Back to Journals » Veterinary Medicine: Research and Reports » Volume 15
Evaluating the Knowledge, Practice, and Regulatory Situation of Veterinary Experts Regarding Counterfeit Veterinary Medications in the Selected Districts of Central Gondar Zone, Ethiopia
Authors Tefera Mekasha Y , Nigussie S, Ashagre W, Getahun Feleke M , Wondie A, Mulaw A , Dessalegn B
Received 1 December 2023
Accepted for publication 22 March 2024
Published 4 April 2024 Volume 2024:15 Pages 91—108
DOI https://doi.org/10.2147/VMRR.S450560
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Yesuneh Tefera Mekasha,1 Sete Nigussie,2 Wegayehu Ashagre,3 Melaku Getahun Feleke,4 Abibo Wondie,5 Asnakew Mulaw,6 Bereket Dessalegn6
1Department of Veterinary Pharmacy, Pharmaceutical Quality Assurance, and Regulatory Affairs, University of Gondar, Gondar, Ethiopia; 2Department of Veterinary Pharmacy, University of Gondar, Gondar, Ethiopia; 3Doctor of Veterinary Medicine, University of Gondar, Gondar, Ethiopia; 4Department of Veterinary Pharmacy, Pharmaceutical Analysis, and Quality Assurance, University of Gondar, Gondar, Ethiopia; 5Department of Veterinary Pharmacy, Drug Supply Chain Management, University of Gondar, Gondar, Ethiopia; 6Department of Veterinary Pathobiology, University of Gondar, Gondar, Ethiopia
Correspondence: Yesuneh Tefera Mekasha, University of Gondar, Gondar, Amhara Regional State, Ethiopia, Email [email protected]
Background: The intentional and illegal misrepresentation of fake medications involves falsely indicating their source. These fraudulent medications can include products that contain either accurate or incorrect ingredients, lack proper labeling, have insufficient quantities of ingredients, and are packaged with counterfeit packaging. This unlawful activity has led to treatment failures, the development of antibiotic resistance, adverse effects, and even deaths. Hence, the objective of this study was to assess the knowledge, practice, and regulatory status of veterinary drug experts in the central Gondar zone of Ethiopia regarding counterfeit veterinary medications.
Methods: From January 2023 to July 2023, a self-administered structured questionnaire was utilized to conduct a cross-sectional study in Central Gondar Zone, Ethiopia. The analysis of the data involved the application of descriptive and chi-square tests.
Results: The study revealed that the majority of professionals possessed a work experience ranging from 5 to 9 years (23; 56.1%). Additionally, a total of 25 individuals (61.0%) who participated in the research had not undergone any kind of training. It was observed that a significant proportion of participants (82.9%) possessed knowledge about counterfeit veterinary drugs. Only about 63% and 36% of respondents had high knowledge and good practice concerning veterinary counterfeit drugs, respectively. Only 29.3% of participants have reported practices. Furthermore, a poor regulatory level of coordination was detected (85.4%). The study revealed a significant (χ 2 =7.6165; p = 0.022) disparity between the respondents’ practice levels and training. Respondents’ regulatory levels were also significantly associated (p < 0.05) with their sex (χ 2 = 13.34; p = 0.001) and work experience (χ 2 = 13.64; p = 0.033). The research findings also revealed a noteworthy correlation between practice and regulatory activity (χ 2 = 15.0463; p = 0.005).
Conclusion: The study outlines the necessity of awareness initiatives, with a focus on the significance of veterinary experts’ knowledge, practice, and regulatory efforts in addressing the issue of counterfeit veterinary medications.
Keywords: counterfeit veterinary drug, veterinary drug professionals, cross-sectional study, knowledge, practice, regulatory activity, central Gondar zone, Ethiopia
Introduction
Ethiopia has a huge livestock population and is ranked among the top ten countries in the world and the first country in Africa with an estimated population of 70 million cattle, 42.9 million goats, and 52.5 million sheep.1 Ethiopia’s livestock sector is of paramount importance to its agricultural industry, as it makes a substantial impact on the national economy and the well-being of countless Ethiopians. Additionally, it possesses immense potential to continue bolstering the country’s economic growth.2 Livestock diseases pose a major obstacle, especially for rural communities lacking access to proper veterinary services. They present an ongoing threat to both domestic animals and livestock used for food production, thereby impeding the growth of the livestock sector. This is particularly significant in Sub-Saharan African nations, where livestock development plays a crucial role in the socio-economic landscape.3 The prevalence of livestock diseases in Africa is alarmingly high, with over 90% of the diseases listed by the World Organization for Animal Health (OIE) affecting the continent. Consequently, the use of veterinary drugs has become imperative to combat these diseases. Nevertheless, it is crucial to acknowledge that chemoprevention and chemotherapy, although effective in controlling animal diseases, come with inherent risks for the animals undergoing treatment.4
Medications are utilized for the purpose of healing or managing illnesses, alleviating symptoms, alleviating pain, preventing illnesses or symptoms, reducing or eliminating symptoms, and slowing down the progression of diseases.5 In order to ensure the therapeutic significance, medications must possess attributes of safety, efficacy, and acceptable quality, and should be administered judiciously to achieve the intended results with favorable clinical and therapeutic consequences. In order to guarantee the delivery of effective animal health services, it is crucial to maintain a steady and ample supply of veterinary medications that are safe, efficient, and of top-notch quality.6 Nevertheless, the pharmaceutical industry faced significant challenges in terms of safety, quality, and effectiveness due to the widespread distribution of counterfeit products. Among all criminal activities, the act of drug counterfeiting poses a grave threat to public health, as it is a deliberate and highly profitable endeavor.7,8 There exist two classifications of inferior medications: substandard drugs and counterfeit drugs. Substandard drugs are legitimate medications that do not adhere to quality standards. Conversely, counterfeit drugs are deliberately misrepresented in terms of their origin and/or identity. These fraudulent medications may contain accurate or incorrect ingredients, lack active ingredients, have insufficient or inadequate ingredient quantities, or be packaged with fake labeling.9
