Back to Journals » Clinical Optometry » Volume 15
Effect of Contact Lens Solutions in Stabilizing the Activity of Tear Lysozyme
Authors Scheuer CA, Barniak VL, Phatak NR , Rah MJ , Reindel W
Received 10 January 2023
Accepted for publication 17 April 2023
Published 12 May 2023 Volume 2023:15 Pages 119—127
DOI https://doi.org/10.2147/OPTO.S404261
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Mr Simon Berry
Catherine A Scheuer, Vicki L Barniak, Nitasha R Phatak, Marjorie J Rah, William Reindel
Vision Care, Bausch & Lomb Inc., Rochester, NY, USA
Correspondence: Nitasha R Phatak, Vision Care, Bausch & Lomb Incorporated, Rochester, NY, USA, Tel +1 585 413-6397, Email [email protected]
Purpose: Interactions between tear proteins and the interfaces of contact lenses can be complex and can influence contact lens wear success. Tear proteins, including lysozyme, function to maintain the balance of ocular surface homeostasis, as evidenced by the effects of its conformation relative to stabilizing the tear film and its potential impact on corneal epithelial cells. Contact lens manufacturers include components in lens care and blister package solutions to help stabilize the tear film and preserve homeostasis. This in vitro study was performed to evaluate the ability of daily disposable contact lens package solutions to stabilize lysozyme and preserve its native conformation under denaturing conditions.
Methods: Lysozyme was added to contact lens solutions sampled from kalifilcon A, etafilcon A, senofilcon A, narafilcon A, nelfilcon A, verofilcon A, delefilcon A, somofilcon A, and stenfilcon A blister packages, then mixed with the protein denaturant sodium lauryl sulfate. Lysozyme activity was evaluated by adding test solutions to a suspension of Micrococcus luteus. Native lysozyme lyses the Micrococcus luteus cell wall, which decreases suspension turbidity. Stabilization of lysozyme activity was determined by comparing suspension turbidity before and after exposure to test solutions.
Results: Lysozyme stabilization was 90.7% for kalifilcon A solution, a statistically significant improvement (p < 0.05) compared to phosphate buffered saline (PBS, negative control). No significant improvement was observed with any other contact lens solution (all lysozyme stabilization < 5.00%).
Conclusion: The representative tear protein lysozyme was significantly more stable in the novel kalifilcon A contact lens solution containing multiple moisturizers and osmoprotectants than in PBS or other daily disposable contact lens solutions. The lysozyme activity assay provides mechanistic evidence that the kalifilcon A contact lens solution can stabilize proteins under conditions that typically denature proteins, which may contribute to maintaining ocular surface homeostasis.
Keywords: kalifilcon A, silicone hydrogel, homeostasis, protein stabilization
A Letter to the Editor has been published for this article.
A Response to Letter by Mr Chaurasiya has been published for this article.
Introduction
In biology, homeostasis is defined as,
the ability or tendency of the body or a cell to seek and maintain a condition of equilibrium - a stable internal environment - as it deals with external changes.1
Ocular surface homeostasis is complex, as multiple cellular and anatomical constituents including cornea, conjunctiva, lacrimal glands, and tears must work together to maintain and restore a condition of equilibrium. The introduction of a contact lens (CL) onto the eye can represent a change that alters the equilibrium of the ocular surface.2
Tears cover the ocular surface not as a homogenous static film, but rather as a more complex, dynamic, layered construct. The tear film was traditionally modeled as a tri-layered film comprising the mucin layer in contact with the cornea, the central aqueous layer, and the lipid layer in contact with air,3 but it may be even more complex,4 both chemically and physically. A CL perturbs the natural tear film by splitting it into two distinct films that differ chemically and physically, the pre-lens (in contact with the lens and ambient air) and post-lens (in contact with the lens and cornea) tear films.4,5 While the pre-lens film is reformed and replenished with each complete blink, the post-lens film is more stagnant, which can result in an osmolarity gradient between the anterior and posterior lens surfaces.6
Normal human tears comprise an aqueous solution of proteins (including mucins), enzymes, lipids, metabolites, and electrolytes that maintain osmolarity and pH.7,8 Lipidomic methods were used to identify over 600 lipids in human tears, metabolomic methods to identify 85 metabolites, and proteomic methods to identify 1543 proteins.4 Of the latter, 4 key proteins (lysozyme, lactoferrin, lipocalin, and secretory immunoglobulin A [sIgA]) are produced in relatively large amounts.7 Lipid–protein interactions play a role in the tear film lipid layer. Lysozyme and other lachrymal origin proteins contribute to tear film stability by adsorbing to and penetrating the tear film lipid layer, reducing surface tension.8 Mathematical modeling suggests that restructuring of the tear film upon blinking leads to incorporation of lysozyme (a hydrophilic protein) into the lipid film as inverse lipid micellar structures.9
The tear film serves as the eye’s innate defense system, as well as performs other important functions, including lubrication and protection of the eye, and delivery of nutrients and growth factors to the corneal epithelium.10 Functions of major tear film components and their contributions to ocular surface homeostasis are described in the literature.11 The lipid layer retards water evaporation and stabilizes the tear film at the air interface, primarily by reducing surface tension. Tear proteins in the aqueous layer perform various functions, including contributing to tear film stability and clearing microbial contamination at the ocular surface.
