.27 A recent study from 2022 included 40 patients with hourglass deformity.23 This study does not report on improvement in girth, indentation, or hourglass deformity and found no significant difference of curvature improvement after CCH therapy in patients with or without hourglass deformity (21.5% vs 15.3%, respectively, p=0.23).23
Additional studies including patients with unilateral indentation or hourglass deformity do measure penile curvature as an endpoint, but most fail to report improvements in indentation deformities. To that end, Ziegelmann et al conducted a retrospective study of 20 patients with unilateral indentation and 20 with hourglass deformity (defined as unilateral or bilateral subjective >10% girth discrepancy relative to the corpora proximal and distal to the area of maximum curvature).32 From 40 patients, only 3/40 had significant improvement in indentation deformity after a mean number of 3.8 injections, with no improvement in penile curvature. 64% of patients with moderate or severe hourglass deformity required subsequent corrective surgery.32 The authors conclude there are low rates of CCH success on improving the volume loss in these patient cohorts with PD. Finally,
The majority of current studies solely focus on curvature improvement and do not focus on assessment of improvement in hourglass or indentation after CCH, which is an important factor in sexual satisfaction. Patients may be counseled that while their penile curvature may be improved with CCH, there is currently a lack of substantial evidence suggesting intralesional CCH therapy corrects curvature deformities concurrent with volume loss deformities. For patients primarily bothered by volume loss deformity and instability or hinge effect, intralesional CCH therapy should not be recommended.
Intralesional Use of CCH in Calcified Plaque
Calcification of penile plaques has commonly been used as a predictor of CCH failure.29 Levine et al established one of several published grading classification systems for plaque calcification: grade 1 (<0.3 cm calcified plaque), grade 2 (>0.3 cm to <1.5 cm), grade 3 (>1.5 cm or two plaques >1.0 cm).33 While patients with stippled calcification were included in the IMPRESS trials, the definition of stippled calcification is unclear – with PD patients having complete calcification excluded altogether due to concerns of interference with the injections. Post-hoc subgroup analysis of the IMPRESS trials was done on the patients initially recruited with contiguous calcification (but excluded in the trials), noncontiguous stippling, and no calcification.34 Patients with contiguous calcification receiving CCH had a similar 28.7% improvement in penile curvature compared to patients receiving CCH with no calcification having a 29.8% improvement in curvature. However, statistical analysis of penile curvature improvement with CCH (28.0%) vs placebo (19.5%) in the contiguous calcification group showed no significant difference (p = 0.231), making the results of treatment success in this cohort unclear. Another study analyzed 34 patients, controlling for baseline curvature, with calcified plaques of varying severity and found severe plaque calcification to be associated with significantly lower success rates of CCH therapy.29 However, they did reaffirm the secondary outcome that greater baseline curvature is associated with the greatest degrees of improvement. The most recent study to assess curvature improvement in calcified PD plaques treated with CCH used an alternative calcification grading system. This study included a prospective cohort of 60 men with moderately calcified plaque and typical ultrasound shadow (type 2) or severely calcified plaque with complete ultrasound shadowing (type 3); 39 of which received complete treatment with eight injections. Results showed statistically significant improvement of 17.5° in patients and the improvement in curvature was not affected by the calcification grade (p<0.0001).35 While these results are promising, 35% of patients were excluded from analysis as they withdrew from the study prior to receiving 8 injections for reasons including but not limited to difficulty in contact and dissatisfaction from minimal improvement.
The most recent study by Masterson et al suggests that CCH may have a role in the treatment of some PD patients with calcified plaques, however, the high degree of patient drop out raises concerns about the validity and generalizability of these results.35 Limitations with the available studies include small sample sizes and insignificant results when compared to placebo, thus, in the context of the current literature, there is no clear indication for use of intralesional therapy among patients with plaque calcification.
