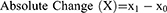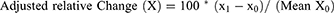Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 16
Efficacy of Adipocyte-Derived Stem Cells-Conditioned Media in Telogen Effluvium
Authors Zari S
Received 29 August 2023
Accepted for publication 7 November 2023
Published 20 November 2023 Volume 2023:16 Pages 77—89
DOI https://doi.org/10.2147/SCCAA.S432179
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Shadi Zari
Department of Dermatology, College of Medicine, University of Jeddah, Jeddah, Saudi Arabia
Correspondence: Shadi Zari, Department of Dermatology, College of Medicine, University of Jeddah, Jeddah, Saudi Arabia, Email [email protected]
Objectives: To assess the effectiveness of adipocyte-derived mesenchymal stem cells-conditioned media (ADSC-CM) formulation in telogen effluvium patients.
Methods: A retrospective cohort study was conducted at a dermatology clinic in Jeddah, Saudi Arabia. The study included 50 consecutive patients aged 20– 70 years, who were diagnosed with telogen effluvium. All patients received five monthly sessions of the same commercial ADSC-CM formulation, using a standardized application protocol. Pre- and post-intervention changes in trichometry parameters were analyzed.
Results: There was a significant increase in mean hair density (up to 29.01 hair/cm2; effect size 0.7– 1.0), cumulative hair thickness (up to 2.67 units; effect size 0.7– 1.4), and the number of follicular hair units (up to 19.96%; effect size 1.0– 1.3) in all scalp regions (p < 0.001), associated with a decrease in mean trichometry-derived Sinclair scale by 0.8– 1.3 (p < 0.001). Positive outcomes were observed in 70%– 92% of the patients depending on the parameter and scalp region. There was no impact of the patient’s age on ADSC-CM efficacy.
Conclusion: ADSC-CM was successfully applied as a new treatment option for patients with telogen effluvium. These findings provide another therapeutic and research area for dermatologists to optimize the management of telogen effluvium and reduce its impact on patients.
Plain Language Summary: Hair loss can be a distressing experience. Telogen effluvium, a common type of hair loss, was the focus of a study in Saudi Arabia. The goal? To see if a new treatment using a mixture of cytokines, growth factors, and proteins from fat cell-derived stem cells (known as ADSC-CM) could help.
The researchers worked with 50 people aged between 20 and 70, all of whom were experiencing telogen effluvium. The treatment involved five monthly sessions of the ADSC-CM product. The team measured aspects of hair growth before and after the treatment.
What did they find? Encouraging signs! Hair density and thickness significantly improved across the scalp. Additionally, the severity of the hair loss condition was reduced. Between 70% and 92% of people saw positive changes depending on the area of the scalp and the specific measurement.
The exciting part is that the age of the person did not affect how well the treatment worked. This suggests it could benefit a wide range of people.
In a nutshell, this study suggests ADSC-CM may be a new and effective way to help people experiencing telogen effluvium. More research is needed, but it is a promising avenue for dermatologists to explore and could potentially help reduce the impact of hair loss on people’s lives.
