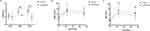Back to Journals » Drug Design, Development and Therapy » Volume 18
Effect of S-Ketamine on Postoperative Nausea and Vomiting in Patients Undergoing Video-Assisted Thoracic Surgery: A Randomized Controlled Trial
Authors Qi Y , Zhou M, Zheng W, Dong Y, Li W, Wang L, Xu H, Zhang M, Yang D, Wang L, Zhou H
Received 14 November 2023
Accepted for publication 7 April 2024
Published 16 April 2024 Volume 2024:18 Pages 1189—1198
DOI https://doi.org/10.2147/DDDT.S449705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Yu Qi,1– 3,* Meiyan Zhou,1– 3,* Wenting Zheng,1,2,* Yaqi Dong,1,2 Weihua Li,2,4 Long Wang,1,2 Haijun Xu,1,2 Miao Zhang,1,5 Dunpeng Yang,1,5 Liwei Wang,1– 3 Hai Zhou1– 3
1The Xuzhou Clinical College of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, Xuzhou Central Hospital, Xuzhou, Jiangsu, People’s Republic of China; 3Jiangsu Province Key Laboratory of Anesthesiology Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 4College of Anesthesiology, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 5Department of Cardiothoracic Surgery, Xuzhou Central Hospital, Xuzhou, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hai Zhou; Liwei Wang, The Xuzhou Clinical College of Xuzhou Medical University, Department of Anesthesiology, Xuzhou Central Hospital, Xuzhou, Jiangsu, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Postoperative nausea and vomiting (PONV) frequently occur in patients after surgery. In this study, the authors investigated whether perioperative S-ketamine infusion could decrease the incidence of PONV in patients undergoing video-assisted thoracoscopic surgery (VATS) lobectomy.
Patients and Methods: This prospective, randomized, double-blinded, controlled study was conducted a total of 420 patients from September 2021 to May 2023 at Xuzhou Central Hospital in China, who underwent elective VATS lobectomy under general anesthesia with tracheal intubation. The patients were randomly assigned to either the S-ketamine group or the control group. The S-ketamine group received a bolus injection of 0.5 mg/kg S-ketamine and an intraoperative continuous infusion of S-ketamine at a rate of 0.25 mg/kg/h. The control group received an equivalent volume of saline. All patients were equipped with patient-controlled intravenous analgesia (PCIA), with a continuous infusion rate of 0.03 mg/kg/h S-ketamine in the S-ketamine group or 0.03 μg/kg/h sufentanil in the control group. The primary outcome was the incidence of PONV. Secondary outcomes included perioperative opioid consumption, hemodynamics, postoperative pain, and adverse events.
Results: The incidence of PONV in the S-ketamine group (9.7%) was significantly lower than in the control group (30.5%). Analysis of perioperative opioid usage revealed that remifentanil usage was 40.0% lower in the S-ketamine group compared to the control group (1414.8 μg vs 2358.2 μg), while sufentanil consumption was 75.2% lower (33.1 μg vs 133.6 μg). The S-ketamine group demonstrated better maintenance of hemodynamic stability. Additionally, the visual analogue scale (VAS) scores on postoperative day 1 (POD-1) and postoperative day 3 (POD-3) were significantly lower in the S-ketamine group. Finally, no statistically significant difference in other postoperative adverse reactions was observed between the two groups.
Conclusion: The results of this trial indicate that perioperative S-ketamine infusion can effectively reduce the incidence of PONV in patients undergoing VATS lobectomy.
Keywords: S-ketamine, postoperative nausea and vomiting, hemodynamics, non-opioid analgesic, postoperative pain, video-assisted thoracoscopic surgery lobectomy
Introduction
Postoperative nausea and vomiting (PONV), which refers to a combination of nausea, vomiting, and/or retching occurring after surgery, is reported to affect 12% to 38% of patients.1,2 The incidence of PONV is significantly higher, reaching up to 70–80%, in high-risk patients, such as females, those with a history of PONV and/or motion sickness, nonsmokers, and younger individuals.3,4 PONV has been associated with complications including pulmonary embolism, dehydration, disturbances in water and electrolyte balance, increased physical burden on patients, prolonged hospitalization, and higher medical costs.5–7 Among the various factors influencing PONV, anesthesia-related factors such as inhaled anesthetics, intraoperative and postoperative opioid use, duration and type of anesthesia, and intraoperative infusion volume have been found to be particularly significant.6,7 Multiple clinical studies have consistently identified opioid dose as the strongest independent risk factor for PONV in surgical patients.8,9 Despite advances in prevention and treatment, the incidence of PONV in thoracic surgery remains as high as 30%. While numerous studies have focused on PONV prophylaxis in thoracic surgery, research on opioid reduction remains limited.