Alternative Intralesional Therapies
Verapamil is a calcium channel blocker that modifies the activity of fibroblasts, collagenase and transforming growth factor-ß (TGF-ß), and possibly slows or reverses plaque formation.36–38 In vitro, verapamil reduces incorporation of proline into collagen and reduces sulfate incorporation into glycosaminoglycans.39 Additionally, verapamil downregulates the release of interleukin-6, interleukin-8, and plaque growth factor which led to its initial clinical use in the treatment of PD.40,41 One of the earliest studies on this agent found a 97% reduction in pain after 2.5 injections and improvement in ability to engage in sexual intercourse in 72% of patients.42 There is some data to suggest that verapamil use has high efficacy in the active phase of PD and softer plaques or hourglass deformities, however, the literature over the last 20 years has not been conclusive. With the introduction of CCH, the use of intralesional verapamil has declined, however its low cost, efficacy on noncalcified plaques, and reduction of pain in most patients justify its continual use as a secondary option for therapy in financially restricted patients (Table 6). The AUA guidelines state clinicians may offer intralesional verapamil for the treatment of PD with Grade C recommendation given its inferior efficacy compared to other treatments and conflicting findings between studies.9
 |
Table 6 Efficacy of Intralesional Verapamil on Decreasing Penile Curvature |
Interferon α-2b (IFN) is a cytokine with utility in alteration of collagen proliferation and the metabolic activity of myofibroblasts. The use of IFN in PD activates collagenases and inhibits fibroblast proliferation to reduce the plaque and curvature of the disease. IFN is not FDA approved and no longer available in the United States, but is included as an off-label treatment option by the AUA. Stewart et al conducted one of the more recent studies on IFN and retrospectively studied the use of IFN in 21 PD patients with a ventral plaque.45 They reported ≥20% reduction in curvature in 54% of patients, with no statistically significant difference between the curvature improvements in this group versus cohorts of dorsal or lateral plaques. Despite this study showing promising results compared to placebo, its inferior efficacy compared to alternative therapies available, increased side effects of flu-like symptoms, myalgias, and high-cost result in infrequent use.
Hyaluronic acid (HA) is a glycosaminoglycan that maintains the hydration of the extracellular matrix in connective tissue and is protective against inflammatory proteins and reactive oxygen species.46,47 Two studies on the efficacy of intralesional HA compared their results to a group of patients receiving intralesional verapamil. Cocci et al included 125 patients in their prospective case-control study and found a decrease in median penile curvature of −9.5° with HA versus −4.5° with verapamil (p< 0.01); and the mean difference in plaque size (mm) of −1.50 with HA vs −1.20 with verapamil (p = 0.10).48 Another study followed a similar design and showed mean change in curvature with HA to be −4.60° compared to 0.0° with verapamil (p < 0.001); and the mean difference in plaque size to be −1.80 mm with HA vs −1.36 mm with verapamil (p< 0.001).47 Gennaro et al utilized a different design for their case control study by comparing the results of HA to no treatment and found a significant reduction in penile curvature of −9.10° (p < 0.0001); this group also noted a significant reduction in plaque volume of −93.8% (p < 0.0001).49 Finally, the study by Zucchi et al showed the greatest decrease in penile curvature of −10° with HA but did not have any control groups in the study, which is a significant limitation.50 Although these studies all showed volumetric reduction of the penile plaques and curvature severity, their level of improvement is inferior to that achieved with intralesional CCH or verapamil (Table 7). Accordingly, there is insufficient evidence to justify the use of HA in PD patients currently.
 |
Table 7 Efficacy of Intralesional Hyaluronic Acid on Decreasing Penile Curvature |
Onabotulinumtoxin A, or Botox (AbbVie, Chicago, Illinois), is a neurotoxin that works at the neuromuscular junction to inhibit presynaptic release of acetylcholine (ACh). Onabotulinumtoxin A is currently used with high efficacy in patients with detrusor overactivity and pelvic-floor disorders.51 A validation study hypothesized this neurotoxin to help treat hypertrophic scars by acting on fibroblasts and downregulating connective tissue growth factor (CTGF), a regular of TGF-β1, by more than 50%. This finding suggests the neurotoxin would be most efficacious in the active phase of disease, however, in the sole study that used Onabotulinumtoxin A in PD, all 22 patients were in the stable phase of disease.52 This study used 1 dose of 100 U of onabotulinumtoxin A and reported statistically significant improvement in penile curvature of 7.85° from baseline (p= 0.025) and decreased plaque thickness of 0.7 mm (p = 0.014). Limitations of the conclusions from this study are the small sample size and lack of control group for comparison. Currently, there is insufficient evidence to recommend Botox as a first-line choice intervention to patients with PD. Additional research is necessary for any consideration of this non-FDA-approved neurotoxin to be a potential treatment modality for patients with typical PD.
