Back to Journals » Drug, Healthcare and Patient Safety » Volume 16
Evaluation of Seven Different Brands of Metformin Hydrochloride Tablets Available in the Market in Gondar City, Ethiopia
Authors Flatie Alemu A, Tegegne AA , Getaw NS
Received 8 November 2023
Accepted for publication 27 January 2024
Published 1 February 2024 Volume 2024:16 Pages 19—28
DOI https://doi.org/10.2147/DHPS.S419522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Siew Siang Chua
Adane Flatie Alemu,1 Addisu Afrassa Tegegne,2 Nurahmed Seid Getaw1
1Pharmaceutical Analysis, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Pharmaceutical Quality Assurance and Regulatory Affairs, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Addisu Afrassa Tegegne, Email [email protected]
Background: WHO estimates that 15.8% of substandard or falsified medical products are used to treat non-communicable diseases which account for 80% of the global burden including diabetes. The increased level of use of metformin hydrochloride tablets in clinical practice creates the need to monitor and ascertain the quality of the various brands available in the drug market for quality control assessment and generic substitution. This study aims to assess the pharmaceutical quality of seven brands of metformin tablets circulating in pharmacy outlets in Gondar City, North West Ethiopia.
Methods: Official Pharmacopoeia tests such as uniformity of weight, disintegration, assay, and dissolution tests were used to assess the physicochemical quality control parameters of metformin hydrochloride tablet brands. The unofficial tests conducted included crushing strength/hardness and friability.
Results: In all seven tests, the tested brands passed the BP official tests for uniformity of weight, friability, disintegration, and dissolution. Each product had a friability of less than 1% with a maximum of 0.385%. In contrast, none of the brands passed the non-official hardness test. Each product disintegrated in seven to twelve minutes, meeting the USP standards. Drug release rates in 45 min ranged from 78.9 to 92.6%, and drug content results were within the USP guidelines (96.55– 102.76%).
Conclusion: The current study demonstrated that all seven brands of metformin hydrochloride 500 mg tablets adhered to the quality control parameters specified in the pharmacopeia, except for the hardness test across all brands.
Keywords: metformin, quality control, substandard or falsified, Gondar, Ethiopia
Introduction
When medicines are the second highest household expense in the poorest economies, resources devoted to poor-quality medicines come at a tremendous opportunity cost, in addition to the risk that a person will be injured or not receive the treatment they need.1,2 A growing number of non-communicable and communicable diseases are left untreated in the market due to the market penetration of such poor-quality pharmaceuticals.3 The WHO reported that 15.8% of the products reported as being substandard or false were used to treat non-communicable diseases, which represent 80% of the global burden, including diabetes, cancer, mental health issues, and cardiovascular diseases, leading to higher mortality rates.4
A disease such as diabetes mellitus has a high incidence and no cure, requiring patients to manage their condition for the rest of their lives.5 It results from the body failing to produce insulin and/or being unable to respond adequately to circulating insulin in a chronic metabolic disorder.6 The number of people living with diabetes worldwide is expected to rise to 643 million by 2030, and 783 million by 2045, with approximately 3 in 4 adults living with diabetes in low- and middle-income countries.7 Oral antidiabetic metformin is widely prescribed for treating type II (non-insulin-dependent diabetes mellitus) since it reduces hepatic glucose production, increases intestinal glucose absorption, and increases insulin sensitivity by improving peripheral glucose uptake and utilization.8 Additionally, it is prescribed for polycystic ovarian disease, impaired fasting glucose, impaired glucose tolerance, and prediabetes.9