S-ketamine is a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist that exhibits high affinity for NMDA and opiate receptors. Compared to racemic ketamine, S-ketamine possesses twice the anesthetic potency and higher in vivo clearance rate, resulting in a lower incidence of psychotropic adverse reactions at equivalent doses.10–12 The effectiveness of perioperative intravenous S-ketamine as an adjunctive analgesic has been well established. Previous studies have demonstrated that low-dose S-ketamine (0.5mg/kg) can serve as an appealing additive to propofol sedation, offering adequate sedation and analgesia with reduced propofol requirements and fewer cardiopulmonary adverse effects.13 Furthermore, an investigation found that intraoperative intravenous infusion of 0.25 mg/kg/h S-ketamine could decrease postoperative opioid consumption and contribute to postoperative recovery in thoracic surgery patients.14 Recent research has shown that perioperative use of S-ketamine in video-assisted thoracoscopic surgery (VATS) may provide superior pain relief and promote rapid postoperative recovery, likely due to its potent analgesic and anti-inflammatory effects.15
Evidence suggests that opioid-sparing anesthesia is an effective approach to prevent PONV.16 Previous clinical studies have demonstrated the safety and efficacy of S-ketamine in surgical settings. Therefore, the current study was conducted to investigate whether perioperative continuous intravenous S-ketamine infusion can effectively reduce the incidence of PONV in postoperative thoracic surgery patients.
Materials and Methods
Study Design and Participants
This study was a prospective, randomized, double-blind, controlled clinical trial aimed at evaluating the effect of perioperative S-ketamine infusion on postoperative nausea and vomiting (PONV) after video-assisted thoracoscopic surgery (VATS) lobectomy for non-small cell lung cancer, and the control group was infused with the same dose of saline. This study is in line with the principles of the Declaration of Helsinki. The study received approval from the institutional ethics committee of Xuzhou Central Hospital and was registered prior to patient enrollment (No: XZXY-LJ-20210331-044). Registration was completed on the Chinese Clinical Trial Registry (CHICTR) (ChiCTR2100051000). Written informed consent was obtained from all participants before their inclusion in the trial. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials. The trial protocol is available in Supplement 1.
This randomized controlled trial was conducted between September 2021 and May 2023. The trial concluded after completion of the follow-up for the last participant. A total of 445 participants aged between 30 and 75 years, with American Society of Anesthesiologists (ASA) physical status classification ranging from I to III, and scheduled for elective VATS lobectomy under general anesthesia with endobronchial intubation, were evaluated. Patients who were willing to use patient-controlled intravenous analgesia (PCIA) and scheduled to be hospitalized within 72 hours of the procedure were included. The exclusion criteria consisted of the following: (1) known allergy to S-ketamine and non-steroidal anti-inflammatory drugs (NSAIDs); (2) history of uncontrolled or malignant hypertension; (3) risk of increased intracranial pressure; (4) untreated or under-treated hyperthyroidism or gastrointestinal bleeding; (5) psychiatric disease or chronic pain that could confound the analgesic response; (6) inability to read, write, and understand Chinese.
Eligible patients were randomly assigned to either the S-ketamine group or the control group in a 1:1 ratio using a computer-generated allocation sequence (Figure 1). To maintain allocation concealment, group assignments were sealed in sequentially numbered envelopes and handed to a nurse who was not involved in the study. The participants, anesthesiologist, and outcome assessor were blinded to the group assignments. PCIA pumps were placed in opaque bags to ensure blinding.