Interactions between tear proteins and the CLs can influence lens wear success. Upon contact with the tear film, a CL placed on eye rapidly sorbs proteins present within the tears.12 Once sorbed, a protein can diffuse from the lens surface into the bulk, desorb back into the medium, or rearrange and change its conformation, becoming more tenaciously bound to the surface.12 The composition of the protein layer adsorbed at the surface is dynamic, driven by the respective concentrations and surface affinities of the individual tear proteins, with the most abundant proteins adhering first, and those with lower surface affinity later displaced by those with higher affinity.13 Proteins sorbed on CLs can either support or disturb ocular surface homeostasis, depending upon the composition and nature of the protein layer. In general, conventional hydrogel CLs sorb more total protein than do silicone hydrogel CLs.14,15 The affinity of each individual tear protein for the lens surface is highly dependent upon lens material. Lysozyme is of particular interest due to its abundance in natural tears,3 its antimicrobial properties,3,16 and its tendency to sorb in high quantity on anionic lenses due to its positive charge.14,17 This protein represents a significant fraction of the total protein sorbed on many conventional poly(2-hydroxyethyl methacrylate) (pHEMA)-based hydrogel CLs and silicone hydrogel CLs;14 it accounts for up to 90% on average of the total protein deposited on clinically worn etafilcon A lenses,18 and up to 50% on balafilcon A lenses.15
Over the years, CL manufacturers have developed new lens materials and designs, as well as provided affordable, frequent replacement lens options to help reduce changes to the ocular surface, such as occur when tear film components accumulate on and within the lens. Protein deposits on CLs participate in lens fouling, and denatured proteins including lysozyme (along with other degraded proteins and lipids) may elicit an immune response,19–21 promoting the release of inflammatory cytokines that adversely affect human corneal epithelial cell (HCEC) viability.22 Several researchers proposed that preventing tight protein binding and denaturation, and preserving the native conformation (and thus activity) of proteins that deposit on lenses during wear should be a goal in CL development,14,23 as such CLs may be less likely to promote ocular inflammation and more likely to support ocular surface homeostasis.
Studies of lysozyme interaction with etafilcon A, an FDA Group IV material (high water, ionic) are illustrative, as this protein retains its activity once associated with this lens. Both in vitro12,16,24 and clinical23,25,26 studies report relatively high amounts of lysozyme sorbed on this material, the majority preserving its activity and presumably its native state, as disruption in protein conformation and structure alter protein function.24 Thus, the total amount of protein sorbed on a lens is not predictive of lens discomfort.23 Unlike etafilcon A, many silicone hydrogel CLs sorb relatively little total lysozyme, with much of it losing its biological activity, coinciding with a higher fraction of denatured lysozyme.14
With the goal of stabilizing tear proteins, CLs can be infused with isotonic buffered saline solution containing additional components to help maintain homeostasis. Numerous in vitro studies of the effects of contact lens multi-purpose solution (MPSs) upon protein in solution are published,19,27 as are the effects of MPSs25,28,29 and CL rewetting drops30 upon clinically sorbed protein. In contrast, there exists little published literature describing the effects of daily disposable CL blister package solutions upon proteins in general or lysozyme in particular. Therefore, the purpose of this study was to measure tear lysozyme stabilization in daily disposable CL blister package solutions under denaturing conditions to determine if any of the solutions can help maintain the protein in its native state.
