Back to Journals » Research and Reports in Urology » Volume 15
Epithelioid Angiomyolipoma with Tumor Thrombus into Inferior Vena Cava Presurgically Treated with Combination Therapy of Pembrolizumab and Axitinib: A Case Report
Authors Kawasoe C, Miyamoto Y, Ito K, Murashima T, Nagai T , Takamori H, Kiwaki T , Kamimura T, Mukai S, Kamoto T
Received 30 June 2023
Accepted for publication 18 September 2023
Published 5 October 2023 Volume 2023:15 Pages 447—452
DOI https://doi.org/10.2147/RRU.S425887
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Chihiro Kawasoe,1,2 Yoshi Miyamoto,1,3 Kaoru Ito,1 Takaya Murashima,1 Takahiro Nagai,1 Hiroki Takamori,1 Takumi Kiwaki,4 Toshio Kamimura,1 Shoichiro Mukai,1 Toshiyuki Kamoto1
1Department of Urology, Faculty of Medicine, Miyazaki University Hospital, Miyazaki, Japan; 2Department of Urology, Koga General Hospital, Miyazaki, Japan; 3Department of Urology, Miyazaki Prefectural Nichinan Hospital, Miyazaki, Japan; 4Section of Oncopathology and Regenerative Biology, Faculty of Medicine, Miyazaki University, Miyazaki, Japan
Correspondence: Shoichiro Mukai, Department of Urology, Faculty of Medicine, University of Miyazaki, 5200 Kihara, Kiyotake, Miyazaki, 889-1692, Japan, Tel +81-985-85-2968, Fax +81-985-85-6958, Email [email protected]
Abstract: Epithelioid angiomyolipoma (EAML) is a rare variant of AML with malignant potential. It is occasionally difficult to distinguish EAML from renal cell carcinoma (RCC) on imaging. A 72-year-old woman was admitted to our hospital for the treatment of a left renal tumor with relatively high blood flow and a tumor thrombus extending to the inferior vena cava, suggesting RCC. The patient underwent presurgical combination therapy with axitinib and pembrolizumab. This treatment significantly shortened the thrombus, and radical nephrectomy was performed. The pathological findings were compatible with EAML, and the treatment effects were observed. We report a case treated pre-surgically with a combined therapy of pembrolizumab and axitinib, with a favorable response as a treatment option for EAML.
Keywords: epithelioid angiomyolipoma, axitinib, pembrolizumab, tumor thrombus, presurgical treatment
Introduction
Epithelioid angiomyolipoma (EAML) is a rare variant of the AML.1 At least 80% of EAML components consist of epithelioid cells. Unlike ordinary AML, EAML has malignant potential; however, aggressiveness has been reported to vary by case.2,3 It is difficult to distinguish EAML from renal cell carcinoma (RCC) on imaging examinations owing to its poor fatty components. There is no established treatment for unresectable cases; however, mTOR inhibitors have been reported to be effective in AML associated with tuberous sclerosis.4–9 There have also been reports of responses to tyrosine kinase inhibitors in some cases.7–13 Unlike RCC, intravenous extension to inferior vena cava (IVC) is extremely rare in EAML.14–17 Here, we report a case of EAML initially diagnosed as RCC, with a tumor thrombus extending to the inferior vena cava (IVC) pre-surgically treated with a combination of pembrolizumab and axitinib with a favorable response.
Case Presentation
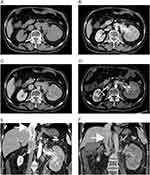
A 72-year-old Japanese woman visited her family doctor with a chief complaint of gross hematuria. The patient had no family history of tuberous sclerosis. Abdominal computed tomography (CT) revealed a 10-cm left renal tumor extending to the IVC (Figure 1A–C and E). The tumor had no fatty components and was diffusely enhanced on contrast CT (Figure 1A–C), suggesting RCC. The patient was then referred to our urology department. Because the tumor thrombus extended to the bifurcation of the hepatic vein, immediate surgical intervention was considered highly invasive. Therefore, we decided to initiate pre-surgical pharmacotherapy. As complete resection was a prerequisite, a biopsy was not performed because of the possibility of peritoneal dissemination.