Counterfeit pharmaceuticals, as per the World Health Organization (WHO) definition, refer to pharmaceutical products that are intentionally mislabeled in terms of their origin and identity. In essence, these deceptive medications are produced with inferior quality, safety, and effectiveness when compared to the established standards. Based on reports received by WHO from 20 different countries, the majority of falsified drugs can be categorized into three groups: (1) approximately 30% of the products contain no active ingredient, (2) around 20% of the products contain an incorrect quantity of the active ingredient, and (3) about 20% of the products contain incorrect contents.10 Livestock production sector is continuously affected by several challenges, from these, providing treatments of animals with poor quality (counterfeit) drugs are the major one. This challenge may give rise to various issues, including higher animal mortality rates, drug resistance, diminished trust in the healthcare system, increased burden on healthcare professionals and facilities like clinics and pharmacies in their efforts to regain trust, toxicity, organ damage, economic losses, and potential fatalities.11 Undesirably, the dangers associated with unlawful veterinary medications extend beyond the mere absence of effectiveness and safety for animals receiving these products. They also pose a risk to human safety through the consumption of food derived from animals treated with such medications. Moreover, the inadequate control of zoonotic infections and the potential escalation of antimicrobial and antiparasitic resistance further contribute to the risks involved.11
Combating the worldwide spread of counterfeit medications will prove to be a challenging task, requiring a comprehensive strategy.12 The various countries employ different approaches, which vary in their effectiveness, to address the issue at hand. These approaches primarily rely on the regulatory status and level of expertise within each country. However, it is important to note that the African Region faces significant challenges due to the presence of weak medicines regulatory authorities and the widespread availability of illicit medicines.13 In Ethiopia, the issue of falsified medicines poses a significant challenge to professional awareness, which has the potential to escalate into a public health crisis.14 Information regarding the knowledge and practice of veterinary professionals toward counterfeit drugs as well as their regulatory status has not been assessed yet. As evidence, the study conducted on the identification, assay, and organoleptic quality of veterinary medicines in Ethiopia showed that more veterinary drug products were under surveillance, which necessitates extensive surveillance on knowledge, practice, and the collaboration of regulatory bodies with different stakeholders for combating such pharmaceutical defects.15
All of the mentioned counterfeit issues are reduced if skilled veterinary drug professionals and the veterinary drug regulatory body work in collaboration in the pharmaceutical environment. Consequently, the objective of this study was to evaluate the knowledge, practices, and regulatory status of veterinary drug experts concerning counterfeit veterinary drugs. This was achieved by addressing four research inquiries: (1) Do veterinary drug professionals working in the selected North Gondar zone districts possess adequate knowledge about counterfeit veterinary drugs? (2) To what extent do veterinary drug professionals rely on their experience to distinguish between authentic and counterfeit veterinary drugs? (3) Does the veterinary drug regulatory body actively participate in the regulation of counterfeit veterinary drugs? (4) Is there effective collaboration between the veterinary drug regulatory body and veterinary drug professionals in combating counterfeit veterinary drugs?
Materials and Methods
Study Setting
From January 2023 to July 2023, a research was carried out among veterinary drug experts employed in the central Gondar zone. Gondar, situated in the northern part of Ethiopia within the Amhara regional state, is positioned 725 km away from Addis Ababa, the federal government’s capital city, and 175 km away from Bahir-Dar, the capital city of Amhara National Regional State. The population of Gondar amounted to 500,788 individuals, with 300,000 being men and 200,788 being women.16
Various institutions offer animal health services, such as private pharmacies, government clinics, and government pharmacies. The livestock population in the region consists of 8202 cattle, 22,590 goats, 2695 sheep, 1065 horses, and 9001 donkeys.17 These animals can offer diverse benefits, including supplying traction power, yielding meat and milk, and enabling the movement of both goods and individuals across different places.
Study Design
From January 2023 to July 2023 G.C., a comprehensive study was undertaken to assess the knowledge, practice, and regulatory compliance pertaining to counterfeit veterinary drugs among veterinary drug experts employed within the selected Central Gondar zone District.
Sources, and Study Populations
All veterinary drug professionals working in the chosen Central Gondar Zone Districts were considered as the sources of population. The veterinary drug professionals employed in the designated regions of the Central Gondar zone districts, including Gondar town, Azezo, Maksegnite, Kola Diba, and Tseda, constituted the study populations.
Sample Size and Sampling Method
Census sampling methods were employed to select the study sites, taking into account the number of animal health service providers, the presence of private drug outlets, and the size of the livestock population. The purposeful sampling technique was then used to select a veterinary drug professional working in the animal health sector and veterinary drug outlets, as they were expected to possess more comprehensive information compared to other health professionals.