Discussion
The pathophysiology and etiology of PD is still not yet completely understood, and patients can present with deformities of various types. Ideal candidates, techniques and dosing regimens for intralesional therapies remain debated. There is currently no gold standard treatment for patients with features of PD that deviate from those included in the IMPRESS trials. By providing an up-to-date assessment of all recent available studies, we recommend that providers consider expanding the use of these therapies to some subsets of patients with PD features that are currently exclusionary. However, as CCH is currently the only FDA-approved intralesional therapy for PD, its use in PD patients with features outside the scope of the IMPRESS trials remains off-label and may pose significant financial barriers to patients seeking treatment.
Limited data is available on CCH in patients with PD features of acute disease, ventral plaques, curvature greater than 90°, hourglass deformity, or calcified plaques. Many of the initial concerns such as urethral injury in ventral plaques or insignificant improvement in acute phase disease are called into question based on this narrative review of the current literature. Based on these recent studies, we found acute-phase and PD patients with ventral plaques to have positive results with intralesional CCH therapy comparable to patients with typical PD features, supporting use in this subgroup of patients. Studies suggest patients with a higher degree of curvature have increased improvement compared to patients with lower baseline curvatures. However, there are minimal studies that distinctly include patients with > 90° curvature. As a result, the use of CCH in this population is not yet justified but warrants further investigation in this subgroup of PD patients.
Recent data on the use of CCH in calcified plaques may lend some support to its use in this population, however, due to the small sample size, high attrition rates, and lack of additional supporting data, this indication remains controversial.35 We found that despite improving penile curvature, there is a lack of data studying the efficacy of intralesional therapies on correcting girth-loss / indentation deformities in patients with hourglass deformity, unilateral indentation, or volume loss deformities. When exploring alternative non-FDA approved intralesional therapies, we found HA and Botox to have inferior efficacy, higher cost, and increased side effect profile compared to CCH or verapamil. However, intralesional verapamil remains the most used intralesional alternative to CCH in patients with PD on account of its efficacy in decreasing acute pain.
Inherent limitations to this narrative review are the result of heterogenous reporting and analysis of symptoms, variable and short follow up in a large number of included studies, treatment intervention technique, and treatment success. There are several studies that include more liberalized inclusion criteria, but many do not perform subgroup analyses. For example, Yang and Bennett, include patients with hourglass deformities, penile curvatures greater than 90 degrees, and ventral curvature, however, these patients were all analyzed together.21 These studies are less helpful in drawing conclusions on how successful intralesional therapies may be in specific PD cohorts with features excluded from the IMPRESS trials. Another difficulty in comparing the results between studies in patients of varying PD presentation is the heterogenous reporting of treatment success. Some studies report >20% improvement as treatment success while others define >15% improvement to be statistically and clinically significant. There was also inconsistent reporting between studies on whether patients were in active treatment at both initiation and completion of the study, total cycles of therapy received, and whether the ability for penetrative sex was restored, which are all important clinical factors to justify treatment expansion and use in patients with atypical PD. Monitoring both subjective and objective responses to all PD treatments is important as some patients may have a perceived changed that differs from the objective measurements reported in the study. The current Peyronie’s Disease Questionnaire (PDQ) fails to ask questions about volume loss deformities, resulting in many of the included studies not fully capturing patient perception of their condition. Despite the PDQ questionnaire not being used, several studies subjectively found patient perception of improvement may positively or negatively affect the results of the intervention therapies as demonstrated with the studies incorporating placebo groups. Thus, prior to any therapies for patients with PD, it is imperative to counsel the patient on having realistic expectations of treatment success and that there is a possibility of subsequent surgical intervention being required in order to achieve their treatment goals.
Conclusion
PD can have a significant impact on a man’s quality of life, affecting not only physical health but also emotional well-being. While there are various surgical and non-surgical treatment options available, collagenase Clostridium histolyticum (CCH) injections have emerged as an especially promising therapy. The landmark IMPRESS trials established the indications for the use of CCH but did not study the use of CCH in patients with curvature greater than 90°, ventral plaques, hourglass deformities, or during the acute phase. This narrative review explores the utility and efficacy of CCH in these cohorts to provide better understanding of whether patients suffering with these features of PD may be treated with similar success. Contemporary evidence for other non-CCH intralesional therapies is reviewed. There is promising data that CCH can significantly improve curvature and bother in patients in the acute phase PD and ventral curvature. Further investigation is needed to determine whether CCH has utility for curvature greater than 90° or calcified plaques, although the limited available literature is promising. When offering off-label treatment of CCH for patients with these features, it is pertinent for the provider to discuss the risks and benefits of utilizing this therapeutic intervention so that clear expectations are established, and patients understand subsequent intervention may still be required.