Materials and Methods
Study Material
In this experiment, CL solutions were sampled from fifteen blister packages of each of nine different daily disposable CLs (Table 1). Variation in solution components can be observed in both legacy CLs, such as nelfilcon A and etafilcon A and more recently developed CLs such as verofilcon A, kalifilcon A, and somofilcon A, as disclosed in their respective package inserts.31–39 The nelfilcon A (Alcon, Ft. Worth, TX) solution contains hydroxypropyl methylcellulose (HPMC) and polyethylene glycol (PEG), as well as poloxamer 108; the delefilcon A and verofilcon A (Alcon) solutions contain copolymers of polyamidoamine and poly(acrylamide-acrylic) acid (PAO-PAAA); the kalifilcon A (Bausch & Lomb Incorporated, Rochester, NY) solution contains poloxamer 181, poloxamine 1107, glycerin, and erythritol; somofilcon A and stenfilcon A (CooperVision, Pleasanton, CA) solutions contain poloxamer 407 and polysorbate, respectively; the etafilcon A solution contains polyvinylpyrrolidone (PVP), while the narafilcon A and senofilcon A (Johnson & Johnson Vision Care, Inc., Jacksonville, FL) solutions contain methyl ether cellulose (MEC). The water-soluble phospholipid sodium coco PG dimonium chloride phosphate (Siltech, Toronto, Ontario, Canada) in phosphate buffered saline (PBS, Gibco – Thermo Fisher Scientific, Waltham, MA) was used as a positive control. Other materials used and were Micrococcus luteus (M. luteus, Sigma-Aldrich, St. Louis, MO), sodium lauryl sulfate (SLS, Fluka, Honeywell Research Chemicals, Morris Plains, NJ) denaturing agent, lysozyme (chicken egg white, Sigma), and an ultraviolet/visible spectrometer at 450 nm.
 |
Table 1 Contact Lens Solutions Evaluated |
Lysozyme Activity Assay
The assay is based on lysozyme’s enzymatic cleavage of peptidoglycan, resulting in the digestion of the cell wall of M. luteus in a suspension. M. luteus is sensitive to active lysozyme and is highly susceptible to lysozyme-mediated killing. Native lysozyme attacks M. luteus cells, which decreases suspension turbidity as the cell walls are lysed by the enzyme.40 The decrease in optical density (OD) is a relative indicator of enzymatic activity and can be measured by following the decrease in absorbance at 450nm (OD450) over time using a spectrophotometer. When the conformation of the lysozyme changes, the enzymatic activity decreases.41 Therefore, enzymatic activity against M. luteus was used to assess changes in the conformation of the lysozyme from the active native form to the inactive denatured form (Figure 1).
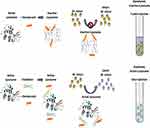 |
Figure 1 Lysozyme activity assay. Notes: Active native lysozyme image courtesy of Biological Magnetic Resonance Data Bank (2020, October 7). BMRB Featured System: Lysozyme. https://bmrb.io/featuredSys/Lysozyme/, used with permission. CC0 1.0 Universal (CC0 1.0) Public Domain Dedication.42 |
Experimental Procedure
A 20% solution of SLS detergent in distilled water and a 25mg/mL solution of lysozyme in PBS were prepared. A 0.03% M. luteus suspension in PBS was prepared from lyophilized cells and mixed for at least 1 hour before use. The initial time point absorbance (T=0 OD450) of the M. luteus suspension was determined by obtaining the average of at least 9 samples of absorbance OD450 of the suspension. Solution was removed from 15 CL blister packages to ensure an adequate volume of solution to prepare 2.5mL solution aliquots, and 2.5mg solubilized lysozyme was added to triplicate samples to achieve a final concentration of 1mg/mL lysozyme in test solutions, which is within the range of its reported 0.7 to 4.6 mg/mL concentration in normal tears.43 To the test sample/lysozyme solution were added 20 µL of SLS denaturant solution, and the mixture was vortexed and incubated at room temperature. After 5 minutes, 0.5mL of this solution were added to 4.5mL aliquots of M. luteus, and the mixture was vortexed and incubated at room temperature. After 5 mins, approximately 1mL of the resulting suspension was measured at OD450. (As the assay reaction is time-dependent, a consistent reaction time for each sample is essential for comparison. In pilot experiments, 5 minutes were determined to be sufficient for cell lysis to occur.) The percentage of lysozyme maintained in native form was determined by calculating the ratio of the difference of suspension turbidity before and after exposure to test solutions (T=0 OD450 – T=5 OD450) relative to the initial turbidity (T=0 OD450).