Inclusion and Exclusion Criteria
Inclusion Criteria
The study encompassed veterinary drug experts employed in various positions within central Gondar, including private veterinary pharmacies, governmental veterinary clinics, governmental veterinary pharmacies, and other relevant roles. These professionals possessed fundamental knowledge pertaining to veterinary drug information. The study also incorporated voluntary veterinary drug professionals who participated in the data collection process.
Exclusion Criteria
Veterinary drug professional declined to take part in the data collection process and was therefore excluded from the study. Furthermore, individuals who were not veterinary drug professionals were also not included in the study.
Study Variables
Independent Variables
The independent variables in this study encompassed various socio-demographic characteristics of the study population, such as age, gender, occupational status, educational attainment, work experience, and training.
Dependent Variables
The knowledge, practice and regulatory status of participants towards counterfeit veterinary drugs were grouped under dependent variables.
Data Collection Methods
Structured questionnaires were used to collect data, with participants completing them on their own. The questionnaires were prepared in English and focused on gathering information about the basic demographic characteristics of the study participants. Additionally, they aimed to assess the knowledge, practice, and regulatory status of veterinary drug professionals regarding counterfeit veterinary drugs. The questionnaire consisted of four parts, primarily consisting of closed-ended questions. These parts covered the socio-demographic characteristics of the study population, as well as their knowledge, practice, and regulatory activities related to counterfeit drugs. The study questionnaire was created using items that had been previously published in the literature.5,11,18–21
Measurement Tools
Self-administered surveys were created to evaluate the knowledge, behavior, and regulatory engagement of residents regarding veterinary practices in the Central Gondar zone, Ethiopia.
Demographic Information
This section consisted of a total of seven questions. The questions covered various aspects such as age, gender, marital status, job position, educational background, work experience, and training in veterinary drug professions.
Assessing Knowledge
The questionnaire’s knowledge section consisted of eight questions. Out of these, seven questions were close-ended, comprising five yes/no questions and two multiple-choice questions. Additionally, an open-ended question was included in this section. The cumulative score of the knowledge questions was used to assess the respondent’s understanding of counterfeit veterinary drugs. The highest possible score for knowledge was 23, while the lowest possible score was 13. Bloom cut-off value22 for knowledge level: - high knowledge level, >80%,19–23 Medium knowledge level, 60–80%,14–18 and low knowledge level, <60%.13 One of the 8 questions was open- ended and was analyzed using descriptive statistics.
Assessing Practice
The practice part of the questionnaire included eight questions. All of questions were close ended, which include seven yes/no and one multiple-choice questions were encompassed in this part. Thus, the maximum attainable score for practice was 19 while the minimum attainable score was 9. Cut-off value22 for practice levels followed: good practice level, >80%,16–19 medium practice level, 60–80%,11–15 and poor practice level, <60%.9,10,23 The participants were categorized into poor, moderate, and good practice groups by dividing the three scores into three categories using the visual binning option in SPSS software.
Assessing Regulatory Activity
The regulatory activity part of the questionnaire included six questions. All of questions were close ended, which include five yes/no and one multiple-choice questions were encompassed in this part. Thus, the maximum attainable score for regulatory status was 16 while the minimum attainable score was 8. Cut-off value for regulatory activity was also based on Bloom cut-off value as followed: strong regulatory coordination, >80%,12–16 medium regulatory coordination, 60–80%,10,11 and poor regulatory coordination, <60%.8,9,22 The determination of regulatory activity was used to estimate the collaboration of regulatory bodies with veterinary drug professionals in the prevention of counterfeit veterinary pharmaceutical distribution.
Data Quality Assurance
The study questionnaires were created after reviewing various literature sources and a veterinary drug regulatory guideline that was published in English. To ensure the data’s quality, several strategies were implemented. Initially, the data was coded and checked for accuracy, consistency, and omissions. It was then prepared for analysis using validated data collection forms from the selected study areas. Before collecting the data, a data collector underwent two-day training on the data collection instruments and processes. A pilot study was conducted to pre-test the research instrument, which involved distributing the questionnaires to thirteen veterinary drug professionals working in the selected study area. The reliability test analysis, specifically Cronbach’s alpha analysis, was utilized to assess the instrument’s internal consistency. The result of 0.712 indicated an acceptable level of internal consistency. The findings from the pilot study were used to make necessary edits to the questionnaire, improving its clarity and flow. Finally, the investigators double-checked the quality of the data to ensure its accuracy.
Data Analysis
The data collected underwent coding, entry, error editing, and analysis through the utilization of Statistical Package for Social Sciences (SPSS) Version 26 (IBM Corporation, Armonk, NY, USA). Descriptive statistics were generated, along with the usage of graphs, tables, and numerical summaries to present the results. The chi-square test was utilized to ascertain the correlation between independent variables (Socio-demographic characteristics) and outcome variables (knowledge, practice, and regulatory activity) (p<0.05). Additionally, the connections between participants’ knowledge level and their practice, knowledge level and regulatory activity, as well as practice level and regulatory activity, were examined to determine the presence of associations in the identification and understanding of counterfeit veterinary drugs in pharmaceutical markets.
Results
The Socio-Demographic Profile of Study Participants
A total of 41 individuals were enrolled in this research. Among the 41 professionals involved, 61.0% (25 individuals) fell within the age range of 26 to 36, whereas 19.5% were aged between 37 and 47. The male gender constituted the majority of study participants, accounting for 80.5% (33 individuals).