Disclosure
Petar Bajic has served on an advisory board for Endo Pharmaceuticals, personal fees from Endo Pharmaceuticals, grants from Coloplast Corporation, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Bjekic MD, Vlajinac HD, Sipetic SB, Marinkovic JM. Risk factors for Peyronie’s disease: a case-control study. BJU Int. 2006;97(3):570–574. doi:10.1111/j.1464-410X.2006.05969.x
2. Kadioglu A, Oktar T, Kandirali E, Kendirci M, Sanli O, Ozsoy C. Incidentally diagnosed Peyronie’s disease in men presenting with erectile dysfunction. Int J Impot Res. 2004;16(6):540–543. doi:10.1038/sj.ijir.3901247
3. Randhawa K, Shukla CJ. Non-invasive treatment in the management of Peyronie’s disease. Ther Adv Urol. 2019;11:1756287218823671. doi:10.1177/1756287218823671
4. Winter CC, Khanna R. Peyronie’s disease: results with dermo-jet injection of dexamethasone. J Urol. 1975;114(6):898–900. doi:10.1016/s0022-5347(17)67169-6
5. Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie’s disease. J Urol. 1994;151(6):1522–1524. doi:10.1016/s0022-5347(17)35291-6
6. Xiaflex prescribing information; 2010. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125338s109lbl.pdf.
7. Cwikla DJ, Yafi FA. Intralesional collagenase Clostridium histolyticum in the management of Peyronie’s disease: current best practice. Ther Adv Urol. 2018;10(4):139–153. doi:10.1177/1756287218755020
8. Gelbard M, Goldstein I, Hellstrom WJG, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled Phase 3 studies. J Urol. 2013;190(1):199–207. doi:10.1016/j.juro.2013.01.087
9. Nehra A, Alterowitz R, Culkin DJ, et al. Peyronie’s disease: AUA guideline. J Urol. 2015;194(3):745–753. doi:10.1016/j.juro.2015.05.098
10. Chung E, Ralph D, Kagioglu A, et al. Evidence-based management guidelines on Peyronie’s disease. J Sex Med. 2016;13(6):905–923. doi:10.1016/j.jsxm.2016.04.062
11. EAU. EAU guidelines on sexual and reproductive health.
12. Martínez-Salamanca JI, Egui A, Moncada I, et al. Acute phase Peyronie’s disease management with traction device: a nonrandomized prospective controlled trial with ultrasound correlation. J Sex Med. 2014;11(2):506–515. doi:10.1111/jsm.12400
13. Chung E, De Young L, Solomon M, Brock GB. Peyronie’s disease and mechanotransduction: an in vitro analysis of the cellular changes to Peyronie’s disease in a cell-culture strain system. J Sex Med. 2013;10(5):1259–1267. doi:10.1111/jsm.12082
14. Ziegelmann MJ, Viers BR, Montgomery BD, Avant RA, Savage JB, Trost LW. Clinical experience with penile traction therapy among men undergoing collagenase Clostridium histolyticum for Peyronie disease. Urology. 2017;104:102–109. doi:10.1016/j.urology.2017.01.054
15. Alom M, Sharma KL, Toussi A, Kohler T, Trost L. Efficacy of combined collagenase Clostridium histolyticum and RestoreX penile traction therapy in men with Peyronie’s disease. J Sex Med. 2019;16(6):891–900. doi:10.1016/j.jsxm.2019.03.007
16. Ziegelmann M, Savage J, Toussi A, et al. Outcomes of a novel penile traction device in men with Peyronie’s disease: a randomized, single-blind, controlled trial. J Urol. 2019;202(3):599–610. doi:10.1097/JU.0000000000000245
17. Yafi FA, Hatzichristodoulou G, DeLay KJ, Hellstrom WJG. Review of management options for patients with atypical Peyronie’s disease. Sex Med Rev. 2017;5(2):211–221. doi:10.1016/j.sxmr.2016.07.004
18. Abern MR, Larsen S, Levine LA. Combination of penile traction, intralesional verapamil, and oral therapies for Peyronie’s disease. J Sex Med. 2012;9(1):288–295. doi:10.1111/j.1743-6109.2011.02519.x
19. Yafi FA, Pinsky MR, Stewart C, et al. The effect of duration of penile traction therapy in patients undergoing intralesional injection therapy for Peyronie’s disease. J Urol. 2015;194(3):754–758. doi:10.1016/j.juro.2015.03.092