Keywords: hair loss, alopecia, stem cells, stem-cell conditioned media, hair regeneration
Introduction
Telogen effluvium (TE) is characterized by an abnormal behavior of hair follicles under stress, where follicles in the growing stage (anagen phase) are prematurely transformed into the resting stage (telogen phase), leading to temporary hair loss.1 Clinically, TE presents as a diffuse, non-scarring alopecia that occurs 2–3 months after a stressful event acting as a triggering factor, and typically lasts less than 6 months except in chronic forms.2 A range of physical or psychological stressors can trigger TE.2 Although TE affects both genders, women tend to be more susceptible and anxious to this condition.3
Epidemiological data of TE are limited. However, in clinical settings, it is recognized as one of the most common causes of diffuse alopecia among adults.2 Regarding pathogenesis, TE is believed to possess several pathogenic types that involve perturbations of the follicular cycle at different stages.4
Despite that acute TE is usually self-limited, the identification or exclusion of triggering factors is essential to prevent progression to chronic, more difficult to manage forms.5 Therefore, the first-line management is to identify and manage the underlying cause.6,7 Besides, there are no current US FDA approved treatments, and only a few drugs have demonstrated their efficacy, all being used off-label.6 These include topical and oral minoxidil, corticosteroids, and CNPDA (caffeine, niacinamide, panthenol, dimethicone and an acrylate polymer).5,8,9 Nevertheless, these therapeutic modalities have efficacy limitations and significant side effects.10 Platelet-rich plasma (PRP) aiming to improve the angiogenesis and to reduce the inflammation could be useful in managing acute/chronic TE triggered by COVID-19 or its psychological consequences, but there are no published studies on the use of PRP for TE to date.11
Recently, adipocyte-derived mesenchymal stem cells-conditioned media (ADSC-CM) have attracted the attention as a hair regeneration therapy.12 These molecules, based on a mixture of cytokines, growth factors, and other proteins secreted by adipose-derived mesenchymal cells, have been demonstrated to promote the proliferation of hair follicle cells.12–14 Recent clinical studies reported encouraging results for treating alopecia with adipose-derived stem cell (ADSCs) extracts.12,13,15 These results are mostly obtained from patients affected by androgenetic alopecia.16
However, the benefits of ADSC-CM in TE have not been investigated in previous studies. Hence, the present study assessed the efficacy of a commercialized ADSC-CM formulation in TE by measuring the subsequent changes in trichometry parameters, and analyzed the effect of the patient’s age and TE type on the therapeutic response.
Materials and Methods
Design and Setting
A retrospective cohort study was conducted at the author’s dermatology clinic in Jeddah, Saudi Arabia, between August 2020 and December 2021.
Population
We included 50 consecutive patients aged 18–65 years, who were diagnosed with TE at the clinic, and who received five monthly sessions of ADSC-CM during the study period. We excluded patients who had one of the following conditions: another adjacent hair or scalp disease; any hair treatments or procedures in the past six months; active cancer; previous radiotherapy or chemotherapy; other causes of hair loss including scarring and other non-scarring alopecia such as androgenetic alopecia, alopecia areata, and trichotillomania.
Case Definition
TE was diagnosed based on a detailed history including identification of triggering factors, physical examination including a positive hair pull test, clinical photography, and confirmed by trichoscopy;17 noting that a negative hair pull test does not exclude the diagnosis.4 Hair pull test may also be negative in chronic TE.18 On trichoscopy, TE can show the presence of empty follicles and numerous short regrowing hairs of normal thickness. These findings are usually diffuse and include the occipital area. Furthermore, TE is sometimes a diagnosis of exclusion on trichoscopy.
ADSC-CM Procedure
All included patients underwent five sessions of ADSC-CM with a one-month interval between each two consecutive sessions. The efficacy and potential adverse effects of ADSC-CM were explained to the patients, who signed a written consent. All patients received the same commercial ADSC-CM formulation, namely Advanced Adipose-derived stem cell Protein Extract (AAPE®) (Prostemics Co., Ltd., Seoul, South Korea). The scalp area was gently cleansed prior to the application of 3 mL of the ADSC-CM solution with an automated microneedling device (Dermapen, USA) at 1.0-mm depth throughout the scalp. No topical or intralesional anesthesia was needed.
Trichometric Examination
Trichometry parameters for the participants were determined using trichoscopic images captured with a Medicam 800 computerized video dermatoscope (FotoFinder Systems GmbH, Bad Birnbach, Germany), in conjunction with Tricholab digital image analysis (TrichoLAB, Warsaw, Poland). For each participant, one image was acquired at 20x magnification and four additional images at 70x magnification; these were captured from both the frontal midline and occipital midline regions. This standardized image acquisition protocol ensured consistent and reliable measurements of trichometry parameters throughout the study population.
Data Collection
Relevant data was extracted on an Excel sheet from the patient medical records. These included age and gender and pre- and post-treatment trichometry findings for all three scalp regions (frontal, temporal and occipital). Trichometry parameters included hair density (N/cm2), average hair shaft thickness (AHST), percentage of thin (<30μm), medium (30–50μm), and thick (>50μm) hairs, cumulative hair thickness (CHT, mm/cm2), number of follicular units (NFU, %), and trichometry-derived Sinclair scale (TDSS).