Most animal healthcare professionals worked in government clinics 26(63.45%) followed by private veterinary pharmacies (12; 29.3%). The least number of professionals worked in Government pharmacy (3; 7.3%). Majority of respondent educational level was; level-4, (15; 36.6%) and DVM (14; 34.1%), also (6; 14.6%) and (2; 9.4%) were MSc and others respectively. Regarding work experience, the majorities of the respondents were in between 5 and 9 years (23; 56.1%) and followed by above 10 years (10; 24.4%). Majority of the study participants (61.0%) had never taken any training about counterfeit veterinary drugs (Table 1).
 |
Table 1 The Socio-Demographic Study Participants Profile in the Study Area |
Knowledge of Study Participants Towards Counterfeits Veterinary Drug
The majority of the study participants (82.9%) had information about counterfeit veterinary drugs. According to the participant’s responses, the major source of information about counterfeit medicine is education (56.1%), followed by friends (19.5%) and others (14.6%). Most of the respondents (70.7%) believed that there was a method for detecting counterfeit drugs. Almost half of the study participants (56.1%) responded, as there was a penalty for supplying veterinary drugs without a manufacturing or entrance license. Most of the participants (82.9%) believed counterfeit drugs affected the quality of the drugs. Also, the majority of respondents (70.7%) believed that there were factors escalating the distribution of counterfeit drugs. The majority of study participants correctly answered (58.5%) what counterfeit medicines are. As per the responses gathered from the study participants, the vast majority (80.5%) were of the opinion that the dangers associated with counterfeit drugs encompassed treatment failure, increased side effects, safety and efficacy issues, higher treatment costs, and economic difficulties (Table 2).
 |
Table 2 Knowledge of Study Participants Towards Veterinary Drug Counterfeits |
The understanding of the elements boosting the spread of counterfeit veterinary pharmaceuticals, as well as the definition of counterfeit medications, varied. Aside from that, although they work in veterinary medication stores, some responders do not know what is meant by a counterfeit drug. Some of responses forwarded from study participants as follows:-
Respondent A said; “factors catalyzing the distribution of veterinary counterfeit drug was trade for lucrative purpose, and weak veterinary drug control policy as well as weak national regulatory policies on the manufacturing and marketing of medications. Counterfeit drugs mean drugs that are not effective in the disease management”. Additionally, the respondents don’t know authorized or licensed veterinary pharmaceutical importer and wholesaler.
Respondent B mentioned that; “illegal trade was without licensed was the most factor contributing the distribution of veterinary drugs across the pharmaceutical market”.
Respondent C said that; “factor contributing for distribution of counterfeit veterinary drug was to be economical benefit by using cheap raw material without active ingredients, and lack of robust veterinary drug regulatory activity”.
Respondent D also mentionded that; “lack of coordination between regulatory body, and licensed veterinary drug professionals may contribute in the distribution of veterinary drugs. In addition to this, defined the counterfeit drugs as; Counterfeit medicines are products that deliberately or fraudulently misrepresent their identity, composition or source”.
In this study, the respondents’ knowledge level was assessed using specific tools designed for knowledge measurement. These tools categorized the knowledge level into higher, medium, and low levels, specifically in relation to counterfeit veterinary drugs. Accordingly, out of 41 respondents, the majority (63.4%) had higher knowledge, followed by medium knowledge (31.7%), and about 4.9% of respondents had the lowest knowledge towards veterinary counterfeit drugs, with 13, 23, 19.2, and 2.4 of the minimum, maximum, mean, and SD values, respectively, Figure 1.
 |
Figure 1 Knowledge distribution level of respondents to ward counterfeit medicine. |
The Practice of Study Participants Towards Veterinary Drug Counterfeits
In this particular study, a group of 41 individuals took part. The findings revealed that a mere 36.6% of these participants were confident in their ability to distinguish between counterfeit drugs and authentic medicine. Moreover, a majority of the respondents, amounting to 68.3%, expressed their reluctance in distributing drugs that had been illegally introduced into the country. Regarding the identification of counterfeit medicines, the participants primarily relied on physical observation, which accounted for 26.8% of their chosen method, as well as unexpected side effects and a lack of effectiveness. Approximately 31.7% of the individuals had undergone training on this subject, although the majority had never received any form of instruction relating to the identification of counterfeit drugs or how to handle such issues. It is important to highlight that 51.2% of the respondents reported the presence of counterfeit drugs within their drug outlets to the relevant drug regulatory authority.
Approximately 68.3% of the individuals involved in the study refrained from participating in any economic transactions with drug wholesalers and suppliers who lacked a valid manufacturing license or distribution permit from the health ministry. As per the respondent’s feedback, 63.4% of these individuals consistently verify the batch number of the medicines they receive to ensure its alignment with the information provided in the purchasing invoices (Table 3).
 |
Table 3 Practice of Study Participants Towards Veterinary Drug Counterfeits |
The study area’s veterinary professional practice experienced evaluation through practice-related questions, resulting in the classification of practice into three levels: poor, fair, and good. The average practice score among all participants was 14.5, with a standard deviation of 2.5. Among the respondents, 36.5% exhibited good practice, 44% had fair practice, and 19.5% had poor practice. The range of practice scores varied from 9 to 19, as shown in Figure 2.