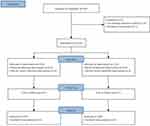 |
Figure 1 Flow diagram. |
Anesthesia and Monitoring
During anesthesia induction, all patients received intravenous midazolam (0.05 mg/kg), propofol (2–3 mg/kg), sufentanil (0.4–0.6 μg/kg), and cis-atracurium besylate (0.2 mg/kg) followed by double-lumen bronchial intubation based on surgical requirements. In the S-ketamine group, patients received an intravenous bolus injection of 0.5 mg/kg S-ketamine after anesthesia induction, while patients in the control group received an equivalent volume of saline. Lung-protective strategies with volume-controlled ventilation were implemented for one-lung ventilation, including a tidal volume of 6–8 mL/kg predicted body weight and peak airway pressure below 30 cmH2O. All participants received an intravenous bolus injection of 0.075 mg palonosetron at the start of the procedure. Anesthesia was maintained using remifentanil (0.1–1.0 µg/kg/min), propofol (4–8 mg/kg/h), and S-ketamine (0.25 mg/kg/h) in the S-ketamine group, while continuous intravenous infusion of remifentanil (0.1–1.0 µg/kg/min) and propofol (4–8 mg/kg/h) was administered in the control group. Intraoperatively, we will dynamically adjust intraoperative analgesic medications based on blood pressure, heart rate, and mean arterial pressure, and we will also monitor intraoperative injury perception using the surgical volume tracing index (SPI) to assess the patient’s level of intraoperative analgesia. A value between 20 and 50 under general anesthesia indicates an appropriate level of analgesia, >50 indicates insufficient analgesia and requires an increase in the dose of remifentanil, and <20 indicates too much analgesia and requires a slower infusion rate. Additional cis-atracurium besylate (0.05–0.1 mg/kg) was given as required for intraoperative muscle relaxation. Infusion of S-ketamine was discontinued 15 minutes before the end of the procedure. Considering the potential impact of fluid therapy on PONV, all patients in this trial followed a restrictive fluid management strategy (crystalloid-to-colloid ratio of 2:1). During the procedure, vital signs including heart rate, oxygen saturation, respiratory rate, body temperature, end-tidal carbon dioxide, mean arterial pressure, electrocardiogram, fluid infusion volume, urinary volume, and bispectral index (BIS) were continuously monitored.
Postoperative Pain Management
In this study, all patients received a patient-controlled intravenous analgesia (PCIA) device. In the control group, patients were equipped with a PCIA consisting of sufentanil (0.03 µg/kg/h) and tropisetron (10 mg) in a total volume of 100 mL, with a continuous infusion rate of 1.5 mL/h for 48 hours. The self-controlled capacity was set at 1.5 mL, with a locking time of 15 minutes. In the S-ketamine group, patients were equipped with a PCIA consisting of S-ketamine (0.03 mg/kg/h) and tropisetron (10 mg) in a total volume of 100 mL, with a continuous infusion rate of 1.5 mL/h for 48 hours. The self-controlled capacity was set at 1.5 mL, with a locking time of 15 minutes. If the Visual Analogue Scale (VAS) score is greater than or equal to 4, an additional 50 mg of flurbiprofen would be administered intravenously, and the PCIA settings would be adjusted to a self-controlled capacity of 2 mL and a locking time of 10 minutes. Patients were also allowed to take acetaminophen orally as supplemental analgesics, with the type and dose of the drug recorded. If the VAS score remained consistently below 1, and symptoms such as respiratory depression, confusion, or unstable blood pressure occurred, the PCIA infusion would be temporarily suspended. The use of PCIA would be resumed once these manifestations improved.
Outcome Measurements
The primary outcome assessed in this study was the incidence of postoperative nausea and vomiting (PONV), which was evaluated using the simplified PONV impact scale(Appendix 1) on postoperative day 1 (POD-1) and postoperative day 3 (POD-3). The simplified PONV impact scale, developed by Myles et al,17 involved a structured interview consisting of two questions. Question 1 addressed whether the patient had vomited or experienced dry-retching, while Question 2 focused on the presence of nausea (“an unsettled feeling in the stomach and a slight urge to vomit”) and its impact on daily activities, such as getting out of bed, moving freely in bed, walking normally, or eating and drinking. Numerical responses to both questions were necessary to calculate the PONV Impact Scale score. A score of 5 on the scale indicated clinically significant PONV.18
The secondary outcomes encompassed several measures, including the total consumption of perioperative opioids, postoperative pain scores at 24 hours and 72 hours (assessed using the Visual Analog Scale or VAS), hemodynamics, and postoperative adverse events. The VAS score(Appendix 2)ranges from 0 (indicating no pain) to 10 (representing the most excruciating pain).19 To assess hemodynamic variables, such as mean blood pressure, systolic blood pressure, diastolic blood pressure, heart rate, and pulse oxygen saturation, measurements were taken at specific time points: before anesthesia induction (T0), before endobronchial intubation (T1), immediately after intubation (T2), after positioning the patient in the lateral decubitus position following anesthesia (T3), 5 minutes after the start of the operation (T4), 30 minutes after the start of the operation (T5), 1 hour after the start of the operation (T6), 90 minutes after the start of the operation (T7), and at the end of the operation (T8). Additionally, the Mini-Mental State Examination Score (MMES), surgery duration, and postoperative adverse events were also monitored.
