Back to Journals » Journal of Inflammation Research » Volume 17
Endovascular Repair and Prognosis of Patients with Brucella abortus Infection-Induced Aorto-Iliac Aneurysm
Authors Zhang Y , Wang H, Bai L, Li X, Liu L, Wang L
Received 18 November 2023
Accepted for publication 9 April 2024
Published 17 April 2024 Volume 2024:17 Pages 2353—2363
DOI https://doi.org/10.2147/JIR.S450573
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yujing Zhang,1,* Haiqian Wang,2,* Lei Bai,1 Xiaodong Li,1 Li Liu,1 Liang Wang3
1Department of Cardiovascular Surgery, General Hospital of Ningxia Medical University, Yinchuan, 750004, People’s Republic of China; 2Department of Outpatient, General Hospital of Ningxia Medical University, Yinchuan, 750004, People’s Republic of China; 3Department of Interventional Therapy and Vascular Surgery, Dongguan People’s Hospital, Dongguan, 523059, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang Wang, Department of Interventional Therapy and Vascular Surgery, Dongguan People’s Hospital, Dongguan, 523059, People’s Republic of China, Email [email protected]
Objective: To establish the endovascular repair and prognosis of patients with aorto-iliac aneurysm and Brucella abortus infection.
Methods: From September 2018 to September 2021, seven cases of Brucella abortus infection with aorto-iliac aneurysm were treated by the endovascular aneurysm repair (EVAR) procedure. Clinical and imaging data were collected to evaluate the therapeutic results, including body temperature, blood culture, imaging manifestations, stent patency and endoleak during the postoperative and follow-up periods.
Results: Except for one patient who died of acute hematemesis and hematochezia just after the admission, seven patients were treated successfully. The aneurysms were completely excluded, and all stent grafts were patent. Patients were followed up for 12– 32 months, with an average follow-up of 18.5 ± 9.1 months. There were no cases of endoleak, infection recurrence, gluteal muscle ischemia or spinal cord ischemia during the follow-up period.
Conclusion: It is feasible to treat Brucella abortus-infected aneurysms with the EVAR procedure. The results were optimistic in the short and medium-term. The application of sensitive antibiotics before and after the operation is the cornerstone of endovascular therapy. However, the long-term results require further follow-up.
Keywords: Brucella abortus infection, aorto-iliac aneurysm, endovascular aneurysm repair, bacterial infection, zoonosis, mycotic aneurysm
Introduction
An aortic aneurysm is a focal dilation of the aorta with a diameter >3 cm or >50% larger than normal.1,2 The pathogenesis of aortic aneurysm is a complex multi-factorial process, which is usually accelerated by several risk factors, including smoking, sex, age and hypertension. Bacterial infections can also cause aortic aneurysms. However, these cases are rare and only account for 0.7% to 4.5% of aortic aneurysms.3 Although the exact mechanism of bacterial infection-induced aortic aneurysms remains unclear, it is supposed that bacterial embolism, vascular wall infections, bacteremia, direct infection and trauma contribute to this pathological process. Compared to the common bacteria-induced aortic aneurysms, for example, Staphylococcus, Gram-negative Bacillus and Salmonella infections,3 Brucella abortus infection-induced aortic aneurysms are rare.4–17 Usually, in Brucella abortus infection-induced aortic aneurysms, the abdominal aorta is more susceptible than the thoracic aorta regarding the typical imaging findings, for example, saccular aneurysm or pseudoaneurysm. Since the standardized treatment of Brucella abortus infection-related aortic aneurysms is lacking, it remains a challenge for clinicians to improve the outcomes of this disease.