Statistical Analysis
The differences in lysozyme stabilization between each test lens solution (n=3) and the PBS control were tested for significance using a two-sample Student’s t-test; a significance level of α = 0.05 was utilized.
Results
The PBS control solution retained 4.5% of lysozyme activity. Lysozyme stabilization was 90.7% for kalifilcon A solution, a statistically significant improvement (p<0.05) compared to PBS (Figure 2).
All other solutions provided <5.0% stabilization. The etafilcon A, verofilcon A, senofilcon A, delefilcon A, somofilcon A and narafilcon A solutions retained lysozyme activity that was not significantly different than that of the PBS control (p>0.05). Both nelfilcon A and stenfilcon A solutions had significantly less retained lysozyme activity when compared to the PBS control (p<0.05).
Discussion
Ocular surface homeostasis is complex. The placement of a CL on the surface of the eye changes the state of homeostasis, and lens materials, lens designs, and solutions integrated into the lens material can play a role in helping to maintain ocular surface homeostasis. The state of tear proteins plays a key role in preserving homeostasis, and it remains an area to be explored further. The solutions supporting daily disposable CL materials can play an important role in stabilizing tear proteins.
In this study, lysozyme was incubated with respective solutions sampled from the blister packages of nine daily disposable CL models, two of which were traditional hydrogels (nelfilcon A and etafilcon A) and the remaining seven silicone hydrogels, as well as with PBS as control. Retained lysozyme activity after incubation of the test solutions with SLS was greater with the kalifilcon A solution compared with PBS control, and less with the stenfilcon A and narafilcon A solutions compared with PBS control (all p < 0.05; Figure 2); activity with the etafilcon A, verofilcon A, senofilcon A, delefilcon A, somofilcon A and narafilcon A solutions did not differ from PBS control (all p ≥ 0.05).
Lysozyme was selected as the model protein in this study for three reasons. First, along with lipocalin and lactoferrin, it is abundant in natural tears;7 second, it is known to deposit on soft CLs;29 third, an assay of the enzyme’s biological activity provides an indicator of native versus denatured protein conformation.29 As a model protein, the results can be indicative of outcomes associated with other proteins found in tears.
Denatured lens-bound protein can affect homeostasis by changing CL surface wetting characteristics or the coefficient of friction (COF) between the lens and the ocular surface, dependent upon both the specific adsorbed proteins and their conformational state, as well as the specific lens. For example, model pHEMA and polymethyl methacrylate (PMMA) lens surfaces became more hydrophobic (less wettable) upon adsorption of albumin.44 COF increased concomitantly at high surface coverage but was not significantly different at lower coverage when the protein denatured.44 In comparison, when lysozyme sorbed on CLs, COF increased at high surface coverage of ocufilcon D and helfilcon A but not nelfilcon A lenses.45 The researchers proposed that increased COF might be related to denatured lysozyme rather than total lysozyme sorbed. Increased friction can elicit cytokine and chemokine mediated ocular surface inflammation, perpetuating a vicious cycle of hyperaemia and dryness.20
Comparison of the publicly disclosed solution formulations of the lenses evaluated suggests that not all surfactants are effective in this lysozyme stabilization model system. All of the daily disposable CL blister packages evaluated in this study contain phosphate or borate buffered saline, and each contains one or more additional ingredients intended to improve lens performance. These include surfactants such as poloxamers and poloxamines, and humectants such as PEG and HPMC. The kalifilcon A solution also contains glycerol and erythritol osmoprotectants, which are used as protein stabilizers in the pharmaceutical industry.46 Because surfactants are known to mediate protein refolding,47 surfactant-containing solutions might potentially help prevent tear protein denaturation. However, neither the poloxamer 108 in the solution for nelfilcon A lenses, nor the poloxamer 407 in the solution for somofilcon A lenses at their respective concentrations did so for lysozyme in this study (4.0% ± 1.0% and 4.4% ± 0.6% retained activity, respectively). Only the kalifilcon A lens solution, which includes dual surfactants/moisturizers and dual osmoprotectants largely retained lysozyme activity.