Metformin hydrochloride ((N, N-dimethyl-imido-dicarbonimidic diamide hydrochloride) has a chemical name of 1,1-Dimethylbiguanide hydrochloride with a molecular formula of C4H11N5 • HCl and a molecular weight of 165.62 g/mole (Figure 1). It is a white crystalline powder that is freely soluble in water and soluble ethanol (95%), and practically insoluble in acetone, chloroform, dichloromethane, and ether. The melting point is 232 °C. Metformin hydrochloride, USP exhibits high solubility in water while demonstrating negligible solubility in acetone, ether, and chloroform. The pKa value associated with Metformin is 12.4. The pH value of a 1% aqueous solution containing metformin is measured to be 6.68.10 Metformin HCl, classified as a biguanide, is an oral medication commonly prescribed as an antidiabetic drug. According to biological classification systems, metformin hydrochloride falls under Class-III drugs, characterized by high solubility and lower permeability. Despite metformin being a hydrophilic compound, it is typically formulated in its hydrochloride salt form for oral administration. This characteristic indicates that metformin has limited lipophilicity, resulting in a low diffusion rate across cell membranes. Permeability plays a crucial role as the rate-limiting step in drug absorption. Hence, it is necessary to have strict requirements for the duration of dissolution testing for class III drugs. Since drug permeation acts as the controlling factor, it is not anticipated to establish an in-vivo-in-vitro (IVIV) correlation.11
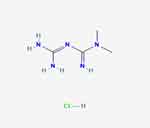 |
Figure 1 Chemical structure of metformin hydrochloride. |
The presence of poor-quality medicines, particularly substandard and falsified ones, can pose significant risks to patients. Insufficient dosage of a medication can results in therapeutic failure, while ineffective treatment prolongs hospital stays and may even contribute to mortality. Substandard or falsified medical products were adopted at the Seventieth World Health Assembly on 29 May 2017, a decision that was approved by the Assembly. Falsified products are those that intentionally/fraudulently misrepresent their identity, composition, or source. Those that are substandard are referred to as “out of specification”, which are authorized medical products that do not meet quality standards or specifications.4
Pharmaceutical products must meet essential standards for dosage quality, such as safety, potency, efficacy, consistency, as well as compliance with government regulations.12 In developing countries, where substandard or falsified medicines have become a major challenge for healthcare facilities, routine quality testing of drugs on the market is crucial to protecting public health.13,14 A systematic review done by Ozawa et al, 2018 included 96 studies that tested 50 samples or more, the overall prevalence of poor-quality medicines was 13.6%, with a regional prevalence of 18.7% in Africa.15 In studies conducted to assess the pharmaceutical quality control parameters of metformin hydrochloride brands in the market16–20 more than one sample failed at least one test parameter, as opposed to other studies21–23 passed all the samples metformin hydrochloride brands. As a result of the lack of effective monitoring mechanisms on the market, treatment failures, and drug resistance have become a common occurrence in developing countries.24 Quality assessment studies on marketed drug products could give an insight into the quality of the pharmaceutical products marketed within the distribution chain and consumed.25 Therefore, the purpose of this study was to evaluate the critical quality parameters of different brands of metformin hydrochloride.
Materials and Methods
Study Area and Period
The study was conducted from February to May 2021 at the University of Gondar, which is located North of Gondar city, Ethiopia. Gondar City is located 727 km from the Northwest of Addis Ababa.
Study Design
The experimental in-vitro study design was used to evaluate the in-vitro quality control parameter of the commercially available metformin hydrochloride tablet brands that are available in Gondar City. The study was conducted by performing various test procedures associated with the quality such as weight variations, hardness, and disintegration time, friability, dissolution.
Instruments
UV-spectrophotometer (LT-291, India), analytical balance (JA203P; China), tablet hardness tester (PTB111E, Pharma Test), disintegration tester (Model B.J-3, Shanghai Famo Machinery manufacture), dissolution test apparatus (RC-6, India), tablet grinder (Model 20–120, China), and PH meter (VSI-01ATC, VSI Electronics PVT. Ltd).
Reagents Used
Hydrochloric acid (Blulux Laboratories Pvt. Ltd., India), Potassium Hydrogen Phosphate (Blulux Laboratories Pvt. Ltd., India), and distilled water. The dissolution medium (0.68% w/v of Potassium Hydrogen Phosphate adjusted to pH 6.8 by the addition of 0.2 M hydrochloric acid) was prepared by dissolving 6.8 gm of potassium hydrogen phosphate in sufficient distilled water to make 1000 mL solution.