As a zoonotic disease, Brucella abortus infection usually manifests as undulant fever, headache, muscle pain and arthralgia in patients in the acute phase. After progressing to the chronic phase, it frequently affects the gastrointestinal, cardiovascular, genitourinary, hematologic, nervous and skeletal systems, resulting in multiple comorbidities.18 Brucella abortus infects patients through aerosols, broken skin tissues and the consumption of Brucella-contaminated meat or dairy products. Approximately 500,000 people are infected with Brucella abortus each year all over the world. However, this number is probably under-evaluated because the vulnerable populations are mainly distributed among nomadic herders, animal handlers, raw dairy consumers, slaughterhouse workers and veterinarians, particularly in developing countries.18–20 In these high-risk populations, the average prevalence is increased to 11%,21 which is dramatically different from the urban population in developed countries. Importantly, Brucella abortus infection, including infection induced aortic aneurysms, has become a significant public health threat to socioeconomically underprivileged herdsmen worldwide. Therefore, improvements in the control and treatment of Brucella abortus infection-induced aortic aneurysms have become urgent.
To document the demographic features, explore the therapeutic approaches and observe the prognosis of Brucella abortus infection-induced aortic aneurysms in a pastoral area in China, eight cases of Brucella abortus infectious-related aorto-iliac aneurysm were recruited in the Department of Cardiovascular Surgery of Ningxia Medical University from September 2018 to September 2021. Endovascular aneurysm repair (EVAR) was performed in seven cases, and all patients were followed up for 12 to 32 months. This study provided a certain thread that aims to benefit the patients who are suffering from Brucella abortus infection-induced aortic aneurysms.
Methods
Patient Recruitment
We conducted the study in compliance with recognized international standards, including the principles of the Declaration of Helsinki. The study was approved by the institutional review board at Ningxia Medical University, and all the participants provided informed consent. The demographic and clinical characteristics of participants are listed in Table 1. The individual patient information is listed in Table 2.
 |
Table 1 Demographic and Clinical Characteristics of the Study Participants |
 |
Table 2 Individual Patient Information |
Diagnosis
Computed tomography angiography (CTA) examination (as shown in Table 3, CTA manifestations of the pre-operative aneurysm anatomy), complete blood cell counts, biochemical tests, erythrocyte sedimentation rate, C-reactive protein and the Brucella agglutination test (SAT) were performed immediately after the admission for all aneurysm patients (as shown in Table 4, pre-operative blood test results). Blood cultures were performed in patients with fever. The aortic aneurysm patients were diagnosed according to the criteria established by the 2014 European Society of Cardiology Guidelines on the Diagnosis and Treatment of Aortic Diseases.2,22 The Brucella abortus infected patients were diagnosed by the expert consensus on the diagnosis and treatment of brucellosis,23 combined with epidemiology, clinical manifestations, imaging findings and laboratory findings from each patient.
 |
Table 3 Computed Tomography Angiography (CTA) Manifestations of the Pre-Operative Aneurysm Anatomy |
 |
Table 4 Pre-Operative Blood Test Results |
Anti-Brucella Treatment
All the patients except patient #8 received anti-Brucella treatment and endovascular repair, as described below.
Before admission, patient #1 was misdiagnosed as a standard true abdominal aortic aneurysm, and EVAR was performed. The stent graft-related infection was found 2 weeks after the procedure. After admission, a positive result was obtained from the Brucella agglutination test. The patient was administered anti-Brucella treatment, which included intravenous rifampicin (0.6 g, once a day) combined with oral doxycycline (0.1 g, twice a day) for 1 month and the body temperature was restored to normal. Due to personal selection, patient #1 opted out of laparotomy and decided to continue the oral rifampicin combined with doxycycline for an additional 11 months.
Typically, patients #2–7 received anti-Brucella treatment (intravenous rifampicin (0.6 g, once a day) combined with oral doxycycline (0.1 g, twice a day)) for 1 month or 2 weeks prior to procedure. The duration of the anti-Brucella treatment prior to endovascular repair was at least 2 weeks. If the patients’ condition was stable, without signs of aortic rupture, we preferred 1 month period of anti-Brucella therapy. The patients continued to receive intravenous rifampicin (0.6 g, once a day) combined with oral doxycycline (0.1 g, twice a day) for an additional 1 month and then conferred oral rifampicin and doxycycline for another 5 months.