Statistical Analysis
Based on the preliminary study results, which reported a PONV incidence of 25% on postoperative day 1 (POD 1) in VATS Lobectomy, a sample size of 200 patients per group was calculated to detect a 50% relative risk reduction after surgery, with a two-sided α=0.05 and 90% power. Considering potential loss to follow-up, a total of 445 patients were ultimately included in this trial. The sample size calculation was performed using the PASS software (V.20.0.6, NCSS, Kaysville, USA).
Statistical analysis was conducted using IBM SPSS version 26.0. The Kolmogorov–Smirnov test was utilized to assess the normal distribution of continuous variables. Normally distributed data were presented as mean (SD) and compared using the unpaired, two-tailed t-test. Non-normally distributed data were reported as median (IQR) and analyzed using the Mann–Whitney U-test. Repeated measures analysis of variance was employed to compare different time points within groups. Categorical variables were presented as number (%) and analyzed using the χ2 test or Fisher’s exact test, as appropriate. A two-sided p-value less than 0.05 was considered indicative of a statistically significant difference.
Results
Patients
A total of 445 patients underwent eligibility assessment, and 25 patients were excluded based on the exclusion criteria. Subsequently, 420 patients were randomized into the control group and the S-ketamine group, with 210 patients in each group. Among them, 210 patients were in the control group, and 209 patients were ultimately included in the analysis, as one case was lost to follow-up (Figure 1). Clinical characteristics were comparable between the two groups, with no differences observed in baseline data (Table 1).
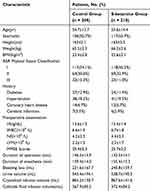 |
Table 1 Patient Demographic and Perioperative Characteristics |
Hemodynamics
There were no significant hemodynamic fluctuations observed among patients before and after anesthesia induction and double-lumen endotracheal tube placement when comparing the two groups. In the control group, we observed hemodynamic fluctuations around ±22% compared to baseline levels after induction. This difference was statistically significant compared to the S-ketamine group (±22% vs ±12%) during induction (Table 2A). Although no statistical differences were observed between the two groups at various time points during maintenance, we found that the control group maintained stable hemodynamics (±16%), whereas the S-ketamine group exhibited hemodynamic fluctuations within ±12% compared to baseline levels (Table 2B).
 |
Table 2 HR, SBP, DBP and MAP at Different Time Points |
Outcome Measures
Firstly, the overall incidence of PONV in the S-ketamine group was significantly lower than that in the control group (9.7% vs 30.5%), indicating a 20.8% reduction in PONV incidence in the S-ketamine group. There were no significant differences in the incidence of PONV in the post-anesthesia care unit (PACU) after surgery. However, the S-ketamine group had a lower incidence of PONV on postoperative day 1 (16.2% vs 2.6%; P < 0.0001) and postoperative day 3 (8.4% vs 1.3%; P<0.0001) compared to the control group (Figure 2A). Furthermore, our study found that the total perioperative consumption of remifentanil (1414.8±296.3 μg vs 2358.2±548.1 μg; P<0.0001) (Figure 2B) and sufentanil (33.1 ± 17.4 μg vs 133.6±28.2 μg; P<0.0001) (Figure 2C) was significantly lower in the S-ketamine group compared to the control group.
Secondly, there were no significant differences in VAS scores in the PACU after surgery between the two groups. However, the VAS scores in the S-ketamine group were significantly lower than those in the control group on postoperative day 1 and postoperative day 3 (Figure 3A). Rescue analgesia was required by 19 patients (12.3%) in the control group, while only 10 patients (6.67%) in the S-ketamine group required it. Additionally, the results showed that the white blood cell and neutrophil counts were significantly lower in the S-ketamine group on postoperative days 1 and 3 (Figure 3B and C).