Studies of the interactions between surfactants and proteins reported in the literature offer some clues as to how surfactants in kalifilcon A might contribute to protection of tear film protein. As molecules of globular lysozyme denature, they tend to aggregate with neighboring molecules. In one study, poloxamer and poloxamine multi-block surfactant copolymers, but not the humectant PEG, were reported to suppress aggregation of heat-denatured lysozyme, with poloxamine 1107 more effective than either poloxamer 108 or poloxamer 188.48 This observation is believed to be due to steric effects, illustrating the importance of the relative sizes of the hydrophobic regions of the protein and the poloxamine 1107 surfactant.49 It was also proposed that this surfactant mimics naturally occurring small heat shock proteins to prevent irreversible aggregation.50 In the case of the kalifilcon A solution, highly hydrophilic poloxamine 1107 (hydrophile-lipophile balance, HLB = 18–23)47 may work in conjunction with hydrophobic poloxamer 181 (HLB = 3)47 to stabilize different parts of the lysozyme molecule.
The ability of the surfactants in the kalifilcon A solution to contribute to stabilized lysozyme activity observed in this study is consistent with other studies of surfactants and lens care products. In one study, MPS formulations that included poloxamine 1107 preserved 90% of lysozyme activity in the presence of sodium dodecyl sulfate (aka SLS), while formulations that included poloxamine 1304, poloxamer 407, or poloxamer 237 preserved no more than 6%.27 In addition, the use of an eye drop containing lauryl ether carboxylic acid and poloxamine 1304 surfactants in conjunction with wear of silicone hydrogel CLs only marginally decreased denaturation of lysozyme sorbed on lenses.30 These studies support the differential performance of solutions containing poloxamine 1107 and 1304 at promoting retention of native lysozyme structure. This difference may reflect differences in relative surfactant concentration is the products studied, as well as the relative difference in HLB between the two surfactants, poloxamine 1107 being highly hydrophilic (HLB = 18–23) and poloxamine 1304 being medium hydrophilic (HLB = 12–18).47 Poloxamine 1107 was reported to be able to disaggregate lysozyme and preserve its secondary structure after exposure to dithiothreitol.51 While not specifically exploring lysozyme stabilization, one study reported that poloxamine 1107 sorbed on etafilcon A lenses increased lens wettability that persisted over the first four hours of wear and was retained by the lens over a full eight-hour wearing period.52 Wettability and subjective comfort were improved compared with the untreated control lens and highlight that various ingredients can have a sustained benefit over the course of the wearing period.
Retaining tear protein structure and function by the kalifilcon A solution supports the possibility of clinical benefits. Contact of the kalifilcon A lens solution that includes protein stabilizing agents with the CL wearer’s ocular surface may help stabilize tear proteins within the tear film and therefore may help to maintain ocular surface homeostasis. Future studies to determine the relative contributions of the components of the solution and effects of potential contributors to protein stabilization versus denaturation are warranted, including studies to determine if the in vitro lysozyme stabilization demonstrated in this study occurs during lens wear.
Conclusion
The representative protein lysozyme, in the presence of a novel CL solution containing dual moisturizers and dual osmoprotectants, was significantly more stable when compared to PBS and other daily disposable CL solutions intended to support various lens materials. The lysozyme activity assay provides mechanistic evidence that the kalifilcon A CL solution can stabilize proteins under conditions that typically denature proteins. While the clinical significance of small amounts of denatured lysozyme remains to be established, native proteins contribute to tear film stability and protects the ocular surface from microbial colonization. Additional research will further the understanding of the benefits of stabilizing proteins in preserving ocular surface homeostasis during CL wear.
