Back to Journals » Clinical Ophthalmology » Volume 16
Effect of OTX-101 in Patients with Dry Eye Disease at Day 14 of Treatment: Ocular Surface Endpoint Results from the Phase 2b/3 Clinical Trial
Authors Schechter BA, Urbieta M, Bacharach J, Toyos M , Smyth-Medina R, Mitchell B, Luchs JI
Received 5 October 2022
Accepted for publication 24 November 2022
Published 13 December 2022 Volume 2022:16 Pages 4145—4151
DOI https://doi.org/10.2147/OPTH.S392315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Barry A Schechter,1 Maitee Urbieta,2 Jason Bacharach,3 Melissa Toyos,4 Robert Smyth-Medina,5 Brittany Mitchell,2 Jodi I Luchs6
1Florida Eye Microsurgical Institute, Boynton Beach, FL, USA; 2Medical Affairs North America, Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA; 3North Bay Eye Associates, Petaluma, CA, USA; 4Toyos Clinic, Nashville, TN, USA; 5North Valley Eye Medical Group, Inc., Mission Hills, CA, USA; 6Florida Vision Institute, West Palm Beach, FL, USA
Correspondence: Barry A Schechter, Florida Eye Microsurgical Institute, 1717 Woolbright Road, Boynton Beach, FL, 33426, USA, Tel +1 561 737-5500, Email [email protected]
Abstract: Dry eye disease (DED) is a multifactorial disorder characterized by loss of tear film homeostasis, which initiates a cycle of ocular surface inflammation and damage. As ocular discomfort symptoms associated with DED can decrease quality of life, affected patients prefer treatments that rapidly improve the underlying disease process. OTX-101 0.09% (CEQUA®) is indicated to increase tear production in patients with DED. The current analysis assessed early efficacy of OTX-101 0.09% in adult patients with bilateral DED by evaluating ocular surface endpoints after 14 days of treatment in the phase 2b/3 trial. In this randomized, double-masked, vehicle-controlled, dose-ranging study, patients received one drop of OTX-101 0.05%, OTX-101 0.09%, or vehicle per eye twice daily for 84 days. Corneal staining, conjunctival staining, tear breakup time (TBUT), and modified Symptom Assessment iN Dry Eye (SANDE) total global symptom score were assessed at baseline and Days 14, 28, 42, 56, and 84/early discontinuation. Overall, 455 patients were randomized (OTX-101 0.05%, n=151; OTX-101 0.09%, n=152; vehicle, n=152); only baseline and Day 14 results for the approved OTX-101 0.09% formulation and vehicle are presented. Least squares (LS) mean (standard error [SE]) change from baseline in conjunctival staining score was − 1.3 (0.1) for OTX-101 and − 1.0 (0.1) for vehicle. LS mean (SE) change from baseline in corneal staining score was − 1.1 (0.17) for OTX-101 and − 0.7 (0.17) for vehicle. LS mean (SE) change from baseline in TBUT was 0.52 (0.15) for OTX-101 and 0.36 (0.15) for vehicle. LS mean (SE) change from baseline in modified SANDE total global symptom score was − 4.93 (1.54) for OTX-101 and − 9.1 (1.54) for vehicle. OTX-101 0.09% demonstrated a numerically greater treatment effect compared with vehicle in conjunctival staining, corneal staining, and TBUT after 14 days.