Combination treatment with pembrolizumab 200 mg/body/3 weeks and axitinib 3 mg bis in die (BID) was initiated and continued for 12 weeks. The objective response to treatment was assessed using contrast-enhanced CT at 4 and 12 weeks. Our protocol called for immediate surgical intervention if disease progression was observed at four weeks; however, no progression was observed at that point. Liver dysfunction (grade 3) occurred after four days as a treatment-related adverse event. Temporary withdrawal of axitinib (1 week) improved liver function, and axitinib was restarted at a dose of 2 mg. Thereafter, the dose of axitinib was increased to 2 mg every two weeks and maintained at 6 mg. Post-treatment CT showed favorable treatment outcomes, including reduction of the contrast effect in the primary tumor and reduction in the length of the tumor thrombus below the bifurcation of the hepatic vein (Figure 1D and F).
We then performed laparoscopic left nephrectomy and thrombectomy, followed by a planned open conversion. IVC filter was placed at the day before surgery to prevent perioperative thromboembolism. After the patient was placed in the lateral position, we began a laparoscopic procedure to mobilize the spleen, pancreatic tail, and descending colon, and ligate the left renal artery. The patient was then repositioned to the supine position, and an open surgical procedure was performed through an upper abdominal incision. IVC and bilateral renal veins were exposed, and the left and short hepatic veins were dissected. After clamping the caudal IVC, left renal vein, and cephalic IVC, the wall of the IVC was cut and the free-floating tumor thrombus was removed. We removed the IVC filter in the surgical field at this point. The total operative time was 9 h and 43 min, and the total bleeding volume was 2040 mL. Despite intraoperative bleeding with blood transfusion, the patient recovered without postoperative complications. There was no apparent recurrence by postoperative CT at one month after surgery; however, multiple recurrences occurred in the pelvic area, and, unfortunately, the patient passed away at 8 months after surgery.
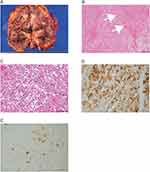
The gross appearance of the cross-section revealed a 15×10 cm tan-brown renal tumor with hemorrhage and necrosis (Figure 2A). The pseudocapsule was absent. These findings differ from those of conventional clear cell RCC. Histological sections demonstrated that tumor cells with severe atypia and abundant eosinophilic cytoplasm proliferated and infiltrated diffusely or formed a vague papillary architecture (Figure 2B and C). Venous invasion, including infiltration into IVC. The tumor cells also invaded the connective tissue of the renal sinus, and perineural invasion was observed. Approximately four mitotic figures per 10 hpf were observed. Metastasis was observed in the resected adrenal glands. More than half of the tumor cells were necrotic, indicating an obvious effect of the presurgical treatment. Immunohistochemical staining revealed tumor cell positivity for Melan A (partly, Figure 2D), cathepsin K (partly, Figure 2E), CD10 (partly), and c-KIT (partly and weakly). However, the staining was negative for AE1/AE3, PAX8, GATA3, CAIX, RCC, CK7, TFE3, SMA, and HMB-45. Based on these findings, the patient was diagnosed with a primary epithelioid angiomyolipoma.