Outcome Definition
Trichometry was performed at baseline and one month following the last session of ADSC-CM. Trichometry parameters were divided into two subgroups: positive and negative parameters. Positive parameters consisted of those for which an increase indicates favorable response; these include: hair density; AHST; % thick hair; cumulative hair thickness; and number of follicular units. Negative parameters consisted of those for which a decrease indicates favorable response; these include: % thin hair; % medium hair; and derived Sinclair grade. For each parameter, we calculated an absolute and adjusted relative change, as follows:
Where X: the given parameter; x0: pre-intervention level of X; x1: post-intervention level of X; Mean X0: the pre-intervention population mean of X.
Statistical Methods
Data were analyzed using the Statistical Package for Social Sciences version 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to summarize the study variables, both in pre- and post-intervention times. Pre-to-post changes in trichometry parameters were analyzed using paired t-test, with calculation of the mean absolute change and the effect size using Cohen’s D method. Favorable response rate was calculated as the percentage of participants who had a favorable response to a given parameter, by scalp region. Likewise, mean absolute changes and mean adjusted relative changes were calculated among favorable responders for each parameter, by scalp region. The effect of age was explored using Pearson’s correlation between age and absolute change of each parameter separately. The effect of TE type, secondary versus idiopathic, was analyzed by comparing the mean absolute change of each parameter between the two types using Mann-Whitney U test. A p-value of <0.05 was considered to reject the null hypothesis.
Ethical Considerations
The present study was designed and conducted in compliance with the standard international ethical principles and in accordance with the Declaration of Helsinki. The study protocol was ethically approved by the Bioethics Committee of Scientific and Medical Research of the University of Jeddah (Ref#UJ-REC-071). It is a standard practice in our clinic to obtain written informed consent from all patients. This consent specifically permits the use of participants’ clinical data for future research activities, ensuring the principles of anonymity, privacy, and autonomy of the individual participants. All consent procedures in this study were conducted in accordance with ethical guidelines and regulations.
Results
Participant’s Characteristics
Majority of the patients were females (96.0%). The mean (SD) age was 37.40 (19.95) years (range 20–70 years). Secondary and idiopathic TE were found in 25 patients each. Anemia (24.0%) and hormonal disorders (26.0%) were the most frequent triggering factors of TE (Table 1).
 |
Table 1 Baseline Demographic and Clinical Parameters (N = 50) |
Pre- and Post-Intervention Trichometry Findings
We observed a significant increase in mean hair density (up to 29.06 hair/cm2), CHT (up to 2.67 mm/cm2), and number of follicular hair units (up to 18.96%) with large effect sizes in all three scalp regions (p < 0.001). We further observed a decrease in the mean TDSS by 0.21–0.36 (effect size 0.8–1.3), depending on the scalp region (p < 0.001). Several other parameters showed improvement, such as an increase in AHST and thick hair and decrease in thin hair; however, these were of small effect size and did not concern all scalp regions (Table 2, Figure 1).
 |
Table 2 Pre- and Post-Intervention Trichometry Findings by Scalp Region (Intrasubject Analysis) |
Figure 2 shows the clinical photography of a 39-year-old female patient before and after ADSC-CM, where we note an aesthetic improvement of the midline and bitemporal areas. Figure 3 shows the trichometry images and parameters of the same patient in pre- versus post ADSC-CM.
 |
Figure 3 Pre- and post ADSC-CM trichometry of a 39-year-old female patient with telogen effluvium. |
Favorable Response Rate and Extents of Change Among Responders
ADSC-CM resulted in a high percentage of responders notably for hair density (78–84%), CHT (78–92%), NFU (80–90%), and TDSS (70–88%), depending on the scalp region. The response rates as well as the mean absolute and adjusted relative changes for each parameter by scalp region for responders are depicted in Table 3.
 |
Table 3 Favorable Response Rate by Trichometry Parameter and Extent of Change Among Responders |
Effect of Age
No significant correlation was found between age and any of the parameters’ absolute change (Pearson’s correlation r = −0.265 to 0.165, p > 0.05), implying that the extent of improvement is not correlated to patient’s age (Results not presented in Tables).