 |
Figure 2 Practice distribution level of respondents to ward counterfeit medicine. |
The Regulatory Activities Towards Veterinary Drug Counterfeits
The majority of the study participants (82.9%) had not received any training from regulatory bodies regarding counterfeit drugs. Furthermore, a significant number of respondents (61.0%) stated that there is no specific regulation of drugs by the regulatory body. Moreover, only 36.6% of respondents were familiar with authorized or licensed pharmaceutical importers and wholesalers. In addition, approximately 65.9% of the study participants were unaware of a regulatory body that authorizes or licenses veterinary drug professionals. The majority of the participants (90.2%) believe that the main factors contributing to the presence of illegal drug sources were a lack of control in the informal market, inadequate control at entry ports, and the profitability of illegal products. Only 29.3% of the participants were reported incidents related to counterfeit drugs to regulatory bodies (Table 4).
 |
Table 4 Regulatory Activities Towards Veterinary Drug Counterfeits |
The study noted as there was no strong connection between the regulating agency and the professionals, the majority of respondents “said that despite the existence of counterfeit pharmaceuticals on the market, there was no reporting procedure”. “They lack trust in their ability to identify fake pharmaceuticals, in addition to that, there is no any guidance on the counterfeit products”.
A study conducted revealed that there was a significant lack of coordination in regulating counterfeit veterinary drugs, with 85.4% of respondents indicating a poor level of coordination. On the other hand, a moderate level of coordination was reported by only 2.4% of the participants. The average regulatory activity score for all respondents was 10.7 out of a total of 16 points, with a standard deviation of 1.5. The regulatory status scores ranged from a minimum of 8 to a maximum of 16 (Figure 3). This suggests that there is a lack of effective regulatory measures in the study area, necessitating immediate regional oversight. Merely 12.2% of the robust regulatory efforts involved cooperation between regulatory bodies and veterinary drug experts in order to address the issue of counterfeit veterinary drugs circulating in the market (Figure 3).
 |
Figure 3 Regulatory distribution level of respondents to ward counterfeit medicine. |
Socio-Demographic Variables Associated with the Knowledge of the Veterinary Drug Professionals Toward Counterfeit Veterinary Drugs
The association between the respondents’ knowledge of counterfeit veterinary drugs and their socio-demographic variables, such as age, gender, work position, education level, work experience, and training on counterfeit veterinary drugs, were examined. The results indicated that there was no significant association (p > 0.05) between the socio-demographic characteristics of the participants and their level of knowledge regarding counterfeit veterinary drugs (Table 5).
 |
Table 5 Association of Knowledge with Socio Demographic Characteristics |
Socio-Demographic Variables Associated with the Practice of the Veterinary Drug Professionals Toward Counterfeit Veterinary Drugs
The respondents’ level of practice showed a significant association with their training, as evidenced by a chi-square value of 7.6165 (p=0.022) (Table 6) while age, sex, marital status, work position, educational level, and work experience were not associated with the level of practice of the respondents toward counterfeit veterinary medicines (P>0.05). Therefore, training was very important in combating the counterfeit veterinary drug distribution in the study area.
 |
Table 6 Association of Practice with Socio Demographic Characteristics |
Socio-Demographic Variables Associated with the Regulatory Activity of the Veterinary Drug Professionals Toward Counterfeit Veterinary Drugs
The successful oversight of veterinary medications necessitates robust regulatory coordination across all regulatory tiers. According to the research findings, the regulatory engagement of the participants were notably linked to gender (χ2=13.3472, P=0.001), marital status (χ2=12.9638, p=0.044), and professional experience (χ2=13.6788, p=0.033) (Table 7). This suggests that, for the successful implementation of regulatory issue in the combating veterinary drugs; qualified, and experience human power, and diversified working environments by far needed in the regulatory environments.
 |
Table 7 Association of Regulatory with Socio Demographic Characteristics |
Association Between Veterinary Professions Knowledge, Practice and Regulatory Activity on Counterfeit Medicine
A statistically significant difference (χ2=15.0463, p=0.005) was observed in the chi-square test of association between the practice and regulatory activity of veterinary professions regarding veterinary counterfeit medicine. However, no association was found between knowledge and practice (χ2=4.7220, p=0.317) or between knowledge and regulatory activity (χ2=0.9912, p=0.911) (Table 8–10).
 |
Table 8 Association Between Knowledge and Practice Level |
 |
Table 9 Association Between Knowledge and Regulatory Level |
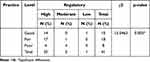 |
Table 10 Association Between Practice and Regulatory Level |
The findings presented in Table 10 indicate that there was a statistically significant correlation between practice and the level of regulatory activity towards counterfeit veterinary drugs among veterinary drug professionals (p=0.005). This suggests that as the level of regulatory activity on counterfeit veterinary drugs increases, the proportion of respondents with good practice also increases among the study participants.
Discussion
The livestock production sector is continuously affected by several challenges, from these, providing treatment of animals with counterfeit drugs is the major one and this challenge can lead to several problems, such as increased mortality of animals, drug resistance, and reduced con.24 Controlling the worldwide counterfeit drug epidemic will prove to be a challenging task, requiring a comprehensive approach. The strategies employed by various countries differ significantly, primarily relying on their regulatory framework and the expertise of their professionals.25
In this investigation, out of the 41 professionals examined, 25 (61.0%) were aged between 26 and 36, while the remaining 16 (39.0%) were between 37 and 47. In terms of professional experience, the majority of respondents had 5–9 years of experience, making up 23 (56.1%), followed by those with over 10 years of experience, totaling 10 (24.4%). A significant portion of the participants (61.0%) had not received any training on counterfeit veterinary drugs. The lack of awareness among professionals in Ethiopia concerning counterfeit medicines is a critical issue that could potentially lead to a public health emergency.11,26 The professionals’ knowledge and practice about drug qualities were found to be less. Also, information regarding the knowledge, practice, and level of regulatory body coordination with veterinary professionals toward counterfeit drugs was poor.