Discussion
AML belongs to the perivascular epithelioid cell tumor (PEComas) family.1–3,18 PEComas are mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular cells.1–3 Other than AML, clear cell sugar tumors of the lung and lymphangioleiomyomatosis are also included in this family.1–3,18 Immunohistochemically, tumor cells are characterized by the expression of melanocytic (HMB-45, Melan-A) and myoid (desmin, actin) markers. The malignant potential is reported to vary from benign (conventional AML, PEComa) to malignant (EAML, malignant PEComa). Although the exact pathogenesis is not well understood, association with tuberous sclerosis complex (TSC) inactivation has been hypothesized.1 Indeed, a favorable response of mammalian target of rapamycin (mTOR) inhibitors to malignant PEComa has been previously reported. Vascular endothelial growth factor receptor (VEGFR)-targeted tyrosine kinase inhibitors including sunitinib, pazopanib, and sorafenib have also been reported to be effective (Table 1). In our case, CT revealed a fat-poor renal tumor with contrast enhancement and early washout, resembling RCC. In addition, the tumor thrombus extended to the bifurcation of the hepatic vein through the IVC. Therefore, we planned presurgical treatment with a combination of pembrolizumab and axitinib without biopsy, as in clear cell RCC. A distinct response was observed in both the imaging examination (CT) and pathological findings, despite the tumor being an EAML. To the best of our knowledge, this is the first report describing a favorable response to combination therapy with pembrolizumab and axitinib for EAML.
 |
Table 1 Summary of Characteristics; Previously Reported Case of EAML/PEComa Treated with TKI |
To date, only four cases of EAML with IVC thrombus have been reported; however, presurgical treatment was not performed.14–17 Nephrectomy and thrombectomy were completed in three cases.15–17
Clinicopathological prognostic factors were analyzed in two studies. Pathological findings suggesting malignant behavior reported by Brimo include the presence of (1) > or = 70% atypical epithelioid cells, (2) > or = 2 mitotic figures per 10 hpf, (3) atypical mitotic figures, and (4) necrosis. The presence of three or four of these features is highly predictive of malignancy.3 For risk classification, Nese advocated five prognostic parameters: (1) tuberous sclerosis complex or concurrent AML, (2) tumor size > 7 cm, (3) extrarenal extension and/or renal vein involvement, (4) carcinoma-like growth pattern, and (5) necrosis. Tumors with fewer than 2 parameters were considered to be in the low-risk group, 15% had disease progression, and tumors with 2–3 parameters were in the intermediate-risk group with 64% disease progression. In addition, tumors with 4–5 parameters were considered to be in the high-risk group with 100% disease progression.2 In our case, the tumor was 15 cm in diameter with distinct venous extension and adrenal metastasis, and the pathological features of our case were compatible with those of the high-risk group. Although the initial diagnosis was different, and if limited to presurgical treatment, combination therapy with pembrolizumab and axitinib was successful without severe adverse events. We believe that this report presents a useful treatment option for cases of EAML with high malignant potential.
Conclusion
We encountered a case of a hypervascular renal tumor with a tumor thrombus extending to the IVC that was initially suspected to be RCC and was later diagnosed as EAML. Presurgical treatment was successfully performed with combined therapy of pembrolizumab and axitinib, which might be a treatment option for locally advanced EAML.
Consent Statement
Written informed consent for publication was obtained from the patient, who consented to the publication of the case details and accompanying images.
Acknowledgments
The authors would like to thank Ms. Miyuki Akino of the Department of Urology, Faculty of Medicine, University of Miyazaki, for her assistance with ethical protocols.
Ethics Approval
This case report was approved by the Ethics Committee of Miyazaki University (approval number: C-0151).
Funding
We report this case for academic purposes and received no external funding for this work. This submission has no commercial interests.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. WHO Classification of Tumours Editorial Board. WHO classification of tumours of urinary and male genital tumours, 5th ed. 2022.