Effect of Telogen Effluvium
Likewise, we observed no significant difference in the absolute change of any of the parameters between secondary and idiopathic TE (p > 0.05); except for thin hair (%) in the occipital region, where there was a significant decrease in secondary TE (median absolute change = −3.0; IQR = 4.50) compared with idiopathic TE (0.0 [3.50]) (p = 0.008) (Table 4).
 |
Table 4 Comparison of the Absolute Change in Trichometry Parameters Between Idiopathic and Secondary Telogen Effluvium After Treatment with ADSC-CM |
Discussion
Summary of the Findings
This is the first study providing clinical evidence of the efficacy of ADSC-CM in TE. The 5-month course of topical scalp application of a commercial ADSC-CM solution, with an automated microneedling device, induced considerable diffuse improvements in all trichoscopy parameters, notably hair density (up to 29 hairs/cm2), CHT (up to 2.7 mm/cm2) and NFU (up to 19%), with large effect size (Cohen’s D 0.7–1.4). This resulted in a decrease of 0.21–0.36 (mean absolute change) of TDSS depending on the scalp region. These positive results were observed in majority of the patients with a response rate ranging between 70% and 92% depending on the parameter and scalp region. Moreover, the efficacy of ADSC-CM was not impacted by age or TE type.
Evidence of ADSC-Based Therapy Efficacy in Different Types of Alopecia
Although there is no data in the literature regarding the clinical efficacy of ADSC-CM in TE, there is growing evidence supporting the utility of ADSC-based therapies in improving different hair loss disorders. In a study involving 40 patients with alopecia, scalp intradermal injections of ADSC-CM were associated with significant increase in hair density and anagen hair rate.19 Fukuoka et al performed several studies using ADSC-CM as treatment for alopecia and reported a remarkable efficacy with convenience and tolerance.12,13,15 Tak et al conducted a randomized controlled trial to determine the efficacy of a 16-week self-application of topical ADSC constituent extract in 38 patients. Authors observed remarkably improved hair count (28.1% vs 7.1%) along with improvement of hair thickness (14.2% vs 6.3%) in the intervention group when compared with the control group, respectively.20 Similarly, a study conducted by Shin et al in 27 female patients with pattern hair loss treated with ADSC-CM for 12 weeks showed an increase of 16.4% and 11.3% in the mean hair density and mean hair thickness, respectively.21
Other researchers used ADSC-based therapies to boost the effect of other therapeutic procedures. Zanzottera et al used ADSC and growth factors after hair transplantation, which accelerated the healing of the micro-wounds (after only 2 weeks) and boosted the growth time of the transplanted hair up to two months after the procedure.22 Lee et al reported improved hair densities and global improvement scores in patients with androgenetic alopecia who received application of ADSC-CM after non-ablative fractional laser treatment.23 Anderi et al presented a study in 20 patients with alopecia areata from the Middle East, who were treated with scalp transplantation of adipose-derived stromal vascular cells, which is a similar method to ADSC but differs in using freshly isolated cells. Postoperative assessments showed significant hair regeneration among all participants, and 6-month assessments showed increased mean hair density from 85.1 to 121.1 hair/cm2, decreased pull test (4.4 to 0.8), and increased hair diameter (60.5 to 80.8μ) with reference to baseline.24 These observations support various clinical applications of ADSC-CM in hair loss.
