Back to Journals » The Application of Clinical Genetics » Volume 16
Eleven Years of Oncogenetic Consultations in a Swiss Center: Patient and Testing Characteristics
Authors Grandjean B , Scherz A , Rabaglio M
Received 20 March 2023
Accepted for publication 12 October 2023
Published 9 November 2023 Volume 2023:16 Pages 205—213
DOI https://doi.org/10.2147/TACG.S410261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Martin Maurer
Bastien Grandjean, Amina Scherz, Manuela Rabaglio
Department of Medical Oncology, Inselspital University Hospital, Bern, Switzerland
Correspondence: Bastien Grandjean; Manuela Rabaglio, Email [email protected]; [email protected]
Introduction: Oncogenetic counseling has been provided at the University Hospital of Bern since 2004. Since the public announcement by Ms. Angelina Jolie in 2013 that she had undergone bilateral prophylactic mastectomy, other oncogenetic centers have reported an increase in consultations. We conducted a retrospective review of the oncogenetic consultations at our center to evaluate the presence and the consequences of a potential “Angelina Jolie effect” and to characterize this patient population over a decade.
Methods: All initial oncogenetic consultations between 2005 and 2015 were collected, using electronic records. Demographics, cancer type, testing, and mutation results, as well as consultation rates, were recorded. The yearly trends were analyzed using Joinpoint regression analysis (JPA).
Results: In total, 823 patient cases were included, mostly women (84%), half of them with a positive personal cancer history. A hereditary breast and ovarian cancer (HBOC) risk was the main reason for consultation (72%). Moreover, 22% of patients had a previously detected familial mutation. Two-thirds underwent testing, which yielded a positive test result in 31% of the cases. According to JPA, the consultation rate increased throughout the decade, with a significant upward trend from 2013. Rates of testing and positive results remained stable over time. Most patients (86%) fulfilled the referral criteria of published guidelines.
Discussion: At our center, we found retrospectively a disproportionate growth in the referral rate for HBOC cases compared to other oncological cases after the year 2013, but overall, no change in testing rates was detected.
Keywords: oncogenetic, family cancer, demographics
Introduction
Since the first description of familial cancer by Henry T. Lynch in the 1960s,1 the field of oncogenetics has grown enormously. The development of age-based risk models allowed the calculation of the cancer risk based on patient and epidemiological data.2 Genetic testing emerged as a result of improved sequencing techniques and the isolation of germ-line cancer mutations with high penetrance, and became part of oncogenetic counseling.3 It has become part of routine care for cancer patients and is included in national prevention guidelines such as those from the US Preventive Services Task Force (USPSTF).4 In Switzerland, centers and physicians providing oncogenetic counseling are listed on the website of the Swiss Group for Clinical Cancer Research (SAKK),5 which acts as a national coordinator.6 In 2017, the SAKK published national guidelines for hereditary breast and ovarian cancer (HBOC).7 Oncogenetic counseling has been offered in all Swiss university hospitals and some regional centers for over 20 years; at our Hospital, the Department of Medical Oncology has offered such counseling since 2004.
Since the public announcement by the prominent US actress Angelina Jolie in 2013 that she was undergoing prophylactic bilateral mastectomy owing to positive BRCA1 testing, other oncogenetic centers have documented a strong increase in breast cancer consultations.8 The so-called “Angelina Jolie effect” has been already described.9 Its effects on the quality of the referral and its consequences in the long term remain unknown.
To evaluate the incidence of new cases and the test rate, we conducted a retrospective study and recorded all consultations at our center over 11 years. Our main objectives were to characterize the population using our services and to detect a potential “Angelina Jolie effect”, as well as its extent and its possible impacts.
Method
After approval of the project by our local Ethical Committee, we screened systematically the consultation agenda of the clinic to find all patients who had their first consultation for oncogenetic counseling between 01.01.2005 and 31.12.2015 at our oncology department. Medical data were gathered using the electronic record system (ERS) and the digital archive system of the hospital. We excluded the following cases: initial consultation before 01.01.2005 or after 31.12.2015, lost to follow-up, not an oncological case, and incomplete/empty records impeding adequate data collection.