Sampling Techniques and Sample Collection
Sampling strategy was conducted as per the guideline proposed by Guidelines for Field Surveys of the Quality of Medicines.26 Metformin hydrochloride, having a label strength of 500mg of 7 different brands was randomly collected from University of Gondar hospital pharmacy, government health centers, and private pharmacies in Gondar City, Ethiopia. All available brands were collected by mystery shopper approach. The shoppers were unaware of the study’s objective and were solely instructed to obtain approximately one hundred tablets (n=102) of each brand of metformin hydrochloride tablets from various drug retail outlets. The tablets were bought in their original packaging as provided by the manufacturers. Throughout the collection process, essential details including the drug substance name, country of origin, manufacturing company, expiry date, manufacturing date, and batch/lot number were systematically recorded (Table 1). After purchase, the shoppers quickly departed from the outlets, ensuring no crucial information was missed. Subsequently, all collected samples were transported to the Pharmaceutical Chemistry Laboratory at the University of Gondar and stored according to the specified storage conditions mentioned on the product label until analysis.
 |
Table 1 Profile of Sampled Metformin Hydrochloride Tablets Marketed in Gondar, Ethiopia |
Uniformity of Weight
Sample tablets20 of each brand were weighed together and the average weight was determined. Each tablet was weighed individually on an analytical balance and the percentage deviation of the tablets was calculated using the following expression.27
(%) deviation = [(individual weight - average weight)/average weight] × 100
Hardness Test
By selecting ten tablets randomly from each brand, the hardness of the tablets was determined using a hardness tester machine (PTB111E Pharma Test, Germany). Tablets were placed between two anvils and forces were applied to the anvils, measuring the crushing strength that caused a tablet to break.
Friability Test
For each of the brands, 15 tablets were selected and carefully dusted before testing, and weighed. Then the tablets were placed in the drum of the friability tester and rotated at the speed of 25rpm for 4 minutes. After 100 revolutions and de-dusting, tablets were re-weighed and the friability percentage was calculated by the following equation.27 As per the guidelines outlined in the USP, the permissible weight loss should not exceed 1%.
% Friability = [(Initial weight – Final weight)/Initial weight] ×100
Disintegration Test
Tablet disintegration was determined at 37±0.5°C using a disintegration apparatus. The disintegration time of randomly selected six tablets of each brand was determined in 900 mL of distilled water. The time taken to disintegrate the tablet and pass through the mesh was recorded and the mean of time taken was calculated.27
Dissolution Test
The dissolution test was conducted according to USP pharmacopeia. In a medium containing 900mL of phosphate buffer (pH 6.8), the basket was rotated at a fixed speed of 100 rpm, and the temperature was maintained at 37 ±0.5°C. Six tablets of each brand were selected randomly and then subjected to the test. Samples were withdrawn at 10, 15, 20, 30, 45 and 60 minutes. The paddle was rotated at 100 Revolutions Per Minute (rpm). 10mL of samples were taken from each dissolution test vessel at each sampling time. An equivalent amount of a fresh 10mL dissolution medium was replaced immediately to maintain the vessel volume constant throughout the analysis. The samples were filtered and assayed for drug content by measuring their absorbance at a maximum of 233 nm. Then the total content of metformin hydrochloride in the medium was calculated taking 806 as the value of A (1%, 1 cm).27
Assay of Metformin Hydrochloride Tablets
Twenty tablets of metformin hydrochloride were weighed and powdered using a mortar and pestle. Then a quantity of the powder containing 0.1 g of Metformin Hydrochloride was shaken with 70 mL of distilled water for 15 minutes, and made the volume to 100 mL with distilled water and filtered then 10 mL of the filtrate was diluted to 100 mL with distilled water and 10 mL of the resulting solution was further diluted to 100 mL with distilled water to give a nominal concentration of 10 µg/mL. The absorbance of the resulting solution was measured at the maximum at 232 nm and the content of metformin hydrochloride was calculated using 798 as the value of A (1%, 1 cm), and this was used to calculate the drug content. Distilled water was used as a blank.27
Results
Uniformity of Weight of Metformin Hydrochloride Tablets
Test results of uniformity of weight in this study showed that all brands fulfill the compendia specification. According to the USP 2015 guidelines, the tablet is considered to pass the test if no more than two individual weights deviate from the average weight by ± 5%, and none deviates by ± 10%. (Table 2).
 |
Table 2 Uniformity of Weight of the Different Brands of Metformin Hydrochloride Tablets Marketed in Gondar, Ethiopia |
Friability Test of Metformin Hydrochloride Tablets
In this study, all brands of metformin hydrochloride tablets passed the friability test with a value ranging from 0% w/w (M1) to 0.385% w/w (M3) (Table 3). According to the USP guidelines, tablets that experience a weight loss of no more than 1.0% are deemed acceptable.