Possible Mechanism of Action of ADSC-CM in TE
Adipose tissue is highly rich in mesenchymal cells. Thus, the number of stem cells found in one cubic centimeter of adipose tissue is 100 to 1,000 times higher than that in bone marrow.16 ADSC promotes hair growth by providing a supportive environment of various growth factors that stimulate hair follicles regeneration. Additionally, ADSC-based therapies appear to have anti-hair loss effects through the protection of human dermal papilla cells (mesenchymal cells in the hair follicle) from androgen and reactive oxygen species-mediated cytotoxicity.25 A study revealed the potential positive effect of ADSCs in increasing the mitogenesis and survival of human dermal papilla cells, while maintaining their trichogenic ability.26 Furthermore, ADSC-CM activates human keratinocyte proliferation and migration.27
Growth factors, whether endogenous or exogenous, enhance the growth and migration of follicular outer root sheath cells by stimulating the nuclear translocation of β-catenin, and the upregulation of Wnt10b, β-catenin, epidermal growth factor (EGF) receptor and SOX9.28 Fibroblast growth factors anticipate and prolong anagen phase in resting (telogenic) hair follicles via earlier induction of β-catenin and Sonic hedgehog in hair follicles.29 Vascular endothelial growth factor (VEGF) stimulates perifollicular vasculogenesis and angiogenesis, which favors hair growth by supplying nutrients to the developing hair follicle, and increases the follicle diameter.16,30 Platelet-derived growth factor (PDGF) signaling was shown to enhance maintenance and self-renewal of adult hair follicle dermal stem cell.31 Insulin-like growth factor 1 (IGF-1) is an important mitogenic and morphogenetic mediator in hair follicle biology that enhances follicular proliferation and differentiation, tissue remodeling, and the hair growth cycle.32
On the other hand, the development of TE is influenced by several growth factors.33 Decreased expression of VEGF, keratinocyte growth factor (KGF), and EGF are associated with higher susceptibility to TE, whereas elevated levels of transforming growth factor-beta 1 (TGF-β1) were found in patients with TE. Hence, growth factors therapy associated with iontophoresis technique allowed early stop of hair shedding in TE-related alopecia.34 This suggests the potential use of ADSC-CM in the management of TE. A randomized trial involving 30 female subjects with TE showed significant increase in anagen hairs and reduction in telogen hairs after treatment with biomimetic peptides that induced an elevation in VEGF and EGF expression and a decrease in KGF and TGF-β1 expression.35 However, evidence on possible pro-alopecic action of growth factors is weak compared with the strong evidence of their substantial anti-alopecic effects.36
Efficacy in Idiopathic versus Secondary TE
In this study, anemia and hormonal disease factors were the most common causes of TE accounting for 50% of the patients. Half of the patients had secondary and other half of the patients had idiopathic TE. All patients with a treatable triggering factor were referred to the corresponding specialist doctor for the management of the given disease in parallel with ADSC-CM treatment. Although the prevalence of either type of TE is not clearly documented, idiopathic TE is likely to be less common especially in acute TE.4
Besides iron deficiency anemia, thyroid disorders, including hyper or hypothyroidism, can also cause TE.37 In Saudi Arabia, a study showed that approximately, 94.9% of females with TE had iron-deficiency anemia and 21.1% had hypothyroidism, while other triggering factors were found in less than 12% of the cases.38 TE may further be triggered by organ failure and systemic conditions such as liver and renal disorders, systemic lupus erythematosus, dermatomyositis, and secondary syphilis.37 Drugs inducing TE include psychotropic drugs, anticoagulants, cardiovascular drugs, retinoids, and antithyroid drugs.39 Local inflammatory conditions of the scalp, mostly seborrheic dermatitis and psoriasis, are also described in TE patients.8
Chronic TE, or primary TE, is an idiopathic form of TE that lasts for more than 6 months, first described by Whiting in 1996.40 It is diagnosed by exclusion and usually affects females aged between 30 and 50 years. It is marked by increased hair shedding, loss of volume, and bitemporal hair thinning. A family history of androgenetic alopecia is much more frequent in female androgenetic alopecia than it is in chronic telogen effluvium.37 From a pathogenesis perspective, it is proposed that chronic TE results from a shortening of the anagen, or active growth phase, in the hair cycle, without a concurrent miniaturization of the hair follicle.41
This study showed no difference in ADSC-CM efficacy between idiopathic and secondary TE. This indicates the potential of ADSC-CM to enhance hair follicle cells regrowth in TE regardless of the underlying triggering mechanism. The efficacy of ADSC-CM in idiopathic TE may be explained by maintaining the anagen stage and preventing early transition to telogen stage, thereby reversing one of the probable mechanisms of idiopathic TE.42 In secondary TE, the triggering factors result in a sustained pro-apoptotic insult that exerts continuous antimitotic effects on hair keratinocytes, which is proportional to the severity and duration of the triggering factor.43 ADSC-CM probably decreases these pro-apoptotic effects by providing potent anti-apoptotic stimuli resulting in hair regeneration.