Keywords: CEQUA, keratoconjunctivitis sicca, corneal staining, conjunctival staining, tear breakup time, early efficacy
Introduction
Dry eye disease (DED) is a multifactorial ocular surface disorder characterized by loss of tear film homeostasis.1,2 In DED, tear film hyperosmolarity and instability cause ocular surface exposure to desiccating stress, which initiates a self-perpetuating cycle of inflammation and damage.1–3 Various chronic symptoms are associated with DED, including ocular fatigue, dryness, stinging, blurred vision, and foreign body sensation.1,2 The resultant patient discomfort and visual dysfunction may reduce workplace productivity and limit ability to perform daily activities.1,3,4 Therefore, patients prefer medications that rapidly improve the underlying disease process by reducing inflammation, restoring tear film homeostasis, and repairing ocular surface damage.3
Multiple tools may be used to diagnose and monitor DED.4,5 Common clinical assessments include ocular surface staining, tear breakup time (TBUT), and patient questionnaires.5 Ocular surface staining utilizes lissamine green and sodium fluorescein dyes to detect the corneal and conjunctival epithelial defects often noted in DED.5 TBUT measures the amount of time between blinking and appearance of a break in the tear film; shorter breakup times indicate less tear film stability.5 Numerous patient questionnaires have been developed to evaluate DED symptoms.4,5 The Symptom Assessment iN Dry Eye (SANDE) questionnaire uses a visual analogue technique to quantify the frequency and severity of ocular discomfort symptoms and is useful for repeat clinical assessments.4–6
OTX-101 0.09% (CEQUA®, Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA) is a novel, aqueous, nanomicellar ophthalmic cyclosporine A solution indicated to increase tear production in patients with DED.7 Cyclosporine, a calcineurin inhibitor immunosuppressant, inhibits T lymphocyte activation and prevents release of proinflammatory mediators to decrease the underlying ocular surface inflammation characteristic of DED.7,8 After 84 days of treatment in a phase 2b/3 clinical trial investigating efficacy and safety of OTX-101 in patients with DED, OTX-101 0.09% produced statistically significant improvements in conjunctival staining, corneal staining, and Schirmer’s score relative to vehicle.8 The current analysis aimed to characterize the early efficacy of OTX-101 0.09% vs vehicle by evaluating ocular surface endpoints—conjunctival staining, corneal staining, TBUT, and modified SANDE total global symptom score—after 14 days of treatment in the phase 2b/3 study.
Materials and Methods
Study Design
This randomized, multicenter, double-masked, vehicle-controlled, dose-ranging, phase 2b/3 study investigated the safety and efficacy of two concentrations of OTX-101 vs vehicle for DED treatment (NCT02254265). The study was conducted in accordance with the ethical guidelines of the latest revision of the Declaration of Helsinki, and with independent review board approval by Alpha IRB. All patients provided informed consent. Study design has been previously described.8 Briefly, enrolled patients were randomized 1:1:1 to OTX-101 0.05%, OTX-101 0.09%, or vehicle treatment groups. Eligible patients entered a 14- to 17-day run-in period where all patients instilled one drop of vehicle into each eye twice daily, followed by an 84-day treatment period where patients instilled one drop of their assigned ophthalmic formulation into each eye twice daily.
Patient Eligibility
Patients were required to be ≥18 years old, with a self-reported history of DED for ≥6 months supported by a clinical diagnosis of bilateral DED. Lissamine green conjunctival staining sum score of 3–9 out of 12 in the same eye at screening and baseline, patient-rated global symptom score of ≥40 on a 0–100 visual analogue scale at screening and baseline, and Snellen visual acuity >20/200 in both eyes were required. Patients were required to discontinue any current DED therapy, including artificial tears or ocular lubricants, and any topical ophthalmic medications other than the study medication, for study duration beginning at initiation of the run-in period. Exclusion criteria included use of cyclosporine ophthalmic emulsion 0.05% within 3 months of screening or previous treatment failure on topical cyclosporine, diagnosis of Sjögren’s syndrome for >5 years, and history of seasonal allergic conjunctivitis or any currently active eye disease other than DED. Additional inclusion and exclusion criteria have been previously described.8
Outcome Measures
Efficacy was assessed via evaluation of DED signs and symptoms collected at all study visits. Clinical signs included corneal fluorescein staining score, conjunctival lissamine green staining score, and TBUT evaluated at baseline and Days 14, 28, 42, 56, and 84/early discontinuation (ED); Schirmer’s test was evaluated at baseline and Day 84/ED. Both eyes were assessed at each visit; the study eye was that with the higher baseline lissamine green conjunctival staining score, or the right eye if scores were equal. Patient symptoms were assessed via modified SANDE questionnaire at baseline and Days 14, 28, 42, 56, and 84/ED. Coprimary efficacy endpoints were mean changes from baseline at Day 84 for total conjunctival lissamine green staining score in the study eye, and global symptom score. Secondary efficacy endpoints included mean change from baseline in TBUT, total corneal fluorescein staining score, and Schirmer’s score (average of both eyes).
Safety assessments included slit-lamp examinations, Snellen visual acuity measurements, and adverse event (AE) recording at all study visits; ophthalmoscopy/dilated fundoscopy at screening and Day 84/ED; and tonometry at screening and Days 28, 56, and 84/ED.