Patient #8 died of acute hematemesis and hematochezia shortly after admission, with no chance for urgent EVAR treatment. The treatment information is summarized in Table 5.
 |
Table 5 Treatment Information of Patients |
Endovascular Repair
The procedure was performed under local anesthesia. According to the angiography and CTA data, an abdominal aortic endograft with 10–20% oversize was introduced and deployed at the level of the renal arteries. The abdominal aortography was performed to observe the immediate results of complete exclusion of the aneurysm without endoleak. The femoral artery was closed percutaneously with one or two Perclose ProGlide closure devices (Abbott, Santa Clara, CA, USA).
Four patients received the Endurant (Medtronic, Minneapolis, MN), two received the C3 Excluder (W. L. Gore & Associates, Flagstaff, Ariz) and one received the Minos (Shanghai MicroPort Endovascular MedTech Co., Shanghai, PRC). The stent graft was pre-treated with 10 mg/mL of rifampicin immersion in four patients (patients #4, #5, #6, and #7). The treatment information is summarized in Table 5.
Follow-Up Terms
In the agricultural and pastoral areas of northwest China, patients’ compliance with blood collection is poor. So, we mainly focused on the CTA data during the follow-up period, including the peripheral aortic fluid dark area, gas and other imaging indicators. After the procedure, 1 week, 1 month, 3 months, 6 months, 12 months and annually follow-ups were performed to record the postoperative survival rate, and aortic CTA was performed to evaluate the result of the endovascular repair, including the stent patency rate, the incidence of endoleak, re-infection and re-intervention. The patients were administered with anti-Brucella treatment during the follow-ups, which consisted of oral rifampicin (0.6 g, once a day) combined with oral doxycycline (0.1 g, twice a day).
Statistical Analysis
All the results are presented as mean ± standard error of the mean (SEM). Initially, the data were tested for normality and equal variance to confirm the appropriateness of parametric tests. All statistical analyses involved were conducted by SPSS 27.
Results
As shown in Table 1, the mean patient age was 65.4 ± 4.9 years old, and all were male. Regarding ethnicity, there were four Hans, three Huis and one Mongolian patients. It is worth noting that the first symptoms included fever and backpain (Table 6). All eight patients (100%) had a history of working in the pastoral area 3 months pre to disease onset. Although all patients denied a history of eating uncooked meat and drinking raw milk, six patients (75%) had experience in family breeding of farmed sheep, and four patients (50%) were breeding dogs (Table 7). These suggest that the cattle exposure history maybe a major etiological factor relating Brucella infection with aortic aneurysms.
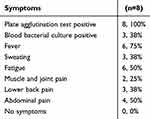 |
Table 6 Symptoms of the study participants |
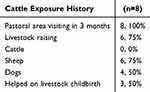 |
Table 7 Epidemiological Data |
All the patients tested positive for Brucella infection (Table 4). Typically, the patients (except #1 and #8) in this group received three phases of treatment. The first phase, these patients received anti-Brucella treatment prior to procedure for 1 month or 2 weeks. In the second phase, the EVAR procedure was performed. After the surgery, in the third phase, the patients continued anti-Brucella treatment for 6 months to 1 year (6 months for patient #2, #3, #4, #5, #6, and #7). Patient #1 was diagnosed with stent graft-related Brucella infection for 2 weeks after the procedure and received anti-Brucella treatment for 1 year. Patient #8 died of acute hematemesis and hematochezia after admission (Table 5).
Patients were followed up for 12–32 months, with an average follow-up of 18.5 ± 9.1 months. Six patients (#2-7,75%) showed no endoleak, spinal cord ischemia and gluteal muscle ischemia during the follow-up. Patient #1 was diagnosed with a stent graft infection 2 weeks after the EVAR procedure. After 1 month of intravenous administration of rifampicin combined with oral doxycycline anti-Brucella treatment, the patients’ body temperature dropped to normal and the treatment regimen was changed to oral rifampicin and doxycycline. As shown in Figures 1–5, after 30 months of follow-up, the CTA exam showed that the gas and low-density shadow around the stent graft had disappeared. After 32 months of follow-up, the patient survived without the recurrence of infection or endoleak. In addition, patient #4, who had bilateral common iliac vein thrombosis, received long-term oral anticoagulant therapy with rivaroxaban (15 mg, twice a day for 3 weeks, which was then changed to 20 mg, once a day).