The knowledge, practices and regulatory status concerning counterfeit drug are critical for combating global antimicrobial resistance, treatment failure, mortality of animals and loss confidence in the health system.11 The results derived from this investigation indicated that 63.4% of the individuals possessed adequate knowledge, 82.9% were aware of counterfeit veterinary drugs, and a majority of the participants (58.5%) accurately described counterfeit medications. Additionally, the present study revealed that education (56.1%) was the primary source of information regarding counterfeit medicine, followed by friends (19.5%) and other channels (14.6%). In contrast, Bashir et al (2020) noted that in Egypt, 84.6% of individuals acquired their awareness and skill to identify counterfeit medicines through personal encounters.21 In this study, deficiency of skills to identify counterfeit medications from the genuine medicine (36.6%) was detected. In line with this study, a similar study reported by El-Dahiyat et al (2021) in United Arab Emirates revealed, only less than one -third of the participants were identified as counterfeit medications from the genuine medicine.27 In Ethiopia, veterinary drug regulatory experts face a significant skills gap in veterinary drug regulatory activity and quality control. A study conducted by Zeru Hailu reveals that experts lack the ability to identify illegal veterinary drugs and have never attempted to distinguish between legal and illegal drugs in the market. Furthermore, they fail to mention the importance of physicochemical analysis methods and the identification of labeling and packaging defects as crucial aspects of product quality control.9
The current investigation further revealed that approximately 26.8% of participants recognized counterfeit veterinary drugs through visual inspection, unexpected adverse reactions, and ineffectiveness. Conversely, a study conducted by Sholy et al (2018) in Lebanon reported that 67.7% of respondents in their study believed that the medicinal impact of counterfeit veterinary drugs can serve as an identifying factor.18 1. Additionally, a different research study conducted by Noun (2021) in Lebanon highlighted the occurrence of negative outcomes as a sign of the utilization of fraudulent drugs. Conversely, a study carried out by Bashir et al (2020) in Egypt revealed that 50% of the participants were able to spot counterfeit medications through personal inspection, while 22.9% identified them based on customer complaints.21 According to the study, the majority of the 41 respondents (63.4%) possessed a higher level of knowledge, while a significant portion (31.7%) had medium knowledge. This suggests that understanding regarding counterfeit drugs is a prevalent concern in the study area. Additionally, the study revealed that approximately 4.9% of respondents had weak knowledge. The presence of inadequate knowledge about counterfeit products in general appears to be a widespread barrier hindering endeavors to combat drug counterfeiting, even in developed nations. Consequently, addressing this issue becomes imperative.28
In developing countries, it is a prevalent practice to engage in the illegal distribution of specific drugs and services, as well as facing challenges in meeting quality standards and obtaining official registration.29 In the current study, the majority of participants (68.3%) indicated their reluctance to distribute a drug if it was unlawfully brought into the country. This discovery contradicts the research conducted by Shahverdi et al (2012) in Iran, where a small percentage of participants (14.5%) were unable to approve the distribution of a drug if it entered the country illegally.20 Around 63.4% of the participants in the study consistently cross-checked the batch number of the medications they received with the one listed in the purchase invoice. This finding is in line with a previous study by Adigwe et al (2022), which found that approximately 92.4% of healthcare professionals also verified the batch number of the medications they received.30 Nevertheless, the research carried out in Egypt revealed that the majority of participants (84.6%) did not consistently verify the batch number of the medications they received with the one mentioned in the purchase invoices.21 The research also aims to evaluate the extent of veterinary professional practice in the study area concerning counterfeit veterinary medications. The findings of the study revealed that 36.5% of the participants demonstrated a commendable level of practice, while 44% exhibited a fair level of practice. On the other hand, 19.5% displayed a poor level of practice. Existing literature suggests that in order to effectively identify substandard or falsified veterinary products, veterinarians should possess knowledge about the specific products that are likely to be targeted. Substandard, falsified, or unregistered/unlicensed veterinary products pose a significant threat to the health and well-being of both animals and humans.28,31 This indicates that further training should be needed to improve the level of practice so as to reduce the circulation of counterfeit veterinary drugs.
Fraudsters are greedy offenders who seek financial gain while facing minimal consequences within the law.20 In this study, 70.7% of respondents did not report about counterfeited drugs to regulatory body. Similar to this 76.6% of participant in Egypt21 and 97.5% of participant in United Arab Emirates did not report about counterfeited drugs to regulatory body. In addition to that, less than half (43.9%) of the study participants were able to tells, there was no penalty for supplying products without manufacture or entrance license.27 This is deviated from the study conducted in Iran by Shahverdi et al (2012) majority of study participants (81.9%) were able to tell, there was no Penalty for supplying products without manufacture or entrance license.20
The study also assessed the level of coordination between regulatory body (experts) and veterinary drug professionals, which found that poor regulatory level of coordination was detected (85.4%) while the moderate level of coordination was of 2.4% in combating counterfeit veterinary drugs. This implies that, there is a weak regulatory activity in the study area which needs urgent regional supervision. Only 12.2% strong regulatory activity was done by collaboration of regulatory body and veterinary drug professionals works for combating the circulation of the counterfeit veterinary drugs. As the study showed that the horizontal communication and coordination of the regional regulatory bodies of Ethiopia are very poor.9 This finding suggests the establishment of mechanism that enabling veterinary drug professionals and community to report illegal and/or defective products to the regional regulatory bodies through strong collaboration with regulatory bodies of the country so far important.