We gathered basic demographic data on the included patients, along with consultation dates. The specialty and origin of the referrer were also recorded, if documented.
Personal and family oncological histories were documented, with tumor entities, age at diagnosis, and whether a previously identified mutation was known. This allowed a division of the cohort into four broad subgroups: I) patients without a personal cancer history and no previously identified familial mutation (ie patients with a family history of cancer, but no previous testing in the family; II) patients with a previously identified familial mutation but no personal history; III) patients with a personal cancer history but no family history of mutation; and IV) patients with both a personal cancer history and a previously identified familial mutation.
Oncological entities were regrouped into three main categories: 1) hereditary breast and ovarian cancer (HBOC); 2) hereditary colorectal cancer (HCC), containing both familial adenomatous polyposis (FAP) and hereditary non-polyposis colon cancer (HNPCC); and 3) all remaining cases as “others”.
To assess the adequacy of the referral, we used previously published Swiss7 and American College of Medical Genetics and Genomics (ACMG)10 guidelines. We considered a referral as appropriate if a single criterion was fulfilled.
Test results were reported as positive, negative, or variant of unknown significance (VUS). In case of multiple results due to the analysis of more than one gene, we recorded only positive results. If no genetic testing was ordered, the reason was recorded.
We analyzed descriptive statistics using the software STATA 14.2 (StataCorp, College Station, TX, USA). Results were reported with the usual parameters.
To analyze a time trend, patient cases were regrouped into 11 year-groups (from 2005 to 2015) based on the first consultation date to calculate an annual consultation rate, similar to an annual incidence. This allowed us to derive an annual testing rate (based on the percentage of patients undergoing testing) as well as an (annual) positive result rate (based on the percentage of tests yielding a mutation).
Since we hypothesized that an “Angelina Jolie effect” was present and would manifest itself as an increase in the consultation rate, we decided to further analyze this rate using Joinpoint trend analysis (JPA) with the software Joinpoint Regression Program 4.6 (Statistical Research and Applications Branch of National Cancer Institute, Bethesda, MD, USA).
JPA is a regression analysis that allows a statistical change in trend to be detected. The software computes several regression models and uses a Monte-Carlo permutation-based significance test to select the best fitting model. The results are shown either as a straight line or as a segmented line with joinpoints marking statistical changes in trend.
Initially described and used in 2000 to analyze prostate incidence and mortality rate,11 JPA has also been applied in other situations; in a setting analogous to our work, Hobbs et al used JPA in 2016 to analyze time trend changes in consultation rates of health providers over a period of 7 years.12
The Joinpoint Regression Program was configured with following technical parameters: count and proportion as types of variable, constant variance assumption, no logarithmic transformation, and number of maximum joinpoints set to one (default setting). The rest of the parameters were left unchanged.
We applied the JPA to the annual consultation rate, testing rate, and positive result rate using all patient cases together, and carried out a subanalysis using our three main categories (HBOC, HCC, and others).
Results
Between 01.01.2005 and 31.12.2015, we found 901 first oncogenetic consultations in the electronic agenda. Of those, 823 were included and 78 were excluded (91% inclusion rate). The reasons for exclusion are detailed in the flowchart (Figure 1). The demographics of the analyzed patients are summarized in Table 1.
 |
Table 1 Demographics of Patients |
 |
Figure 1 Flowchart of cases. |
The annual number of first consultations increased seven-fold over the years: while there were only 24 patients in 2005, there was 172 new cases in 2015. Figure 2 shows the number of new cases per trimester over time.
 |
Figure 2 Consultations over time. |
Over half (56%) of the patients had a personal cancer history, with a mean ± SD age at diagnosis of 44.6±12.1 years. On average, these patients consulted 5 years after the diagnosis of cancer (age at the first consultation 49.5±12.2 years) regardless of the type of cancer. Nine percent of all patients had a second malignancy in their personal history and 2% had a third one. Overall, 558 cancer diagnoses were recorded, including 334 cases of breast cancer, 70 cases of colorectal cancer, and 51 cases of ovarian cancer.