Lastly, we evaluated the MMSE scores of the two groups before surgery and on postoperative day 1 and day 3 (POD-1 and POD-3), and no statistically significant changes were observed. Regarding postoperative adverse events, including nightmares, hallucinations, dizziness, delirium, and others, there were no significant differences between the two groups except for PONV (Table 3).
 |
Table 3 Occurrence of Postoperative Adverse Reactions in Patients |
Discussion
In this trial, we observed that the administration of intravenous S-ketamine during anesthesia induction, intraoperative maintenance, and postoperative analgesia effectively reduced the incidence of postoperative nausea and vomiting (PONV) in patients undergoing thoracic surgery by reducing opioid consumption.
The elderly have degenerative changes in the function of numerous organs and a low tolerance for surgical trauma, thus the consequences produced by PONV will be more severe, and the incidence of PONV in thoracic surgery patients has increased to 30%, which aligns with the findings of our study (30.5% in the control group). We observed a significantly lower overall incidence of PONV in the S-ketamine group compared to the control group (9.7% vs 30.5%). Additionally, we analyzed the incidence of PONV at different time points. Our results showed a significant increase in the number of new PONV cases in the control group on postoperative day 1 (POD-1) and postoperative day 3 (POD-3). Based on our analysis of perioperative opioid usage, we found that the S-ketamine group had a 40.0% reduction in remifentanil usage compared to the control group (1414.8 μg vs 2358.2 μg), and sufentanil consumption was 75.2% lower in the S-ketamine group than in the control group (33.1 μg vs 133.6 μg). Consequently, we believe that two important reasons contributed to the reduced incidence of PONV in the S-ketamine group. Firstly, there was a reduction in perioperative opioid consumption, which has been established as a major risk factor for PONV.20 Secondly, S-ketamine helped maintain hemodynamic stability during anesthesia.21 The mechanism might involve lower mean arterial pressure during surgery, which could lead to intermittent hypoperfusion of the brainstem and vestibular system, triggering the release of cytokines, histamine, and serotonin. These substances can stimulate histamine and serotonin receptors in the chemoreceptor areas, potentially resulting in nausea and vomiting.22 Previous studies have suggested that opioid-free anesthesia significantly reduces postoperative nausea and vomiting, making it an appealing option for high-risk PONV patients.18
During anesthesia induction, the administration of intravenous S-ketamine at a dose of 0.5 mg/kg helped maintain stable hemodynamic levels while implementing opioid-sparing anesthesia. The study results demonstrated that the blood pressure of both groups decreased after induction compared to baseline levels. However, the blood pressure of patients in the S-ketamine group could be stabilized at a more consistent level after induction, and there were no excessive hemodynamic fluctuations following the double-lumen endotracheal tube procedure compared to the control group (±12% vs ±22%). These findings confirm the safety and efficacy of S-ketamine for anesthesia induction, which is consistent with previous studies.13,14,21
During the intraoperative anesthesia maintenance phase, the opioid-sparing anesthesia protocol with continuous infusion of S-ketamine at a rate of 0.25 mg/kg/h was employed in the S-ketamine group. At each observation time point, we did not observe significant hemodynamic fluctuations in the S-ketamine group compared to the control group. While conventional anesthesia was able to maintain stable hemodynamics (±16%), the use of S-ketamine in opioid-sparing anesthesia resulted in hemodynamic fluctuations within ±12% compared to baseline levels. A previous study has indicated that the use of S-ketamine during anesthesia induction and maintenance may improve peripheral perfusion and blood pressure. This improvement may be attributed to the activation of the sympathetic nervous system by S-ketamine, thus reducing the sharp decline in blood pressure during anesthesia maintenance.23
As a notable aspect of this trial, we implemented a non-opioid analgesic protocol for postoperative intravenous analgesia via patient-controlled intravenous analgesia (PCIA) using S-ketamine (0.03 mg/kg/h) in combination with tropisetron (10 mg) in the S-ketamine group. The findings demonstrated that this non-opioid analgesic protocol was both safe and effective for postoperative pain management in patients undergoing VATS lobectomy. However, there is a limited number of studies on non-opioid analgesic protocols in thoracic surgery, and further evidence is required before considering its widespread implementation in clinical practice. It is worth noting that our observation indicated that patients undergoing VATS lobectomy experienced fewer serious surgical perioperative complications when subjected to the non-opioid analgesic protocol compared to opioid-balanced anesthesia. This finding aligns with the recommendations of the Thoracic Enhanced Recovery After Surgery Program, which strongly advocates for minimizing opioid usage to improve outcomes.24
Furthermore, this trial also focused on assessing postoperative pain status and complications in patients undergoing opioid-sparing anesthesia and non-opioid analgesic protocols. There was no statistically significant difference in the Visual Analog Scale (VAS) scores between the two groups when patients returned to the ward from the post-anesthesia care unit (PACU) (3.2 vs 3.1). However, during the postoperative follow-up on postoperative day 1 (POD-1) (3.8 vs 4.1) and postoperative day 3 (POD-3) (2.9 vs 3.5), patients in the S-ketamine group exhibited significantly lower VAS scores. Additionally, we found that the number of white blood cells (WBC) in the S-ketamine group was lower than that in the control group on POD-1 (13*109/L vs 14.3*109/L) and POD-3 (10.2*109/L vs 11.8*109/L). Similarly, our study revealed that the neutrophil count on POD-1 (11.5*109/L vs 13.7*109/L) and POD-3 (8.6*109/L vs 11.2*109/L) in the S-ketamine group was significantly lower than that in the control group.