The current analysis assessed efficacy outcomes, including mean change from baseline in total conjunctival staining score, total corneal staining score, TBUT, and modified SANDE total global symptom score at Day 14.
Assessments
Conjunctival staining was assessed in six conjunctival areas (temporal, nasal, two superior, two inferior) at 1–4 minutes after instilling one drop (10 μL) of 1% lissamine green solution by pipette. Staining was assessed in low-to-moderate intensity white slit-lamp light and scored on a 0–3 scale (0, no stain uptake; 3, dense stain uptake). Scores for each eye, excluding the superior zones, were summed.
Corneal staining was assessed in five corneal areas (superior, inferior, temporal, nasal, central) at 2–2.5 minutes after instilling one drop (10 μL) of 0.5% fluorescein solution by pipette into the conjunctival cul-de-sac, followed by adequate blinking. Staining was scored on a 0–4 scale in increments of 0.5 (0, no punctate stain; 4, severe diffuse macropunctate stain). Scores per region were summed.
TBUT was assessed by observing the tear film via slit-lamp for 10–15 seconds without blinking. Time to development of the first dry spots was measured in seconds. This was repeated three times, and each value was recorded.
Symptoms were rated using a modified SANDE instrument, in which patients were asked two questions: “Please indicate how often, over the past week, your eyes felt dry and/or irritated” and “Please indicate how severe, on average, you felt your symptoms of dryness and/or irritation were over the past week.” Frequency was rated on a scale of 0–100 (0, rarely; 100, all the time). Severity was also rated on a scale of 0–100 (0, very mild; 100, very severe). Scores were recorded, and global symptom score was calculated as the square root of frequency score times severity score.
Statistical Analyses
All statistical methods have been previously described.8 The intent-to-treat population was used for the primary efficacy analysis and included all randomized patients. The safety population included all patients receiving ≥1 dose of study medication. Continuous variables were summarized with descriptive statistics (n, mean, median, standard deviation [SD], standard error [SE], minimum, maximum), and categorical variables were summarized with counts and percentages. Both coprimary endpoints were tested at a significance level of 0.05 without adjustment for multiplicity. All P values other than those for coprimary efficacy endpoints are purely descriptive.
Results
Baseline Patient Characteristics
A total of 455 patients were randomized; 152 received OTX-101 0.09%, 151 received OTX-101 0.05%, and 152 received vehicle. Only results for OTX-101 0.09% (the FDA-approved formulation) and vehicle treatment groups (n=304) are presented here. Demographic characteristics were generally similar between treatment groups; most participants were female (80.3% OTX-101; 78.9% vehicle) and white (82.9% OTX-101; 78.9% vehicle), with mean ages of 59.2 years for OTX-101 and 59.3 years for vehicle (Table 1).
 |
Table 1 Baseline Patient Demographics and Clinical Characteristics |
Efficacy and Safety Outcomes
Mean baseline efficacy outcome scores are reported in Table 1. At Day 14, least squares (LS) mean change from baseline (SE) in total conjunctival staining score was −1.3 (0.1) for OTX-101 and −1.0 (0.1) for vehicle (P=0.1176; Figure 1A). For total corneal staining score at Day 14, the LS mean change from baseline (SE) was −1.1 (0.17) for OTX-101 and −0.7 (0.17) for vehicle (P=0.0557; Figure 1B). The LS mean change from baseline (SE) in TBUT at Day 14 was 0.52 (0.15) for OTX-101 and 0.36 (0.15) for vehicle (P=0.4465; Figure 2), and for modified SANDE total global symptom score was −4.93 (1.54) for OTX-101 and −9.10 (1.54) for vehicle (P=0.0500; Figure 3).
 |
Figure 1 Least squares mean change from baseline in ocular surface staining at Day 14. (A) Total conjunctival staining score. (B) Total corneal staining score. Abbreviation: SE, standard error. |
 |
Figure 2 Least squares mean change from baseline in tear breakup time at Day 14. Abbreviation: SE, standard error. |
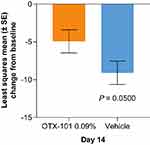 |
Figure 3 Least squares mean change from baseline in modified SANDE total global symptom score at Day 14. Abbreviations: SANDE, Symptom Assessment iN Dry Eye; SE, standard error. |
Throughout the 12-week study, 52 patients receiving OTX-101 0.09% reported 118 AEs, and 51 patients receiving vehicle reported 105 AEs. The most common AE was instillation site pain (15.1% OTX-101 0.09%; 3.3% vehicle), and most ocular AEs were mild or moderate in severity.