Using the three main categories, HBOC represented the majority of cases (72%), followed by HCC (19%); the remaining cases (9%) were heterogeneous and included, for example, hereditary diffuse gastric cancer (HDGC) cases, MEN syndromes, Von Hippel–Lindau syndrome, familial melanoma cases, Li–Fraumeni syndrome, and familial pancreatic cancer cases. Ten heterogeneous cases did not correspond to a clear syndromic familial constellation. Lastly, eight cases were suspected to harbor multiple mutations (eg HBOC and HCC).
A total of 185 patients (22%) had a previously known pathological mutation in their family; of these, 26 patients also had a personal cancer history. Mutations in BRCA2 (n=66) and BRCA1 (n=57) were the most common, followed by MSH2 (n=26). Fourteen other genes of interest were reported (MSH6, CDH1, APC, MLH1, VHL, MEN1, SDHB, FH, NF1, NF2, PMS2, RB1, SKT11, and TP53).
We evaluated the referral as adequate in 86% of the cases. Specifically, 87% of the HBOC cases (n=596), 90% of the HCC cases (n=154), and 67% of the remaining cases (n=73) fulfilled at least one criterion of the Swiss7 and ACMG10 guidelines.
Overall, two-thirds of the patients underwent testing (n=554); the remaining patients (n=269) were not tested because of low pretest probability (36%) or the patient’s own decision (25%), or because testing was preferably indicated in another family member (16%). Lack of cost coverage by the health insurer was reported in 5% as the main reason for not testing. Considering the personal and family history, patients without a personal history of cancer underwent testing less frequently if no mutation had previously been identified in a family member (29%, n=191) than in those with a known familial genetic constellation (96%, n=159). Patients with a personal history of cancer but without a known familial mutation underwent testing in most cases (73%, n=447), whereas all patients with a personal cancer history and a known familial mutation were tested (100%, n=26).
Combined BRCA1 and BRCA2 constituted 55% of all testing; as separated assays, they represented 10% and 12% of the tests, respectively. All testing for HNPCC, including MSH2, MSH6, MLH1, and PMS2, together or separately, represented 13% of the total. Twelve additional genes were tested, with a low frequency (APC, CDH1, VHL, MUTYH, MEN1, SDHB, TP53, FH, JAK2, NF1, RB1, and CDKN2A). Five cases (1%) were tested using next-generation sequencing (NGS) panels during the years 2005 and 2015.
A pathogenic mutation was detected in 31% (n=174) of all tests; additionally, 24 reported a VUS at the time of the analysis. No case of VUS was reported to be reclassified as benign or pathological during the years 2005–2015. Pathogenic mutations were found in BRCA2 (n=68), BRCA1 (n=57), MSH2 (n=23), MSH6 (n=12), CDH1 (n=4), MLH1 (n=3), APC (n=2), VHL (n=2), MEN1, RB1, and SDHB (n=1 each).
The JPA yielded, as shown in Figure 3, a model with two segments and one joinpoint (p-value vs “no joinpoint” hypothesis =0.002) for the year 2012. A similar result was obtained from the subanalysis of HBOC cases (p=0.0002). No significant joinpoints were detected from the subanalyses of HCC (p=0.46) and remaining cases (p=0.25). A significant upward trend during the period 2005–2015 was detected (p<0.05) from all four analyses.
Considering the annual testing rate and annual positive results rate, the analysis yielded, as shown in Figures 4 and 5, an almost flat single slope for both of these. No significant joinpoints were detected, and the hypothesis that the regression slope differs from zero was rejected (p=0.9 and p=0.7, respectively).