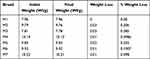 |
Table 3 Friability of the Different Brands of Metformin Hydrochloride Tablets Marketed in Gondar City, Ethiopia |
Hardness of Metformin Hydrochloride Tablets
Typically, oral tablets exhibit a hardness range of 4 to 10 kg according to non-official USP specifications.28 The result of the hardness test in this study showed that all brands failed the non-official test with M1 having the least hardness of 15.325±0.82 Kg and M4 having the highest hardness of 23.588±1.91 Kg. (Table 4)
 |
Table 4 Hardness of the Different Brands of Metformin Hydrochloride Tablets Available in the Gondar City, Ethiopia |
The Disintegration of Metformin Hydrochloride Tablets
All film-coated (M1, M2, M3, M4, M5, and M6) and uncoated (M7) brands passed the disintegration test according to BP which specifies 30 minutes for film-coated tablets and specifies 15 minutes for uncoated tablets. (Table 5)
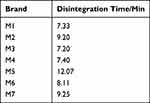 |
Table 5 Disintegration Time of the Different Brands of Metformin Hydrochloride Tablets Available in the Gondar City, Ethiopia |
Assay of Metformin Hydrochloride Tablets
The results obtained from the analysis using UV-Vis Spectrophotometers showed that all brands had values that fell within the monograph specifications according to BP which specifies 95–105% of label claims. (Table 6)
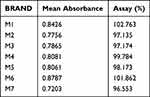 |
Table 6 Assay of the Different Brands of Metformin Hydrochloride Tablets Available in the Gondar City, Ethiopia |
Dissolution of Metformin Hydrochloride Tablets
As per the BP 2007 requirements, the dissolution test mandates that a minimum of 70% of the active ingredient should dissolve within a 45-minute timeframe.29 Thorough evaluation (Table 7) showed that the highest percent release concentration was found in sample M3, (109%) and the lowest percent release concentration was found in sample M5 (78.9%).
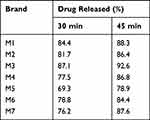 |
Table 7 Percentage Released (Dissolution) of Metformin Hydrochloride Tablets Available in Gondar City, Ethiopia |
Discussion
Consistency in drug dosage across individual tablets is a fundamental requirement in the development of pharmaceutical products.30 If a weight variation test fails, it can lead to the absence of the required quantity of active pharmaceutical ingredient in the unit dose, resulting in the unavailability of the necessary therapeutic effect. The uniformity of weight determination for all seven brands gave values that complied with official specifications for weight uniformity with a deviation less than 5% from the mean value and the lowest deviation is M2 (±0.0037). Brand M5 had the highest standard deviation value of ±0.0103 which could be attributed to the flow properties of the granules even the feeding of granules into the die as well as regular movement of the lower punch to produce uniform weight distribution of the tablets.31 In this study, all brands can withstand abrasion associated with handling, packaging, transportation, and shipping with the friability value for all brands ranging between 0 to 0.385%. Meeting the specification of the friability test may be due to optimum binder concentration, resulting in not losing the interparticle bonding or the use of appropriate compression pressure in the tablet machine.30 The results compare favorably with the results of a study conducted in Kenya that revealed all sample brands achieved BP specifications for uniformity of weight and friability.18
The crushing strength test evaluates the tablet’s ability to withstand pressure and stress encountered during handling, packaging, and transportation. This test measures the tablet’s resistance to permanent deformation and serves as an indicator of its physical robustness. Using the PTB111E hardness tester, the strength of the tablets was tested. A force of about 40 N is the minimum requirement for a satisfactory tablet.32 Hence, tablets of all seven brands were not satisfactory for hardness. Brand M1 had the lowest hardness of 15.325±0.82 Kg and M4 had the highest hardness of 23.588±1.91 Kg. Tablets must be sufficiently hard during handling, packaging, and shipping to avoid damage. Five out of eight Metformin hydrochloride brands tested in Nigeria33 failed the crushing test, while a study done in Sudan in 201734 showed one of five brands failed the hardness test. As seen in all brands of metformin hydrochloride, low crushing strength may result from a poor choice of binding agent, low binder concentration, incorrect incorporation method, inadequately dried granules, and low compression force.35
Disintegration plays a critical role in the release of drugs from immediate-release dosage forms. The speed at which disintegration occurs is directly linked to the rate of dissolution. The disintegration rate is affected by the water influx into the tablets, which, in turn, is influenced by the tablets’ porosity. Disintegration time was measured to determine the time that a drug would disintegrate in the gastric environment, thereby indicating the drug’s release profile. In this study, the mean disintegration time varied between brands M3 (7.20) and M5 (12.07) minutes, less than the standard disintegration time. A study conducted in Sudan34 showed all the metformin hydrochloride brands examined had a suitable disintegration time with the fastest and slowest disintegrated tablets of 6.07 and 13.32 min respectively while in Bangladesh,22 the longest disintegration time (21 minutes) and the shortest disintegration time (5 minutes).