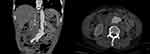 |
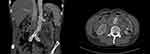
Figure 1 A 62-year-old male patient with abdominal aortic aneurysm. Abdominal aortic lobulated cystic aneurysm, with a surrounding low-density shadow. |
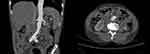 |
Figure 2 Two weeks after endovascular repair of the abdominal aorta, a liquid dark area appeared around the abdominal aorta with visible bubbles. |
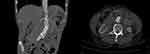 |
Figure 4 Eight months after the procedure and with continuous regular anti-infective treatment. The bubbles and liquid dark areas around the abdominal aorta had disappeared. |
 |
Figure 5 Thirty months after the procedure. There are no bubble and liquid dark area around the abdominal aorta. |
Discussion
Brucellosis is a genus of aerobic Gram-negative bacteria and a pathogen of zoonosis.24 From 2012 to 2016, the average incidence of brucellosis in the Inner Mongolia Autonomous Region of China has increased to 3.79% of the total population.25 Our center is only 30 km away from the pastoral area of Inner Mongolia. People who are most vulnerable to brucellosis are directly exposed to infected animals and are infected by eating unsterilized meat or dairy products. The eight patients included in this study were farmers or herdsmen who raised animals. Infection-induced aneurysms involving the aorta caused by Brucella abortus are rare,4,17 and most cases are reported with spotted patterns. Among the published reports on cases of aortic brucellosis, the most common site is the abdominal aorta, followed by the ascending aorta, descending aorta and iliac artery.5,7
The pathogenesis of infectious aortic aneurysms includes endothelial damage due to atherosclerosis, plaque or ulceration, bacterial colonization of the damaged endothelium and infection of atherosclerotic aneurysms.3,5 In addition, infection can occur because of either the bacteremic or contiguous spread of infection to the vasa vasorum, causing local infection of the aortic wall and progression to mycotic aneurysm.3,26 Local infiltration of a gastrointestinal infection or a vertebral infection can also lead to infection of the abdominal aorta.3,7 All patients in this study were male which is consistent with the reference, suggests that the blood vessel degeneration due to arteriosclerosis, hypertension and smoking makes the aorto-iliac arteries more vulnerable to Brucella infection.26,27 Bacteria are most likely to adhere to the neck of an aneurysm, the posterior lower segment of the aneurysm cavity and the iliac branch with a larger angle between the bifurcation of the iliac aorta and the long axis of the abdominal aorta.26
Stent graft-related infection after EVAR is mainly caused by exposure to exogenous bacteria during the stent deployment and postoperative hematogenous dissemination.1,28,29 However, infection caused by Brucella abortus after EVAR has not been reported. For the patient #1, endovascular repair without the protection of enough anti-Brucella therapy was the cause of stent graft infection.