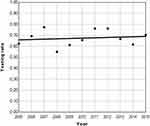 |
Figure 4 Joinpoint analysis of annual testing rate. |
 |
Figure 5 Joinpoint analysis of annual positive results rate. |
To summarize in plain language, the annual consultation rate of new cases was reported as significantly increasing over the years. This upward trend was stronger after the year 2012. In a subanalysis of the three main case categories, a similar pattern was found only in HBOC cases. No change in trend was detected for HCC and remaining cases. The annual testing rate and positive results rate did not change over the years.
Discussion
In our retrospective analysis, we found 823 cases of oncogenetic counseling over 11 years. Our cohort size is similar to that of Henriksson et al,13 who surveyed a Swedish clinic during 10 years. As previously reported by others,14,15 HBOC cases represent by far the largest proportion of all consultations; as expected, our cohort contains mostly women and the determination of BRCA status is the major testing procedure.
Compared to historical cohorts, our consultation rate increased markedly in the years 2013–2015. An initial growth has been reported during the first years of establishment of a new oncogenetic service.13,16 More solid financing of oncogenetic services, as reported in France between 2003 and 2007,17 may also lead to an increase in consultation rates. But neither of these applies to our situation. We can hypothesize that an “Angelina Jolie effect” was the main driver of this growth: using regression analysis, we detected a change in trend occurring only in the HBOC cohort after the year 2012, corresponding to the public announcement by Ms. Jolie in May 2013.
During the decade 2005–2015, the number of prescribed tests grew eight-fold. We feared that such an increased workload would be associated with a lower threshold to refer and thus to test patients. Our data show that most of our patients were referred appropriately according to guidelines and that the testing rates, alongside the positive results rate, remained stable over the years.
Our work is limited by its retrospective design. Seven percent of initially screened patient cases had to be excluded because of incomplete files. Our assumption that an “Angelina Jolie effect” took place in Bern relies only on a temporal association. To prove causality, it would have been necessary to prospectively assess the motivation of the patients or the referrers to undergo oncogenetic counseling.
We nevertheless found a sharp increase in consultation rates within a few years. Aside from a potential mass media-associated phenomenon, we expect a further increase in the demand for such services, based on the following assumptions.
First, some familial cancer syndromes are probably still underdiagnosed. As an example, melanoma is the fourth most frequent cancer in Switzerland, with approximately 2450 cases per year,18 and it is known that a significant share (5–15%) of melanoma cases are hereditary.19 Yet, in our 11-year cohort, we found only two cases of patients referred for familial melanoma. Better identification of such cases is potentially warranted.
Secondly, new indications for oncogenetic testing will probably emerge over time. For instance, determination of germline BRCA mutational status in metastatic pancreatic cancer has been shown to have therapeutic consequences,20 and could lead to a broader testing strategy for this entity. Regarding metastatic prostate cancer, 2019 guidelines recommend genetic testing including germline mutations regardless of family history,21 as therapeutic consequences have been highlighted, for example, with PARP inhibitors.22
Lastly, the increasing use of NGS testing in oncology to guide targeted therapy may reveal unexpected germline mutations,23 and these incidental findings will require oncogenetic counseling to discuss the results with the patient. We therefore expect a further growth in the role of oncogenetic counseling in the coming decades.
Research on Humans
The authors declare that the project complies with the principles of the Declaration of Helsinki.
The project research involved retrospective use of previously recorded patient data. The protocol was submitted to the Ethical Committee of Canton Bern (Switzerland) on the 6th of January 2016. Since most of the participants were no longer in follow-up, for several years, obtaining the prior informed consent of all participants before inclusion was anticipated to be difficult, if not impossible. The requirement of prior informed consent from the participants for this project was waived by the Ethical Committee and the project received approval on the 20th of January 2016, under reference number 2016-00049.