Potential Advantages of ADSC-CM Over Other Therapies in TE
ADSCs and their secretomes media are convenient, non-invasive methods with high tolerability profiles and clinical practicality.12 On the other hand, other available treatment options for TE including topical or oral minoxidil and corticosteroids have several disadvantages and may induce side effects that should be taken into consideration; especially when these approaches do not provide satisfactory results.10 Another important advantage with ADSC-CM is that its effects are not dependent on age as demonstrated in the study findings. Since TE affects patients at any age, this would expand the use of ADSC-CM to all adult age categories.3
Combining Microneedling with ADSC-CM
Microneedling (MN) is a minimally invasive procedure in which multiple fine needles are used to create micropunctures in the skin. MN can be paired with other technologies to enhance drug absorption and reduce pain during the procedure, as demonstrated in our study. Additionally, MN can be used to stimulate neovascularization, promote the expression of Wnt proteins, and facilitate the release of growth factors.44 Microneedling has been most extensively researched in the context of androgenetic alopecia. Dhurat et al compared the outcomes of weekly MN sessions combined with a twice-daily application of 5% minoxidil solution over 12 weeks to the use of 5% minoxidil alone applied twice daily. Results indicated that the MN group experienced better hair growth outcomes compared to the use of minoxidil alone, and MN proved effective for patients who are unresponsive to minoxidil treatment.45 Furthermore, while data on MN efficacy in treating alopecia areata are limited, no studies have assessed its use for the management of acute or chronic TE. The relative effectiveness of MN alone, compared to other treatments for hair loss, remains uncertain. This is because clinical trials investigating MN are frequently small, lack randomization and control, or involve combination therapies.46
Safety of ADSC-CM
No significant safety concerns were observed in the 50 study patients, either in early or late post-intervention time. No major adverse effects have been previously reported in the literature. Fukuoka et al used ADSC-based therapy for over 1,000 patients without observing any case of allergic reaction or infection, or other severe complications.12 However, safety and tolerability profiles can only be confirmed after establishing evidence from large-scale controlled trials with long-term follow-up.
The Need for Further Studies
To support the current study conclusions, further well-designed, large-scale randomized controlled trials are warranted, comparing ADSC-CM with placebo or other treatment options in TE. The importance of controlled trials to establish efficacy is crucial notably in TE, as this condition is characterized by spontaneous resolution in many cases. This implies assessing the eventual benefit of ADSC-CM in accelerating the healing process of TE, besides further rigorous risk-benefit assessment.
Limitations
This study is limited by the retrospective design, absence of a control group, as well as the small size of the cohort, which may impede the generalizability of the findings. Our study’s findings may be also limited by the lack of long-term follow-up, as chronic TE tends to fluctuate over a period of several years. Another limitation includes the concurrent use of MN with ADSC-CM, as the observed improvement may be partially attributed to MN. A comparative study examining the effects of ADSC-CM with MN versus MN alone in managing TE would be useful.
Conclusion
ADSC-CM was successfully applied as a new treatment strategy for patients with TE, showing a high efficacy in inducing hair regrowth among the majority of the study patients. These findings add to the multiple therapeutic applications of ADSC-CM, providing another therapeutic and research area for dermatologists to optimize the management of TE and reduce its aesthetic and psychological impact. Future research and clinical applications will help expanding the current understanding of the pathogenic mechanisms of TE and the potential role of ADSCs secretome in hair regeneration.
Acknowledgments
The author thanks Dr Mohamed Amine HAIRECHE for his valuable assistance in analyzing the data of the present study. The authors also thank TrichoLAB (Warsaw, Poland) for providing the pre- and post trichoscopy report evaluations for the study patients.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Kligman AM. Pathologic dynamics of human hair loss. I. Telogen effluvium. Arch Dermatol. 1961;83(2):175–198. doi:10.1001/archderm.1961.01580080005001
2. Grover C, Khurana A. Telogen effluvium. Indian J Dermatol Venereol Leprol. 2013;79(5):591–603. doi:10.4103/0378-6323.116731
3. Hughes EC, Saleh D. Telogen effluvium. In: Treasure Island (FL); 2022.
4. Headington JT. Telogen effluvium. New concepts and review. Arch Dermatol. 1993;129(3):356–363. doi:10.1001/archderm.1993.01680240096017
5. Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen effluvium: a review of the literature. Cureus. 2020;12(5):e8320. doi:10.7759/cureus.8320
6. Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01–WE03. doi:10.7860/JCDR/2015/15219.6492
7. Burroway B, Griggs J, Martinez-Velasco MA, Tosti A. Telogen effluvium. In: Hair and Scalp Treatments. Cham: Springer International Publishing; 2020:125–138.