An infectious aneurysm or stent graft infection can be diagnosed by typical clinical manifestations, which include imaging evidence, blood tests or local bacterial culture.3,30 Among the eight patients in this study, the blood brucellosis agglutination test was positive, and the blood bacterial culture of two patients was positive. There was no significant increase in white blood cells and procalcitonin, except in patient #8, while the results of serum C-reactive protein levels in four patients were significantly increased. Patient #1 had abdominal pain and fever for 2 weeks. CTA examination showed obvious thickening of the arterial wall and surrounding low-density tissue signs. Patient #2 had abdominal pain for 1 week and fever for 1 day. The CTA examination showed no obvious low-density tissue signs around the aorta, considering the onset time was short. According to the CTA examination results, the presence of abdominal aortic aneurysm and intimal calcification in these two patients may be the susceptible factors for infection. In patients #3, #4, #5 and #7, the aneurysms were characterized by lower abdominal pain and back pain, with fever for 3 weeks to 3 months, respectively. Local infection of the vascular wall caused by pathogens was considered to form the aneurysm. However, due to the lack of imaging data before onset, whether an aneurysm or intimal injury was present in the past is unknown. Patient #6 had lower back pain and recurrent fever for 6 months due to repeated infection caused by pathogenic bacteria. Due to the application of antibiotics, the blood bacteria test or bacteria culture of some infected patients showed negative results. In this study, only two patients had blood bacteria culture-confirmed Brucella abortus infection, and five cases were blood culture negative. No arterial wall specimens were obtained because of the limitation of the endovascular procedure. Open surgical repair is the standard therapy for infected abdominal aortic aneurysms like Salmonella species, Streptococcus and Staphylococcus species. According to the results of near- and medium-term follow-up, Brucella infection with abdominal aortic/iliac aneurysm can avoid open surgical repair and choose less invasive endovascular repair with better prognosis. But long-term results still need to be followed up.5,6,8,9,12,13,31
EVAR has been routinely used to treat non-infectious aortic aneurysms. However, there are few reports on the treatment of Brucella-infected aortic aneurysms with EVAR. Lin Wang et al26 reported 14 cases of Brucella-infected aneurysms and 12 cases of EVAR, none of which had surgical complications. All patients survived during follow-up and three of them exhibited recrudescence. Jianjun Jiang et al32 reported 15 cases of aneurysm infected by Brucella treated with EVAR, among which six cases were emergency operations with a 100% success rate, and no cases of postoperative death. In our study, seven patients were followed up for 12–32 months, with an average of 18.5 ± 9.1 months and all survived, including one case of post EVAR stent graft infection.
For brucellosis, the World Health Organization recommends doxycycline (100 mg twice daily) and rifampicin (600–900 mg once daily) for 6 weeks as the standard therapy.33 The American Heart Association states that for infected aneurysms, vascular graft infections, mycotic aneurysms and endovascular infections,3 antibacterial therapy lasting 6 weeks to 6 months should be considered (class IIb, evidence level B). In some cases, lifelong suppression therapy may be considered. At present, there is no uniform standard for antibiotic treatment after EVAR for Brucella-infected aortic aneurysms, with 6 weeks,10 6 months32 or lifetime administration4 all reported. Our study adopted oral rifampicin (0.6 g, once a day) combined with oral doxycycline (0.1 g, twice a day) for 6 months after EVAR, and 1 year for stent graft infection after EVAR. There were no cases of recrudescence during the follow-up.
The treatment of stent graft Brucella abortus infection after EVAR has not been reported. In this study, Patient #1 showed stent graft infection after EVAR and was administered intravenous combined with oral rifampicin and doxycycline as the anti-infective treatment. The patient was followed up for 32 months and no fever re-occurred. The result of aortic CTA showed that the gas and low-density shadow around the stent graft had disappeared, which may suggest that Brucella abortus has a good response to sensitive antibiotics.
Conclusion
The accumulation of experiences showed that the EVAR procedure was feasible for the treatment of Brucella-infected aorto-iliac aneurysms and that the short and mid-term results were satisfactory. This single center experience showed that the key to improve the long-term success rate of the EVAR procedure was to accurately identify Brucella abortus infection before the operation, administer sensitive antibiotic treatment and continue the standardized anti-infection treatment based on the course of treatment after the operation. Due to the limited number of cases with aorto-iliac aneurysms infected by Brucella abortus in our center, the long-term results require further verification in the subsequent study.
Data Sharing Statement
Due to the nature of this research, participants of this study should be protected for their data to be shared publicly. However, detailed data can be available from Yujing Zhang MD and Li Liu, PhD, MD.