20. Ralph DJ, Abdel Raheem A, Liu G. Treatment of Peyronie’s disease with collagenase Clostridium histolyticum and vacuum therapy: a randomized, open-label pilot study. J Sex Med. 2017;14(11):1430–1437. doi:10.1016/j.jsxm.2017.08.015
21. Yang KK, Bennett N. Peyronie’s disease and injectable collagenase Clostridium histolyticum: safety, efficacy, and improvements in subjective symptoms. Urology. 2016;94:143–147. doi:10.1016/j.urology.2016.04.049
22. Nguyen HMT, Anaissie J, DeLay KJ, Yafi FA, Sikka SC, Hellstrom WJG. Safety and efficacy of collagenase Clostridium histolyticum in the treatment of acute-phase Peyronie’s disease. J Sex Med. 2017;14(10):1220–1225. doi:10.1016/j.jsxm.2017.08.008
23. Hu MYY, Sigalos JT, Walker DT, et al. Intralesional collagenase Clostridium histolyticum for acute phase Peyronie’s disease: a single-center, retrospective cohort study. Transl Androl Urol. 2022;11(8):1074–1082. doi:10.21037/tau-22-188
24. El-Khatib FM, Osman MM, Kopelevich A, Towe M, Yafi FA. Treatment-related outcomes for patients with atypical Peyronie’s disease using xiaflex injections. Urology. 2020;143:153–158. doi:10.1016/j.urology.2020.05.076
25. Alom M, Meng Y, Sharma KL, Savage J, Kohler T, Trost L. Safety and efficacy of collagenase clostridium histolyticum in Peyronie’s disease men with ventral curvatures. Urology. 2019;129:119–125. doi:10.1016/j.urology.2019.01.055
26. Sangkum P, Yafi FA, Kim H, et al. Collagenase Clostridium histolyticum (Xiaflex) for the treatment of urethral stricture disease in a rat model of urethral fibrosis. Urology. 2015;86(3):647.e1–6. doi:10.1016/j.urology.2015.06.013
27. Cocci A, Di Maida F, Russo GI, et al. How atypical penile curvature influence clinical outcomes in patients with Peyronie’s disease receiving collagenase clostridium histolyticum therapy? World J Mens Health. 2020;38(1):78–84. doi:10.5534/wjmh.190026
28. Flores JM, Nascimento B, Punjani N, et al. Predictors of curvature improvement in men with Peyronie’s disease treated with intralesional collagenase clostridium histolyticum. J Sex Med. 2022;19:1680–1686. doi:10.1016/j.jsxm.2022.08.001
29. Wymer K, Ziegelmann M, Savage J, Kohler T, Trost L. Plaque calcification: an important predictor of collagenase clostridium histolyticum treatment outcomes for men with Peyronie’s disease. Urology. 2018;119:109–114. doi:10.1016/j.urology.2018.06.003
30. Margolin EJ, Pagano MJ, Aisen CM, Onyeji IC, Stahl PJ. Beyond curvature: prevalence and characteristics of penile volume-loss deformities in men with Peyronie’s disease. Sex Med. 2018;6(4):309–315. doi:10.1016/j.esxm.2018.07.003
31. Traeger M, Leiber-Caspers C, Chierigo F, Cakir OO, Gratzke C, Schlager D. Penile autophotography underestimates the degree of penile curvature in Peyronie’s disease. Eur Urol Focus. 2023;9(1):64–68. doi:10.1016/j.euf.2022.10.009
32. Ziegelmann MJ, Heslop D, Houlihan M, et al. The influence of indentation deformity on outcomes with intralesional collagenase clostridium histolyticum monotherapy for Peyronie’s disease. Urology. 2020;139:122–128. doi:10.1016/j.urology.2020.01.035
33. Levine L, Rybak J, Corder C, Farrel MR. Peyronie’s disease plaque calcification--prevalence, time to identification, and development of a new grading classification. J Sex Med. 2013;10(12):3121–3128. doi:10.1111/jsm.12334
34. Lipshultz LI, Goldstein I, Seftel AD, et al. Clinical efficacy of collagenase Clostridium histolyticum in the treatment of Peyronie’s disease by subgroup: results from two large, double-blind, randomized, placebo-controlled, Phase III studies. BJU Int. 2015;116(4):650–656. doi:10.1111/bju.13096