A dissolution test for pharmaceutical solid dosage forms plays an important role in testing the quality of the product since it liberates the drug from its dosage form and makes it available for gastrointestinal absorption.36 Furthermore, it can be used as an effective method for comparing formulations of the same therapeutic agent sensitively. It is unlikely that drugs that dissolve poorly will be absorbed into the body system or target organs/tissues to be of therapeutic value.37
All formulations were found to pass the BP general specifications test for dissolution rate for tablets based on the results of the study. The obtained dissolution content at 45 minutes was found to range from 78.9% (Brand M5) to 92.6% (Brand M3). This rate of dissolution precludes any possibility of bioavailability problems resulting from drug dissolution. Similar to this study, researchers found that all six brands of metformin tablets met the dissolution requirement in a study examining physicochemical quality tests in Libya,38 whereas in Nigeria,19 one out of nine tablets released the least amount of drug (93%) that exceeded the official specification lower limit. It is usually a combination of drug substances and excipients that make up an oral dosage form, and the proportions between them, the type, and the manufacturing method of the final product are selected based on their content, their physicochemical and bulk properties, as well as their absorption properties.39 The result is distinct dissolution characteristics for each brand, which are different from one another. In this study, seven brands of metformin were tested for in vitro dissolution, so it is hardly surprising that the dissolution rates varied.
A product lacking the necessary active pharmaceutical ingredient fails to produce the intended therapeutic effect, potentially resulting in treatment failure, increased morbidity, and even mortality for patients. An ultraviolet-visible (UV-VIS) spectrophotometer was used to measure the drug content in the selected brands of metformin hydrochloride tablet formulations. The percentage of the drug content for the selected products is shown in Table 6. It can have severe consequences on the health status of the patient when the concentration of metformin hydrochloride is higher or lower than the specification in the product monograph.14 Across all products, the assay results varied from 96.55% (Brand M7) to 102.76% (Brand M1), which meets USP acceptance criteria of 100 ± 5% per tablet. Comparatively, the study conducted in Nepal revealed that all six samples analyzed fell within 95% to 105% of the acceptable limit, while only one of the nine brands tested in Nigeria failed the percentage content assay test. Different brands comply with the BP/USP specifications, despite being manufactured by different companies. It is possible that weighing the active ingredient with accuracy, mixing the active ingredient effectively during granulation, and incorporating the active ingredient accurately during formulation contribute to this.40
Limitation of the Study
This quality control study has limitations. Using statistical tools to compare weight variation, assay, and dissolution profiles was not conducted, limiting the assessment of brand interchangeability. These limitations should be considered when interpreting the results. Further research is needed to address these limitations and gain a more comprehensive understanding of the pharmaceutical quality of metformin hydrochloride tablets in the market.
Conclusion
Various quality-control tests were conducted on seven generic brands of Metformin hydrochloride tablets circulating in Gondar City, including weight variation, friability, hardness, disintegration time, and assays. Test specifications for all selected products were found to comply with official quality control standards. The hardness test for the pharmaceutical quality of these products deviated considerably from the requirements. There is a considerable case for manufacturers to improve the mechanical strength of their tablets so that the risk of breakage during transportation and handling to consumers is reduced greatly. Drug regulatory authorities need to intensify post-market surveillance, and manufacturers may well be advised to increase post-market surveillance.