Ethics
This study was conducted with approval from the institutional review committee of General Hospital of Ningxia Medical University (Ethical number: KYLL-2021-744). This study was conducted in accordance with the declaration of Helsinki. Written informed consent was obtained from all participants.
Funding
This study was supported by The Ningxia Hui Autonomous Region key research and development plan in the field of social development projects (The general project, No: 2022BEG03122) in 2022 and the postdoctoral project of Dongguan People’s Hospital (K202411) in 2024.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chaikof EL, Dalman RL, Eskandari MK. et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2–77. doi:10.1016/j.jvs.2017.10.044
2. Erbel R, Aboyans V, Boileau C, et al. ESC guidelines on the diagnosis and treatment of aortic diseases. Rev Esp Cardiol. 2014.
3. Wilson WR, Bower TC, Creager MA, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134(20):e412–60. doi:10.1161/CIR.0000000000000457
4. Li HL, Chan YC, Cui DZ, Li N, Cheng SW. Successful endovascular aneurysm repair for Brucella mycotic aneurysm acquired from ingestion of sheep placenta. Ann Vasc Surg. 2019;57:274–e11. doi:10.1016/j.avsg.2018.09.032
5. Kakkos SK, Papadoulas S, Lampropoulos G, Marangos M, Kalogeropoulou C, Tsolakis IA. Aorto-iliac aneurysm infected by Brucella: distinctive presentation patterns of a rare entity. Vascular. 2013;21(5):307–315. doi:10.1177/1708538113478777
6. Alhaizaey A, Alassiri M, Alghamdi M, Alsharani M. Mycotic aortic aneurysm due to brucellosis. J Vasc Surg Cases Innov Tech. 2016;2(2):50–52. doi:10.1016/j.jvsc.2016.03.009
7. Cascio A, De Caridi G, Lentini S, et al. Involvement of the aorta in brucellosis: the forgotten, life-threatening complication. A systematic review. Vector Borne Zoonotic Dis. 2012;12(10):827–840. doi:10.1089/vbz.2012.0965
8. Goudard Y, Pierret C, de La Villéon B, Mlynski A, de Kerangal X. In situ repair of a primary Brucella-infected abdominal aortic aneurysm: long-term follow-up. Ann Vasc Surg. 2013;27(2):241–e1. doi:10.1016/j.avsg.2012.02.028
9. Bergeron P, Gonzalès-Fajardo J, Mangialardi N, Courbier R. False aneurysm of the abdominal aorta due to Brucella suis. Ann Vasc Surg. 1992;6:460–463. doi:10.1007/BF02007004
10. Zhang T, Ji D, Wang F. Endovascular treatment of Brucella-infected abdominal aortic aneurysm: a case report. Med Baltim. 2017;96(42):e7666. doi:10.1097/MD.0000000000007666
11. Alsheef M, Alsaleh S, Alanezi N, et al. Descending Thoracic Aortic Aneurysm due to Brucella melitensis. Case Rep Infect Dis. 2019;2019:4939452. doi:10.1155/2019/4939452
12. Nair HR, Goura P, Pitchai S, Madathipat U. Brucella-induced ruptured infrarenal dissecting abdominal aortic aneurysm. Aorta. 2019;7:56–58. doi:10.1055/s-0039-1688449
13. Quaniers J, Durieux R, de Leval LD, Limet R. Abdominal aortic aneurysm due to Brucella melitensis. Acta Chir Belg. 2005;105(1):93–95. doi:10.1080/00015458.2005.11679674
14. Huang C, Zhao M, Ge Y, et al. Analysis of difficult cases 487 cases of low back pain-fever-infectious thoracic aortic aneurysm. Natl Med J China. 2020;100:57–60.
15. Gao LL, Ma JN, Bao XL. Report of 2 cases of infected abdominal aneurysm caused by Brucella. Chin J Infect Chemother. 2016;16:379–382.
16. Wang JW, Zhai L. Dissecting aortic aneurysm associated with Brucella infection: one case. Chin J Infect Chemother. 2019;19:560–561.