Discussion
The current analysis suggests that OTX-101 0.09% may have early efficacy in improving clinical signs and symptoms of DED. In patients receiving OTX-101 0.09%, numerically greater improvements from baseline were found for total conjunctival staining score, total corneal staining score, and TBUT at Day 14 compared with vehicle. OTX-101 0.09% treatment improved modified SANDE total global symptom score from baseline to Day 14, although this improvement was not greater than that observed with vehicle.
In patients with DED, chronic ocular discomfort and visual dysfunction can negatively impact physical, social, and psychological health, reducing overall quality of life.1,4 In fact, patients with severe DED ranked their loss of utility similarly to those affected by severe angina, disabling hip fracture, and dialysis.9,10 Therefore, patient preferences and concerns should be important considerations in DED management; agents that address unmet needs, including rapid action on the underlying disease process, are more likely to be accepted.3,9
Several studies have investigated the impact of OTX-101 0.09% on ocular surface signs and symptoms in patients with DED. In a pooled analysis of OTX-101 phase 2b/3 and phase 3 clinical trials, Malhotra et al (2019) found that OTX-101 0.09% improved total corneal staining scores in patients with DED within 4 weeks and continued through end of study, with statistically significant improvement vs vehicle at all assessed timepoints (P<0.002 for Days 28, 56, and 84).11 Sheppard et al (2020) found that significantly more eyes receiving OTX-101 0.09% vs vehicle demonstrated an increase in Schirmer’s scores ≥10 mm from baseline to Day 84/ED (P<0.0001) in the pooled phase 2b/3 and phase 3 studies; Toyos et al (2021) also observed significant improvement in tear production (P=0.0168) in pooled patients with severe DED (baseline Schirmer’s score <5 mm).12,13 However, while OTX-101 0.09% treatment improved ocular discomfort symptoms as assessed by global SANDE score in OTX-101 pooled clinical trials, this change was similar to that noted with vehicle at Day 84.12 Additionally, there was no significant difference between OTX-101 0.09% and vehicle treatment groups in change from baseline in TBUT at any time point during the phase 2b/3 study.8
It should be noted that, while numerically greater changes in conjunctival staining, corneal staining, and TBUT were observed for OTX-101 0.09% vs vehicle at Day 14, these differences were not statistically significant. Interestingly, compared to patients on OTX-101 0.09%, patients receiving vehicle showed numerically greater improvement in modified SANDE total global symptom scores. Vehicle may therefore provide palliative lubrication of the ocular surface but does not target the underlying disease pathology.14
This study included limited efficacy assessments; future research may incorporate additional analyses like tear production quantification. Additionally, the modified SANDE instrument used for symptom evaluation only assessed ocular dryness and irritation. Future analyses may use other patient-reported tools to better understand the subjective patient experience.
Conclusion
OTX-101 0.09% appeared to rapidly improve ocular surface signs of DED and demonstrated a numerically greater treatment effect in conjunctival staining, corneal staining, and TBUT compared with vehicle after 14 days; symptom improvement was similar for OTX-101 0.09% and vehicle at Day 14.
Data Sharing Statement
Data and other documents will be made available after publication, with no end date, to anyone who submits a reasonable request to the study sponsor.
Ethics Statement
This study was conducted in accordance with the ethical guidelines of the latest revision of the Declaration of Helsinki, and with institutional review board approval by Alpha IRB. All patients provided informed consent.
Acknowledgments
Medical writing and editorial support were provided by Jennifer Masucci, VMD, of AlphaBioCom, LLC, and were funded by Sun Pharma.
Funding
This study was funded by Sun Pharma, Princeton, NJ, USA.