Data Sharing Statement
All relevant data can be obtained from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240–249. doi:10.1016/S0140-6736(08)61762-6
2. Butt MD, Ong SC, Wahab MU, et al. Cost of Illness Analysis of Type 2 Diabetes Mellitus: the Findings from a Lower-Middle Income Country. Int J Environ Res Public Health. 2022;19(19):12611. doi:10.3390/ijerph191912611
3. Witter S, Zou G, Diaconu K, et al. Opportunities and challenges for delivering non-communicable disease management and services in fragile and post-conflict settings: perceptions of policy-makers and health providers in Sierra Leone. Confl Health. 2020;14:1–14. doi:10.1186/s13031-019-0248-3
4. World Health Organization. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products; 2017.
5. Montori VM, Gafni A, Charles C. A shared treatment decision‐making approach between patients with chronic conditions and their clinicians: the case of diabetes. Health Expect. 2006;9(1):25–36. doi:10.1111/j.1369-7625.2006.00359.x
6. Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42(5):987–1000. doi:10.1002/hep.20920
7. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabet Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
8. Setter SM, Iltz JL, Thams J, Campbell RK. Metformin hydrochloride in the treatment of type 2 diabetes mellitus: a clinical review with a focus on dual therapy. Clin Ther. 2003;25(12):2991–3026.
9. Bosi E. Metformin–the gold standard in type 2 diabetes: what does the evidence tell us? Diabetes Obes Metab. 2009;11:3–8. doi:10.1111/j.1463-1326.2008.01031.x
10. Boldhane SP, Kuchekar BS. Gastroretentive drug delivery of metformin hydrochloride: formulation and in vitro evaluation using 32 full factorial design. Curr Drug Deliv. 2009;6(5):477–485. doi:10.2174/156720109789941641
11. Metry M, Shu Y, Abrahamsson B, et al. Biowaiver monographs for immediate release solid oral dosage forms: metformin hydrochloride. J Pharm Sci. 2021;110(4):1513–1526. doi:10.1016/j.xphs.2021.01.011
12. Kaufman B, Novack GD. Compliance issues in manufacturing of drugs. Ocul Surf. 2003;1(2):80–85. doi:10.1016/S1542-0124(12)70131-3
13. Mackey TK, Liang BA. The global counterfeit drug trade: patient safety and public health risks. J Pharm Sci. 2011;100(11):4571–4579. doi:10.1002/jps.22679
14. Caudron J, Ford N, Henkens M, Macé C, Kiddle‐Monroe R, Pinel J. Substandard medicines in resource‐poor settings: a problem that can no longer be ignored. Trop Med Int Health. 2008;13(8):1062–1072. doi:10.1111/j.1365-3156.2008.02106.x
15. Ozawa S, Evans DR, Bessias S, et al. Prevalence and estimated economic burden of substandard and falsified medicines in low-and middle-income countries: a systematic review and meta-analysis. JAMA Network Open. 2018;1(4):e181662–e181662. doi:10.1001/jamanetworkopen.2018.1662
16. Ajala T, Adebona A, Bamiro O. The pharmaceutical quality of brands of metformin tablets in Ogun-State, Nigeria. Afr J Biomed Res. 2014;17(1):43–48.
17. Sougi A, Ofori-Kwakye K, Kuntworbe N, Kipo S, Boakye-Gyasi M. Evaluation of the physicochemical and in vitro dissolution properties of metformin hydrochloride tablet brands marketed in five cities in Ghana. Br J Pharm Res. 2016;9(1):1–14. doi:10.9734/BJPR/2016/21862
18. Kariuki EM, Thoith GN. Quality of Metformin Tablet Products in the Kenyan Market. East Cent Afr J Pharm Sci. 2021;24(2):85–89.
19. Odeniran OA, Olayemi OJ, Galadima IH, Kirim RA, Isimi CY, Mustapha KB. Quality assessment of nine brands of metformin hydrochloride tablets marketed in Abuja, Nigeria. J Pharm Bioresour. 2021;18(3):216–222. doi:10.4314/jpb.v18i3.6
20. Eraga SO, Idemili CD, Iwuagwu MA. Pharmaceutical Quality Assessment of Brands of Metformin Hydrochloride Tablets Available in South-East Nigeria. Afr J Pharm Res Dev. 2020;12(3):333–341.