Finally, no statistically significant differences were observed between the two groups in terms of S-ketamine-related adverse reactions and postoperative complications, except for PONV. However, additional studies are warranted to confirm the role of anti-inflammatory effects in the prophylactic effect of S-ketamine on PONV.
Limitations
This study has several limitations. Firstly, the sample size was small, and only Chinese adults were included, which means that certain findings may have limited sample sizes and may not reflect significant differences. Secondly, a larger multicenter and multiethnic study is needed to investigate whether alternative dosages of S-ketamine can be safely used in patients undergoing VATS lobectomy and other types of thoracic surgery, as only one dose of S-ketamine was included in this study. Thirdly, since perioperative opioid consumption was significantly reduced and no biological samples were collected, it is challenging to determine the direct effect of S-ketamine itself. Collecting blood samples could have helped elucidate the possible mechanisms underlying the beneficial effect of S-ketamine on PONV.
Conclusion
The results of this placebo-controlled randomized clinical trial revealed that perioperative S-ketamine infusion reduced the incidence of PONV in patients who underwent VAST lobectomy.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request. Any information we share will be deidentified.
Funding
This study received support from the Key Research and Development Program of Scientific and Technological Innovation in Xuzhou City (No. KC21251), grant (Dr. Yu Qi), the Opening Project of Jiangsu Key Laboratory of Anesthesiology (No. XZSYSKF2021011), grant (Dr. Hai Zhou) and Jiangsu Provincial Key Medical Discipline/Laboratory Construction Unit Fund Project(No. JSDW202231), grant (Dr. Liwei Wang).
Disclosure
The authors declare no competing interests in this work.
References
1. Ahn EJ, Choi GJ, Kang H, Baek CW, Jung YH, Woo YC. Comparison of ramosetron with palonosetron for prevention of postoperative nausea and vomiting in patients receiving opioid-based intravenous patient-controlled analgesia after gynecological laparoscopy. Biomed Res Int. 2017;2017:1–6. doi:10.1155/2017/9341738
2. Wang N, Ding P, Zheng DY, et al. Wearable transcutaneous electrical acupoint stimulation bracelet for prevention of postoperative nausea and vomiting in patients undergoing hysteroscopic surgery: a randomised controlled trial. Br J Anaesth. 2022;129(4):e85–e87. doi:10.1016/j.bja.2022.06.028
3. Hari Y, Satomi S, Murakami C, et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J Anesth. 2022;36(2):265–269. doi:10.1007/s00540-022-03041-y
4. Echeverria-Villalobos M, Fiorda-Diaz J, Uribe A, Bergese SD. Postoperative nausea and vomiting in female patients undergoing breast and gynecological surgery: a narrative review of risk factors and prophylaxis. Front Med. 2022;9:909982. doi:10.3389/fmed.2022.909982
5. Chen J, Tu Q, Miao S, Zhou Z, Hu S. Transcutaneous electrical acupoint stimulation for preventing postoperative nausea and vomiting after general anesthesia: a meta-analysis of randomized controlled trials. Int J Surg. 2020;73:57–64. doi:10.1016/j.ijsu.2019.10.036
6. Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia Analg. 2020;131(2):411–448. doi:10.1213/ANE.0000000000004833
7. Wang D, Long Y, Sun Y, et al. Opioid-free total intravenous anesthesia for thyroid and parathyroid surgery: protocol for a randomized, double-blind, controlled trial. Front Med. 2022;9:939098. doi:10.3389/fmed.2022.939098