2. Nese N, Martignoni G, Fletcher CD, et al. Pure epithelioid PEComas (so-called epithelioid angiomyolipoma) of the kidney: a clinicopathologic study of 41 cases: detailed assessment of morphology and risk stratification. Am J Surg Pathol. 2011;35(2):161–176. doi:10.1097/PAS.0b013e318206f2a9
3. Brimo F, Robinson B, Guo C, et al. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010;34(5):715–722. doi:10.1097/PAS.0b013e3181d90370
4. Ikarashi D, Mue Y, Shiomi E, et al. Efficacy of everolimus for treating renal angiomyolipoma with inferior vena cava thrombus associated with tuberous sclerosis. Urol Case Rep. 2017;11:11–13. doi:10.1016/j.eucr.2016.12.003
5. Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28(5):835–840. doi:10.1200/JCO.2009.25.2981
6. Italiano A, Delcambre C, Hostein I, et al. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol. 2010;21(5):1135–1137. doi:10.1093/annonc/mdq044
7. Sanfilippo R, Jones R, Blay JY, et al. Role of chemotherapy, VEGFR inhibitors, and mTOR inhibitors in advanced Perivascular Epithelioid Cell Tumors (PEComas). Clin Cancer Res. 2019;25(17):5295–5300. doi:10.1158/1078-0432.CCR-19-0288
8. Hindi N, Sanfilippo R, Stacchiotti S, et al. Systemic therapy in perivascular epithelioid cell tumors (pecoma). Ann Oncol. 2014;25(supplement 4):494–510. doi:10.1093/annonc/mdu354.39
9. Gao F, Huang C, Zhang Y, et al. Combination targeted therapy of VEGFR inhibitor, sorafenib, with an mTOR inhibitor, sirolimus induced a remarkable response of rapid progressive uterine PEComa. Cancer Biol Ther. 2016;17(6):595–598. doi:10.1080/15384047.2016.1167290
10. Lee DW, Chang H, Kim YJ, et al. Sorafenib-induced tumor response in a patient with metastatic epithelioid angiomyolipoma. J Clin Oncol. 2014;32(11):e42–5. doi:10.1200/JCO.2012.48.1960
11. AIAzab RS, Alorjani MS, Sahawneh FE, et al. Metastatic perivascular epithelioid cell tumor of the kidney: a case report with emphasis on response to the tyrosine-kinase inhibitor sunitinib. Res Rep Urol. 2019;11:311–317. doi:10.2147/RRU.S226005
12. Xu J, Gong XL, Wu H, et al. Case report: gastrointestinal PEComa with TFE3 Rearrangement treated with anti-VEGFR TKI apatinib. Front Oncol. 2020;10:582087. doi:10.3389/fonc.2020.582087
13. Zhang N, Ren Y, Zan L, et al. Case report: kidney perivascular epithelioid cell tumor treated with anti-VEGFR tyrosine kinase inhibitor and MTOR inhibitor. Front Oncol. 2022;12:966818. doi:10.3389/fonc.2022.966818
14. Gupta D, Vishwajeet V, Pandey H, et al. Epithelioid angiomyolipoma with tumor thrombus in IVC and right atrium. Autops Case Rep. 2020;10(4):E2020190. doi:10.4322/acr.2020.190
15. Que X, Zhu Y, Ye C, et al. Invasive epithelioid angiomyolipoma with tumor thrombus in the inferior vena cava: a case report and literature review. Urol Int. 2017;98(1):120–124. doi:10.1159/000434648
16. Li X, Liu R, He D. Malignant epithelioid angiomyolipoma invading the inferior vena cava: using a temporary vena cava filter to prevent tumour emboli during nephrectomy. Can Urol Assoc J. 2014;8(7–8):E564–566. doi:10.5489/cuaj.1814
17. Grant C, Lacy JM, Strup SE. A 22-year-old female with invasive epithelioid angiomyolipoma and tumor thrombus into the inferior vena cava: case report and literature review. Case Rep Urol. 2013;2013:730369. doi:10.1155/2013/730369
18. Bleeker JS, Quevedo JF, Folpe AL, et al. ”Malignant” perivascular epithelioid cell neoplasm: risk stratification and treatment strategies. Sarcoma. 2012;2012:541626. doi:10.1155/2012/541626
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.