8. Bayart C, Bergfeld WF. Telogen Effluvium. In: McMichael AJ, Hordinsky MK, editors. Hair and Scalp Disorders.
9. Perera E, Sinclair R. Treatment of chronic telogen effluvium with oral minoxidil: a retrospective study. F1000Res. 2017;6:1650. doi:10.12688/f1000research.11775.1
10. Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021;20(12):3759–3781. doi:10.1111/jocd.14537
11. Gentile P. Hair Loss and Telogen Effluvium Related to COVID-19: the Potential Implication of Adipose-Derived Mesenchymal Stem Cells and Platelet-Rich Plasma as Regenerative Strategies. Int J Mol Sci. 2022;23(16):9116. doi:10.3390/ijms23169116
12. Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther. 2017;12(7):531–534. doi:10.2174/1574888X12666170522114307
13. Fukuoka H, Suga H, Narita K, Watanabe R, Shintani S. The latest advance in hair regeneration therapy using proteins secreted by adipose-derived stem cells. Am J Cosmetic Surg. 2012;29(4):273–282. doi:10.5992/AJCS-D-12-00015.1
14. Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761–771. doi:10.1016/j.cell.2011.07.019
15. Fukuoka H, Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up with trichograms. Eplasty. 2015;15:e10.
16. Nepal S, Venkataram A, Mysore V. The role of adipose tissue in hair regeneration: a potential tool for management?. J Cutan Aesthet Surg. 2021;14(3):295–304. doi:10.4103/JCAS.JCAS_47_19
17. Shrivastava S. Diffuse hair loss in an adult female: approach to diagnosis and management. Indian J Dermatol Venereol Leprol. 2009;75(1):20. doi:10.4103/0378-6323.45215
18. Cranwell WC, Sinclair R. Telogen effluvium. In: Alopecia. Elsevier Health Sciences; 2019:83–93
19. Narita K, Fukuoka H, Sekiyama T, Suga H, Harii K. Sequential scalp assessment in hair regeneration therapy using an adipose-derived stem cell-conditioned medium. Dermatol Surg. 2020;46(6):819–825. doi:10.1097/DSS.0000000000002128
20. Tak YJ, Lee SY, Cho AR, Kim YS. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl Med. 2020;9(8):839–849. doi:10.1002/sctm.19-0410
21. Shin H, Ryu HH, Kwon O, Park BS, Jo SJ. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: a retrospective case series study. Int J Dermatol. 2015;54(6):730–735. doi:10.1111/ijd.12650
22. Zanzottera F, Lavezzari E, Trovato L, Icardi A, Graziano A. Adipose derived stem cells and growth factors applied on hair transplantation. Follow-up of clinical outcome. J Cosmet Dermatol Sci Appl. 2014;4(04):268–274. doi:10.4236/jcdsa.2014.44036
23. Lee YI, Kim J, Kim J, Park S, Lee JH. The effect of conditioned media from human adipocyte-derived mesenchymal stem cells on androgenetic alopecia after nonablative fractional laser treatment. Dermatol Surg. 2020;46(12):1698–1704. doi:10.1097/DSS.0000000000002518
24. Anderi R, Makdissy N, Azar A, Rizk F, Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther. 2018;9(1):141. doi:10.1186/s13287-018-0889-y
25. Won CH, Park GH, Wu X, et al. The basic mechanism of hair growth stimulation by adipose-derived stem cells and their secretory factors. Curr Stem Cell Res Ther. 2017;12(7):535–543. doi:10.2174/1574888X12666170829161058
26. Nilforoushzadeh MA, Aghdami N, Taghiabadi E. Effects of adipose-derived stem cells and platelet-rich plasma exosomes on the inductivity of hair dermal papilla cells. Cell J. 2021;23(5):576–583. doi:10.22074/cellj.2021.7352
27. Moon KM, Park YH, Lee JS, et al. The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int J Mol Sci. 2012;13(1):1239–1257. doi:10.3390/ijms13011239