17. Liu R, Liu X, Liu B, Song XJ, Zheng YH. Endovascular repair for Brucella-infected abdominal aortic aneurysm: one case report and literature review. Chin J Mult Organ Dis E. 2016;15:225–230.
18. Franc KA, Krecek RC, Häsler BN, Arenas-Gamboa AM. Brucellosis remains a neglected disease in the developing world: a call for interdisciplinary action. BMC Public Health. 2018;18(1):125. doi:10.1186/s12889-017-5016-y
19. Deng Y, Liu X, Duan K, Peng Q. Research progress on brucellosis. Curr Med Chem. 2019;26(30):5598–5608. doi:10.2174/0929867325666180510125009
20. Laine CG, Scott HM, Arenas-Gamboa AM. Human brucellosis: widespread information deficiency hinders an understanding of global disease frequency. PLOS Negl Trop Dis. 2022;16:e0010404. doi:10.1371/journal.pntd.0010404
21. McDermott J, Grace D, Zinsstag J. Economics of brucellosis impact and control in low-income countries. Rev Sci Tech. 2013;32(1):249–261. doi:10.20506/rst.32.1.2197
22. Sörelius K. Editor’s Choice - Infective Native Aortic Aneurysms: a Delphi Consensus Document on Terminology, Definition, Classification, Diagnosis, and Reporting Standards. Eur J Vasc Endovasc Surg. 2023;65(3):323–329. doi:10.1016/j.ejvs.2022.11.024
23. Editorial board of Chinese journal of infectious disease. Expert consensus on diagnosis and treatment of brucellosis. Chin J Infect Dis. 2017;35:705–710.
24. De Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol. 2015;185(6):1505–1517. doi:10.1016/j.ajpath.2015.03.003
25. Liu ZG, Wang M, Ta N, et al. Seroprevalence of human brucellosis and molecular characteristics of Brucella strains in Inner Mongolia Autonomous region of China, from 2012 to 2016. Emerg Microbes Infect. 2020;9(1):263–274. doi:10.1080/22221751.2020.1720528
26. Wang L, Wang Y, Ma T, et al. Brucella infectious aneurysm: a retrospective study of 14 cases and review of the literature - case report and literature review. Infect Drug Resist. 2023;16:87–104. doi:10.2147/IDR.S393060
27. Li X, Li X, Cheng Z. Brucellosis involving the aorta and iliac arteries: a systematic review of 130 cases. Front Bioeng Biotechnol. 2023;11:1326246. doi:10.3389/fbioe.2023.1326246
28. Li HL, Chan YC, Cheng SW. Current evidence on management of aortic stent-graft infection: a systematic review and meta-analysis. Ann Vasc Surg. 2018;51:306–313. doi:10.1016/j.avsg.2018.02.038
29. Murphy EH, Szeto WY, Herdrich BJ, et al. The management of endograft infections following endovascular thoracic and abdominal aneurysm repair. J Vasc Surg. 2013;58(5):1179–1185. doi:10.1016/j.jvs.2013.04.040
30. Lyons OTA, Baguneid M, Barwick TD, et al. Diagnosis of aortic graft infection: a case definition by the management of aortic graft infection collaboration (MAGIC). Eur J Vasc Endovasc Surg. 2016;52(6):758–763. doi:10.1016/j.ejvs.2016.09.007
31. Kwon TW, Kim HK, Moon KM, Cho YP, Park SJ. In situ polytetrafluoroethylene graft bypass for primary infected aneurysm of the infrarenal abdominal aorta. World J Surg. 2010;34:1689–1695. doi:10.1007/s00268-010-0507-3
32. Jiang J, Shao W, Shen S, et al. Endovascular stent graft repair for mycotic aorto-iliac aneurysm due to Brucella. J Endovasc Ther;2023. 15266028231155139. doi:10.1177/15266028231155139
33. Listed N. Joint FAO/WHO expert committee on brucellosis. World Health Organ Tech Rep Ser. 1986;740:1–132.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.