The Chi-square test was utilized to assess the relationship between independent variables (Socio-demographic characteristics) and outcome variables (knowledge, practice, and regulatory activity) (p<0.05). Additionally, the correlation between participants’ level of knowledge and their level of practice, level of knowledge and their level of regulatory activity, and level of practice and their level of regulatory activity were examined to determine if there was an association between them. The respondents’ practice level was significantly linked to their training. As one’s level of training increased, counterfeit practice scores tended to increase as well. Furthermore, the regulatory activity of the study participants was significantly associated with their sex, marital status, and work experience. Participants with more than 5 years of work experience had higher regulatory activity scores compared to those with less than 5 years of work experience. There was also a statistically significant difference between practice and regulatory activity, but no association was found between knowledge and practice, as well as between knowledge and regulatory activity.
The recent study highlighted a significant finding which pointed out a lack of synchronization between veterinary drug experts and regulatory organizations. Furthermore, survey participants demonstrated subpar performance in handling counterfeit medications, despite possessing extensive knowledge in this area. These results underscore the essential requirement to improve communication between the veterinary drug industry and regulatory bodies, along with encouraging the reporting of suspicions and thwarting the entry of fake pharmaceuticals into the drug distribution network.32 In addition, there is a significant need for training for professionals on counterfeit veterinary drugs.
Conclusion
The study attempts to identify the knowledge, practice, and collaboration of veterinary drug professionals with the veterinary drug regulatory body towards counterfeit veterinary pharmaceutical products. It is the first in history; no study yet examines the knowledge, practice, and regulatory activity of Ethiopia’s veterinary drug profession towards counterfeit veterinary drugs. A significant portion of veterinary drug experts possessed knowledge regarding counterfeit veterinary drugs. However, only a limited number of participants in the study were capable of distinguishing between authentic and counterfeit medications. The research also uncovered a lack of collaboration between veterinary drug professionals and regulatory organizations in their efforts to combat counterfeit veterinary drugs. The correlation between the professional training level and the attitudes of veterinary drug practitioners towards counterfeit medicines was deemed significant. This research underscores the crucial need to increase awareness among veterinary drug professionals. Additionally, it stresses the importance of drug regulatory bodies and relevant organizations engaging in the creation of effective programs and policies to improve the expertise, practices, and regulatory measures of veterinary professionals concerning counterfeit veterinary drugs.
Therefore, it is crucial to further empower practitioners by enabling them to identify counterfeit drugs through simple observations and providing training on visual inspection. This represents a major step towards discouraging the marketing of counterfeit veterinary drugs.
Data Sharing Statement
The article contains all the important discoveries from the study. Should readers require more details, the corresponding author is available to address their inquiries.
Ethics Approval and Consent to Participate
The study has no ethical or moral issues with the responses; thus, the University of Gondar College of Veterinary Medicine and Animal Sciences ethical review committee saw the project before it was implemented and authorized it under minute reference number CVMAS ERC/018/2023. Since every study participant has read and comprehended the questions that have been given to them, written consent was generated and self-administered. The study’s goal was widely acknowledged and supported by the participants.
Acknowledgments
We express our gratitude to our data collectors. We also convey our sincere appreciation to veterinary health care professionals for their invaluable assistance, cooperation, and provision of essential information during the data collection process.
Author Contributions
All authors have played a substantial role in the research project’s conceptualization, design, execution, data acquisition, analysis, and interpretation. They have also contributed to the drafting, revising, and critical review of the article. Furthermore, they have given their final approval for the publication of the article and have agreed on the journal to which it has been submitted, and they accept responsibility for all aspects of the work.
Funding
Not applicable.
Disclosure
All authors declare no competing interests in this work.
References
1. Central StatisticL Agency. Ethiopia’s Livestock Systems: overview and Areas of Inquiry; 2021.
2. Asresie A, Zemedu L, Adigrat E The contribution of livestock sector in Ethiopian economy. Advances in Life Science and Technology www.iiste.org. ISSN 2224-7181 (Paper) ISSN 2225-062X (Online) Vol.29; 2015.
3. Mouiche MMM, Njingou BZN, Moffo F, Mpouam SE, Feussom JMK, Awah-Ndukum J. Veterinary pharmacovigilance in sub-Sahara Africa context: a pilot study of adverse reactions to veterinary medicine in Cameroon. BMC Vet Res. 2019;15(1):1–8. doi:10.1186/s12917-019-2043-1
4. Brioudes V. Résultats de la conférence continentale de l’OIE sur les produits et médicaments vétérinaires en Afrique. In: Séminaire de formation Points focaux nationaux OIE pour les Produits Vétérinaires. Johannesburg, 8(3), p. 213–217; 2010.
5. Buowari V. Fake and counterfeit drug: a review. Afrimedic J. 2012;3(2):1–4.
6. Loosli K, Davis A, Muwonge A, Lembo T, Selvapandiyan A. Addressing antimicrobial resistance by improving access and quality of care—a review of the literature from East Africa. PLoS Negl Trop Dis. 2021;15(7):e0009529. doi:10.1371/journal.pntd.0009529
7. Buckley GJ, Gostin LO Countering the problem of falsified and substandard drugs. 2013.
8. Davison M. Pharmaceutical Anti-Counterfeiting: Combating the Real Danger from Fake Drugs. John Wiley & Sons; 2011.
9. Zeru H. Assessment of veterinary drugs regulatory framework in Ethiopia using focus groups. Int J Community Med Public Health. 2020;3(1):123–134.