8. Nakai A, Nakada T, Okamoto S, et al. Risk factors for postoperative nausea and vomiting after thoracoscopic pulmonary wedge resection: pitfalls of an increased fentanyl dose. J Thorac Dis. 2021;13(6):3489–3496. doi:10.21037/jtd-21-296
9. Ramirez MF, Gorur A, Cata JP. Opioids and cancer prognosis: a summary of the clinical evidence. Neurosci Lett. 2021;746:135661. doi:10.1016/j.neulet.2021.135661
10. Fanta S, Kinnunen M, Backman JT, Kalso E. Population pharmacokinetics of S-ketamine and norketamine in healthy volunteers after intravenous and oral dosing. Eur J Clin Pharmacol. 2015;71(4):441–447. doi:10.1007/s00228-015-1826-y
11. Krystal JH, Charney DS, Duman RS. A New Rapid-Acting Antidepressant. Cell. 2020;181(1):7. doi:10.1016/j.cell.2020.02.033
12. Höfler J, Rohracher A, Kalss G, et al. (S)-Ketamine in refractory and super-refractory status epilepticus: a retrospective study. CNS Drugs. 2016;30(9):869–876. doi:10.1007/s40263-016-0371-2
13. Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Euro J Anaesthesiol. 2020;37(5):394–401. doi:10.1097/EJA.0000000000001134
14. Yuan J, Chen S, Xie Y, et al. Intraoperative intravenous infusion of esmketamine has opioid-sparing effect and improves the quality of recovery in patients undergoing thoracic surgery: a randomized, double-blind, placebo-controlled clinical trial. Pain Physician. 2022;2:1.
15. Feray S, Lubach J, Joshi GP, et al. PROSPECT guidelines for video‐assisted thoracoscopic surgery: a systematic review and procedure‐specific postoperative pain management recommendations. Anaesthesia. 2022;77(3):311–325. doi:10.1111/anae.15609
16. Frauenknecht J, Kirkham KR, Jacot‐Guillarmod A, Albrecht E. Analgesic impact of intra‐operative opioids vs. opioid‐free anaesthesia: a systematic review and meta‐analysis. Anaesthesia. 2019;74(5):651–662. doi:10.1111/anae.14582
17. Myles PS, Wengritzky R. Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br J Anaesth. 2012;108(3):423–429. doi:10.1093/bja/aer505
18. Massoth C, Schwellenbach J, Saadat-Gilani K, et al. Impact of opioid-free anaesthesia on postoperative nausea, vomiting and pain after gynaecological laparoscopy - A randomised controlled trial. J Clin Anesthesia. 2021;75:110437. doi:10.1016/j.jclinane.2021.110437
19. Chiarotto A, Maxwell LJ, Ostelo RW, Boers M, Tugwell P, Terwee CB. Measurement properties of visual analogue scale, numeric rating scale, and pain severity subscale of the brief pain inventory in patients with low back pain: a systematic review. J Pain. 2019;20(3):245–263. doi:10.1016/j.jpain.2018.07.009
20. Fiore JF, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399(10343):2280–2293. doi:10.1016/S0140-6736(22)00582-7
21. Li J, Wang Z, Wang A, Wang Z. Clinical effects of low‐dose esketamine for anaesthesia induction in the elderly: a randomized controlled trial. Clin Pharm Ther. 2022;47(6):759–766. doi:10.1111/jcpt.13604
22. Uribe AA, Stoicea N, Echeverria-Villalobos M, et al. Postoperative nausea and vomiting after craniotomy: an evidence-based review of general considerations, risk factors, and management. J Neurosurg Anesth. 2019. doi:10.1097/ANA.0000000000000667
23. Zhou N, Liang X, Gong J, et al. S-ketamine used during anesthesia induction increases the perfusion index and mean arterial pressure after induction: a randomized, double-blind, placebo-controlled trial. Eur J Pharm Sci. 2022;179:106312. doi:10.1016/j.ejps.2022.106312
24. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115. doi:10.1093/ejcts/ezy301
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.