21. Arora A, Parle A, Dahiya M, Rani R. Comparative evaluation of Metformin tablets available under government supply and brands available in open market in Delhi, India. IOSR J Pharm. 2021;11(5):8–20.
22. Prithi IJ, Chowdhury SF, Chowdhury S. Comparative in vitro dissolution test and other physicochemical parameters of some commercially available metformin HCl brands in Bangladesh. Pharma Innov. 2018;7(6):5–8.
23. Tesfay K, Kahsay G, Dinda S. In Vitro Quality Evaluation of Metformin Hydrochloride Tablets Marketed in Western and North Western Tigray, Ethiopia. Austin J Anal Pharm Chem. 2019;6(2):1119.
24. Abushaheen MA, Fatani AJ, Alosaimi M, et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon. 2020;66(6):100971. doi:10.1016/j.disamonth.2020.100971
25. Marucheck A, Greis N, Mena C, Cai L. Product safety and security in the global supply chain: issues, challenges and research opportunities. J Oper Manag. 2011;29(7–8).
26. Newton PN, Lee SJ, Goodman C, et al. Guidelines for field surveys of the quality of medicines: a proposal. PLoS Med. 2009;6(3):e1000052. doi:10.1371/journal.pmed.1000052
27. Stationery Office. British Pharmacopoeia 2013: Published on the Recommendation of the Commission on Human Medicines Pursuant to the Medicines Act 1968 and Notified in Draft to the European Commission in Accordance with Directive 98/34/EEC. London: Stationery Office; 2012.
28. Hani U, Alhamhoom Y, Alqahtani A, et al. In-vitro Comparative Study of Different Brands of Metoclopramide Hydrochloride Tablets Marketed in Saudi Arabia. Curr Drug Ther. 2020;15(5):512–517. doi:10.2174/1574885515666200305101840
29. Hamdan II, Jaber AKB. Pharmaceutical evaluation of metformin HCl products available in the Jordanian market. Jordan J Pharm Sci. 2010;3(22):1–7.
30. Peck GE, Baley GJ, McCurdy VE, Banker GS. Tablet formulation and design. Pharm Dos Forms Marcel Dekker N Y. 1989;75–130.
31. Aulton ME. Pharmaceutics: the science of dosage form design. Royal Soc Chem. 2002.
32. Allen L, Ansel HC. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. Lippincott Williams & Wilkins; 2013.
33. Olusola AM, Adekoya AI, Olanrewaju OJ. Comparative evaluation of physicochemical properties of some commercially available brands of metformin HCl tablets in Lagos, Nigeria. J Appl Pharm Sci. 2012;41–44.
34. Osman Z, Osman A, Abedelghayoum A. Comparative evaluation of physicochemical properties of some commercially available brands of metformin HCl tablets marketed in Sudan. IAJPR. 2017;7:7471–7477.
35. Vandevivere L, Denduyver P, Portier C, et al. The effect of binder types on the breakage and drying behavior of granules in a semi-continuous fluid bed dryer after twin screw wet granulation. Int J Pharm. 2022;614:121449. doi:10.1016/j.ijpharm.2022.121449
36. Long M, Chen Y. Dissolution testing of solid products. Developing Solid Oral Dosage Forms Elsevier. 2009;319–340.
37. Kataoka M, Yano K, Hamatsu Y, Masaoka Y, Sakuma S, Yamashita S. Assessment of absorption potential of poorly water-soluble drugs by using the dissolution/permeation system. Eur J Pharm Biopharm. 2013;85(3):1317–1324. doi:10.1016/j.ejpb.2013.06.018
38. Elghnimi T, Bzezi W, Siaan M, Elgreew W, Benmansour H. Comparative in-vitro Evaluation of Some Commercial Brands of Metformin Tablets Marketed in Tripoli-Libya. Eur J Bio Pharm Sci. 2019;6(6):138–143.
39. Allen LV Jr. Dosage form design and development. Clin Ther. 2008;30(11):2102–2111. doi:10.1016/j.clinthera.2008.11.015
40. Kottke MJ, Rudnic EM. Tablet dosage forms. In: Modern Pharmaceutics, Fourth Edition Revised and Expanded. CRC Press; 1996:458–532.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