10. Ubajaka C, Obi-Okaro A, Emelumadu O, Azumarah M, Ukegbu A, Ilikannu S. Factors associated with drug counterfeit in Nigeria: a twelve year review. Br J Med Med Res. 2016;12(4):1–8. doi:10.9734/BJMMR/2016/21342
11. Siraj J, Gebre A, Shafi M, Birhan A, Ejeta F, Hambisa S. Health care providers’ knowledge, attitude and practice toward counterfeit medicines in Mizan-Tepi university teaching hospital, South west Ethiopia: a cross-sectional study. Inquiry. 2022;59:00469580221108335. doi:10.1177/00469580221108335
12. Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med. 2005;2(4):e100. doi:10.1371/journal.pmed.0020100
13. World Animal Health. Counterfeit Medical Products: Report by the Secretariat. Geneva: World Health Organization; 2010.
14. Seifu A, Kebede E, Bacha B, Melaku A, Setegn T. Quality of albendazole tablets legally circulating in the pharmaceutical market of Addis Ababa, Ethiopia: physicochemical evaluation. BMC Pharmacol Toxicol. 2019;20(1):1–7. doi:10.1186/s40360-019-0299-5
15. Tefera B, Bacha B, Belew S, et al. Study on identification, assay and organoleptic quality of veterinary medicines in Ethiopia. J Pharm Polic Pract. 2022;15(1):17. doi:10.1186/s40545-022-00410-6
16. Central Statistical Agency. Central statistical agency of the federal democratic republic of Ethiopia: agricultural samples enumeration statistical abstract. Addis Ababa; 2019.
17. Teshome D. Prevalence of major skin diseases in ruminants and its associated risk factors at University of Gondar Veterinary Clinic, North West Ethiopia. J Res Dev. 2016;4(1):1–7.
18. Sholy L, Gard P, Williams S, MacAdam A, Saliba C. Public and pharmacist perceptions towards counterfeit medicine in Lebanon using focus groups. Int J Community Med Public Health. 2018;5(2):489–499. doi:10.18203/2394-6040.ijcmph20180224
19. Przyswa E. Counterfeit medicines and criminal organisations: IRCAM; [Research Report] IRCAM study report IRCAM. 129 p. ⟨hal-00958233⟩; 2013. Available from: http://www.iracm.com/.
20. Shahverdi S, Hajimiri M, Pourmalek F, et al. Iranian pharmacists’ knowledge, attitude and practice regarding counterfeit drugs. Iran J Pharm Res. 2012;11(3):963.
21. Bashir A, Galal S, Ramadan A, Wahdan A, El-Khordagui L. Community pharmacists’ perceptions, awareness and practices regarding counterfeit medicines: a cross-sectional survey in Alexandria, Egypt. East Mediterr Health J. 2020;26(5):556–564. doi:10.26719/emhj.19.058
22. Bloom S. Bloom -Taxonomy of Educational Objectives, Handbook 1- Cognitive. Domain-Addison Wesley Publishing Company; 1956:1.
23. Lartey PA, Graham AE, Lukulay PH, Ndomondo-Sigonda M. Pharmaceutical sector development in Africa: progress to date. Pharm Med. 2018;32(1):1–11. doi:10.1007/s40290-018-0220-3
24. Health for Animals. Illegal Veterinary Medicines Impact and Effective Control. Report, 5, 123–127; 2017.
25. Liu R, Lundin S. Falsified medicines: literature review. Work Papers Med Human. 2016;2:1.
26. Duguma B, Abera B, Muktar Y, Adugna S, Kefyalew H, Mengistu S. Knowledge, attitude and practices about quality and management of anthelmintic drugs in Adea Berga District, Central Ethiopia. J Veter Sci Technol. 2018;11:14.
27. El-Dahiyat F, Fahelelbom KM, Jairoun AA, Al-Hemyari SS. Combatting substandard and falsified medicines: public awareness and identification of counterfeit medications. Front Public Health. 2021;9:754279. doi:10.3389/fpubh.2021.754279
28. Rahman MS, Yoshida N, Tsuboi H, et al. The health consequences of falsified medicines‐a study of the published literature. Trop Med Int Health. 2018;23(12):1294–1303. doi:10.1111/tmi.13161
29. Goodman C, Kachur SP, Abdulla S, Bloland P, Mills A. Drug shop regulation and malaria treatment in Tanzania—why do shops break the rules, and does it matter? Health Policy Plann. 2007;22(6):393–403. doi:10.1093/heapol/czm033
30. Adigwe OP, Onavbavba G, Wilson DO. Challenges associated with addressing counterfeit medicines in Nigeria: an exploration of pharmacists’ knowledge, practices, and perceptions. Integrat Pharm Res Pract. 2022;11:177–186. doi:10.2147/IPRP.S387354
31. Kelesidis T, Falagas ME. Substandard/counterfeit antimicrobial drugs. Clin Microbiol Rev. 2015;28(2):443–464. doi:10.1128/CMR.00072-14
32. Haji M, Kerbache L, Sheriff KM, Al-Ansari T. Critical success factors and traceability technologies for establishing a safe pharmaceutical supply chain. Meth Protocols. 2021;4(4):85. doi:10.3390/mps4040085
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