Disclosure
BAS receives consulting fees from Bausch Health; Omeros; and Sun Pharma; and is on the board of Aperta Biosciences and Innovation Pharmaceuticals. MU and BM are employees of Sun Pharmaceutical Industries, Inc. JB reports speaker fees from Aerie Pharmaceuticals; Alcon; Allergan; Bausch & Lomb; Glaukos; New World Medical; and Sun Pharmaceutical Industries, Inc.; consultant fees from Aerie Pharmaceuticals, Alcon, Allergan, Bausch & Lomb, Injectsense, New World Medical, Optovue, and Osmotica Pharmaceuticals; personal fees from Sun Pharma; and research support from Aerie Pharmaceuticals, Allergan, Novartis, Glaukos, Optovue, and Ocular Therapeutix. MT reports consultant fees from Allysta; Bausch & Lomb; Eyevance; Greenlight; Iridex; Mallinckrodt; Novartis; Oyster Point; Sun Pharma; and Zeiss; speaker fees from Bausch & Lomb; Iridex; Lumenis; Mallinckrodt; Sun Pharma; and Zeiss; research fees from Bausch & Lomb; Dompé Farmaceutici; Iridex; Lumenis; Mallinckrodt; MIXTO Lasering; Sun Pharma; and Zeiss; and miscellaneous fees from MIXTO Lasering. RSM is involved in research for Aerie; Aerpio; Alcon; Allergan; Allysta; AT Resolve; Aurinia; Auven; Bausch & Lomb; Eleven; Encore; Eyegate; Evidera; Havione; Hi-Tech; Inotek; Insite; Inspire; Ista; Kala; Nicox; Novartis; Ocular Therapeutix; Ocuphire; OmegaD; Ono; Orasis; OTX; Perrigo; Pharmacal; RevitaLid; RVL; Santen; SARcode; Senju; Shire; Silk; Sun Pharma; Surface; Valeant; VivaVision; and Xigen. JIL receives personal fees from Alcon; Aldeyra; Allergan; Bausch & Lomb; Eyevance; Icare; Santen; Shire; TearLab; Sun Pharma; and Xequel Bio; and equity interest from Calhoun Vision, CLXO, Insightful Solutions, Ocular Sciences, Omega Ophthalmics, Trefoil Therapeutics, and RPS. The authors report no other conflicts of interest in this work.
References
1. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011
2. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
3. Asbell P, Messmer E, Chan C, Johnson G, Sloesen B, Cook N. Defining the needs and preferences of patients with dry eye disease. BMJ Open Ophthalmol. 2019;4(1):e000315. doi:10.1136/bmjophth-2019-000315
4. Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1(2):51–57. doi:10.1007/s40135-013-0009-1
5. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–574. doi:10.1016/j.jtos.2017.05.001
6. Schaumberg DA, Gulati A, Mathers WD, et al. Development and validation of a short global dry eye symptom index. Ocul Surf. 2007;5(1):50–57. doi:10.1016/s1542-0124(12)70053-8
7. Sun Pharmaceutical Industries, Inc. CEQUA® (cyclosporine ophthalmic solution) 0.09% full prescribing information. 2022.
8. Tauber J, Schechter BA, Bacharach J, et al. A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–1929. doi:10.2147/OPTH.S175065
9. Buchholz P, Steeds CS, Stern LS, et al. Utility assessment to measure the impact of dry eye disease. Ocul Surf. 2006;4(3):155–161. doi:10.1016/s1542-0124(12)70043-5
10. Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–1419. doi:10.1016/s0161-6420(03)00462-7
11. Malhotra R, Devries DK, Luchs J, et al. Effect of OTX-101, a novel nanomicellar formulation of cyclosporine A, on corneal staining in patients with keratoconjunctivitis sicca: a pooled analysis of phase 2b/3 and phase 3 studies. Cornea. 2019;38(10):1259–1265. doi:10.1097/ICO.0000000000001989
12. Sheppard J, Kannarr S, Luchs J, et al. Efficacy and safety of OTX-101, a novel nanomicellar formulation of cyclosporine A, for the treatment of keratoconjunctivitis sicca: pooled analysis of a phase 2b/3 and phase 3 study. Eye Contact Lens. 2020;46(Suppl 1):S14–S19. doi:10.1097/ICL.0000000000000636
13. Toyos M, Gupta PK, Mitchell B, Karpecki P. The effect of OTX-101 on tear production in patients with severe tear-deficient dry eye disease: a pooled analysis of phase 2b/3 and phase 3 studies. Curr Eye Res. 2022;47(2):220–224. doi:10.1080/02713683.2021.1966477
14. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