Funding
No external sources of funding were received.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Cantor D. The frustrations of families: Henry Lynch, heredity, and cancer control, 1962–1975. Med Hist. 2006;50(3):279–302. doi:10.1017/S0025727300009996
2. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi:10.1093/jnci/81.24.1879
3. Biesecker BB, Boehnke M, Calzone K, et al. Genetic counseling for families with inherited susceptibility to breast and ovarian cancer. JAMA. 1993;269(15):1970–1974. doi:10.1001/jama.1993.03500150082032
4. Moyer VA; US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(4):271–281. doi:10.7326/M13-2747
5. SAKK. Genetic counseling, Swiss group for clinical cancer research. Available from: https://www.sakk.ch/en/patients/genetic-counseling.
6. Pichert G, Stahel RA. Organizing cancer genetics programs: the Swiss model. J Clin Oncol. 2000;18(21 Suppl):65S–9S.
7. Chappuis P, Bolligerb B, Bürkic N, et al. Genetic predisposition to breast and ovarian cancer. Bulletin des Médecins Suisses. 2017;98(2122):682–684.
8. Jolie A. My medical choice. NY Times. 2013;14(05):2013.
9. Evans DG, Barwell J, Eccles DM, et al. The Angelina Jolie effect: how high celebrity profile can have a major impact on provision of cancer related services. Breast Cancer Res. 2014;16(5):442. doi:10.1186/s13058-014-0442-6
10. Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med. 2015;17(1):70–87. doi:10.1038/gim.2014.147
11. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi:10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z
12. Hobbs FDR, Bankhead C, Mukhtar T, et al. Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet. 2016;387(10035):2323–2330. doi:10.1016/S0140-6736(16)00620-6
13. Henriksson K, Olsson H, Kristoffersson U. The need for oncogenetic counselling. Ten years’ experience of a regional oncogenetic clinic. Acta Oncol. 2004;43(7):637–649. doi:10.1080/02841860410018520
14. van Riel E, van Dulmen S, Ausems MG. Who is being referred to cancer genetic counseling? Characteristics of counselees and their referral. J Community Genet. 2012;3(4):265–274. doi:10.1007/s12687-012-0090-4
15. Selkirk CG, Vogel KJ, Newlin AC, et al. Cancer genetic testing panels for inherited cancer susceptibility: the clinical experience of a large adult genetics practice. Fam Cancer. 2014;13(4):527–536. doi:10.1007/s10689-014-9741-4
16. Sobol H, Bignon YJ, Bonaiti C, et al. Four years analysis of cancer genetic clinics activity in France from 1994 to 1997: a survey on 801 patients. French Cooperative Network/Groupe Genetique et Cancer de la Federation Nationale des Centres de Lutte Contre le Cancer. Dis Markers. 1999;15(1–3):15–29. doi:10.1155/1999/140498
17. Institut National du Cancer. Synthèse nationale: évolution de l’activité d’oncogénétique 2003–2007 [National synthesis: changes in oncogenetic activity 2003-2007]. Institut National du Cancer; 2008. French.
18. Arndt V. Swiss Cancer Report 2015 - Current situation and developments. Feder Statist Off. 2016;2016:1.
19. Debniak T. Familial malignant melanoma - overview. Hered Cancer Clin Pract. 2004;2(3):123–129. doi:10.1186/1897-4287-2-3-123
20. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317–327. doi:10.1056/NEJMoa1903387
21. Giri VN, Knudsen KE, Kelly WK, et al. Implementation of germline testing for prostate cancer: Philadelphia Prostate Cancer Consensus Conference 2019. J Clin Oncol. 2020;38(24):2798–2811. doi:10.1200/JCO.20.00046
22. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. doi:10.1056/NEJMoa1911440
23. Meric-Bernstam F, Brusco L, Daniels M, et al. Incidental germline variants in 1000 advanced cancers on a prospective somatic genomic profiling protocol. Ann Oncol. 2016;27(5):795–800. doi:10.1093/annonc/mdw018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