35. Masterson TA, Atuluru P, Zucker I, et al. Collagenase Clostridium histolyticum treatment improves degree of curvature in Peyronie’s disease with calcified plaques. Eur Urol Focus. 2022;S2405-4569(22)00228–0. doi:10.1016/j.euf.2022.09.019
36. Teloken P, Katz D. Medical management of Peyronie’s disease: review of the clinical evidence. Med Sci. 2019;7(9):96. doi:10.3390/medsci7090096
37. Ehrlich HP, Ross R, Bornstein P. Effects of antimicrotubular agents on the secretion of collagen. A biochemical and morphological study. J Cell Biol. 1974;62(2):390–405. doi:10.1083/jcb.62.2.390
38. Levine LA, Estrada CR. Intralesional verapamil for the treatment of Peyronie’s disease: a review. Int J Impot Res. 2002;14(5):324–328. doi:10.1038/sj.ijir.3900917
39. Lee RC, Ping JA. Calcium antagonists retard extracellular matrix production in connective tissue equivalent. J Surg Res. 1990;49(5):463–466. doi:10.1016/0022-4804(90)90197-a
40. Roth M, Eickelberg O, Kohler E, Erne P, Block LH. Ca2+ channel blockers modulate metabolism of collagens within the extracellular matrix. Proc Natl Acad Sci U S A. 1996;93(11):5478–5482. doi:10.1073/pnas.93.11.5478
41. Chong W, Tan RBW. Injectable therapy for Peyronie’s disease. Transl Androl Urol. 2016;5(3):310–317. doi:10.21037/tau.2016.03.15
42. Levine LA. Treatment of Peyronie’s disease with intralesional verapamil injection. J Urol. 1997;158(4):1395–1399. doi:10.1016/S0022-5347(01)64224-1
43. Gallo L, Sarnacchiaro P. Ten-year experience with multimodal treatment for acute phase Peyronie’s disease: a real life clinical report. Actas Urol Esp. 2019;43(4):182–189. doi:10.1016/j.acuro.2018.08.005
44. Wolff B, Peyronnet B, Cattarino S, et al. Intralesional injections for early peyronie disease: standardized assessment and analysis of predictive factors for treatment response. Urology. 2015;86(1):57–61. doi:10.1016/j.urology.2015.03.010
45. Stewart CA, Yafi FA, Knoedler M, et al. Intralesional injection of interferon-α2b improves penile curvature in men with Peyronie’s disease independent of plaque location. J Urol. 2015;194(6):1704–1707. doi:10.1016/j.juro.2015.06.096
46. Yu CJ, Ko CJ, Hsieh CH, et al. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid-regulated proteins involved in chondroprotective effect under oxidative stress. J Proteomics. 2014;99:40–53. doi:10.1016/j.jprot.2014.01.016
47. Favilla V, Russo GI, Zucchi A, et al. Evaluation of intralesional injection of hyaluronic acid compared with verapamil in Peyronie’s disease: preliminary results from a prospective, double-blinded, randomized study. Andrology. 2017;5(4):771–775. doi:10.1111/andr.12368
48. Cocci A, Di Maida F, Cito G, et al. Comparison of intralesional hyaluronic acid vs. verapamil for the treatment of acute phase Peyronie’s disease: a prospective, open-label non-randomized clinical study. World J Mens Health. 2021;39(2):352–357. doi:10.5534/wjmh.190108
49. Gennaro R, Barletta D, Paulis G. Intralesional hyaluronic acid: an innovative treatment for Peyronie’s disease. Int Urol Nephrol. 2015;47(10):1595–1602. doi:10.1007/s11255-015-1074-1
50. Zucchi A, Costantini E, Cai T, et al. Intralesional injection of hyaluronic acid in patients affected with Peyronie’s disease: preliminary results from a prospective, multicenter, pilot study. Sex Med. 2016;4(2):e83–88. doi:10.1016/j.esxm.2016.01.002
51. Apostolidis A, Dasgupta P, Denys P, et al. Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol. 2009;55(1):100–119. doi:10.1016/j.eururo.2008.09.009
52. Reddy AG, Dick BP, Natale C, Akula KP, Yousif A, Hellstrom WJG. Application of botulinum neurotoxin in male sexual dysfunction: where are we now? Sex Med Rev. 2021;9(2):320–330. doi:10.1016/j.sxmr.2020.05.004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