28. Liu J, Xiao Q, Xiao J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. doi:10.1038/s41392-021-00762-6
29. Hong LW, Xiang LJ, Shi HX, et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015;2015:730139. doi:10.1155/2015/730139
30. Gnann LA, Castro RF, Azzalis LA, et al. Hematological and hepatic effects of vascular epidermal growth factor (VEGF) used to stimulate hair growth in an animal model. BMC Dermatol. 2013;13(1):15. doi:10.1186/1471-5945-13-15
31. González R, Moffatt G, Hagner A, et al. Platelet-derived growth factor signaling modulates adult hair follicle dermal stem cell maintenance and self-renewal. NPJ Regen Med. 2017;2(1):11. doi:10.1038/s41536-017-0013-4
32. Trüeb RM. Further clinical evidence for the effect of IGF-1 on hair growth and alopecia. Skin Appendage Disord. 2018;4(2):90–95. doi:10.1159/000479333
33. Kubanov AA, Gallyamova YA, Korableva OA, Kalinina PA. The role of the VEGF, KGF, EGF, and TGF-β1 growth factors in the pathogenesis of telogen effluvium in women. Biomed Pharmacol J. 2017;10(1):191–198. doi:10.13005/bpj/1097
34. Alessandrini AM, Bruni F, Piraccini BM, Starace M. The effectiveness and tolerability of preformed growth factors vehiculated through iontophoresis on patients with androgenetic alopecia and telogen effluvium: a clinical study. Dermatol Pract Concept. 2021;11(3):e2021082. doi:10.5826/dpc.1103a82
35. Kubanov AA, Gallyamova YA, Korableva OA. A randomized study of biomimetic peptides efficacy and impact on the growth factors expression in the hair follicles of patients with telogen effluvium. J Appl Pharm Sci. 2018;8(4):15–22.
36. El-Refai AM, Elhabak DM, Khashaba RA. More is not always better in hair growth factors. epidermal growth factor: hair growth factor involved in alopecia areata pathogenesis. Int J Trichol. 2020;12(4):182–187. doi:10.4103/ijt.ijt_51_20
37. Harrison S, Sinclair R. Telogen effluvium. Clin Exp Dermatol. 2002;27(5):389–395. doi:10.1046/j.1365-2230.2002.01080.x
38. Fatani MI, Bin Mahfoz AM, Mahdi AH, et al. Prevalence and factors associated with telogen effluvium in adult females at Makkah region, Saudi Arabia: a retrospective study. J Dermatol Dermatol Surg. 2015;19(1):27–30. doi:10.1016/j.jdds.2014.04.002
39. Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31(1):67–73. doi:10.1016/j.det.2012.08.002
40. Whiting DA. Chronic telogen effluvium. Dermatol Clin. 1996;14(4):723–731. doi:10.1016/S0733-8635(05)70398-3
41. Glimore S, Sinclair R. Chronic telogen effluvium is due to a reduction in the variance of anagen duration. Australas J Dermatol. 2010;51(3):163–167. doi:10.1111/j.1440-0960.2010.00654.x
42. Xiao S, Deng Y, Mo X, et al. Promotion of hair growth by conditioned medium from extracellular matrix/stromal vascular fraction gel in C57BL/6 Mice. Stem Cells Int. 2020;2020:1–11. doi:10.1155/2020/9054514
43. Rebora A. Telogen effluvium: a comprehensive review. Clin Cosmet Investig Dermatol. 2019;12:583–590. doi:10.2147/CCID.S200471
44. Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss?. J Eur Acad Dermatol Venereol. 2018;32(4):564–569. doi:10.1111/jdv.14722
45. Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. 2013;5(1):6–11. doi:10.4103/0974-7753.114700
46. Gupta AK, Quinlan EM, Venkataraman M, Bamimore MA. Microneedling for hair loss. J Cosmet Dermatol. 2022;21(1):108–117. doi:10.1111/jocd.14525
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.