Acknowledgment
The abstract of this paper was presented at the 2021 British Contact Lens Association (BCLA) Virtual Clinical Conference and Exhibition held June 13–14, 2021. (Scheuer C, Barniak V, Rah M. Reindel W. Effect of daily disposable contact lens solutions in stabilizing the activity of a tear film protein. Cont Lens Anterior Eye 2022;45 Suppl 1: 101638.)
Funding
This work was funded by Bausch & Lomb Incorporated.
Disclosure
All authors are employees of Bausch & Lomb Incorporated. Ms Catherine A Scheuer reports a patent 17/398556 pending to Bausch+Lomb, a patent 110129328 pending to Bausch+Lomb, a patent PCT/EP2021/072140 pending to Bausch+Lomb. Ms Vicki L Barniak reports a patent 17/398,556 pending to Bausch & Lomb, a patent 110129328 pending to Bausch & Lomb, a patent PCT/EP2021/072140 pending to Bausch & Lomb. Dr William Reindel reports a patent 17/398556 pending to Bausch+Lomb, a patent 110129328 pending to Bausch+Lomb, a patent PCT/EP2021/072140 pending to Bausch+Lomb. The authors report no other conflicts of interest in this work.
References
1. biologyonline.com. Homeostasis; 2022. Available from: https://www.biologyonline.com/dictionary/homeostasis.
2. Efron N, Brennan NA, Bright FV, et al. 2. Contact lens care and ocular surface homeostasis. Cont Lens Anterior Eye. 2013;36(Suppl 1):S9–13. doi:10.1016/S1367-0484(13)60004-1
3. Farris RL. Tear analysis in contact lens wearers. Trans Am Ophthalmol Soc. 1985;83:501–545.
4. Willcox MDP, Argüeso P, Georgiev G, et al. TFOS DEWS II tear film report. Ocul Surf. 2017;15(3):366–403. doi:10.1016/j.jtos.2017.03.006
5. Guillon M, Dumbleton K, Theodoratos P, Patel K, Gupta R, Patel T. Pre-contact lens and pre-corneal tear film kinetics. Cont Lens Anterior Eye. 2019;42(3):246–252. doi:10.1016/j.clae.2019.02.001
6. Muntz A, Subbaraman LN, Sorbara L, Jones L. Tear exchange and contact lenses: a review. J Optom. 2015;8(1):2–11. doi:10.1016/j.optom.2014.12.001
7. Tiffany JM. Tears in health and disease. Eye. 2003;17(8):923–926. doi:10.1038/sj.eye.6700566
8. Mudgil P, Torres M, Millar TJ. Adsorption of lysozyme to phospholipid and meibomian lipid monolayer films. Colloids Surf B Biointerfaces. 2006;48(2):128–137. doi:10.1016/j.colsurfb.2006.01.017
9. Wizert A, Iskander DR, Cwiklik L. Interaction of lysozyme with a tear film lipid layer model: a molecular dynamics simulation study. Biochim Biophys Acta Biomembr. 2017;1859(12):2289–2296. doi:10.1016/j.bbamem.2017.08.015
10. Lawrenson JG. Anterior eye. In: Efron N, editor. Contact Lens Practice.
11. Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res. 2020;197:108115. doi:10.1016/j.exer.2020.108115
12. Hall B, Jones LW, Forrest JA. Competitive effects from an artificial tear solution to protein adsorption. Optom Vis Sci. 2015;92(7):781–789. doi:10.1097/OPX.0000000000000618
13. Latour RA. Fundamental principles of the thermodynamics and kinetics of protein adsorption to material surfaces. Colloids Surf B Biointerfaces. 2020;191:110992. doi:10.1016/j.colsurfb.2020.110992
14. Luensmann D, Jones L. Protein deposition on contact lenses: the past, the present, and the future. Cont Lens Anterior Eye. 2012;35(2):53–64. doi:10.1016/j.clae.2011.12.005
15. Subbaraman L, Glasier MA, Dumbleton K, Jones L. Quantification of protein deposition on five commercially available silicone hydrogel contact lens materials. Optom Vis Sci. 2007;84:E–abstract070031.
16. Omali NB, Subbaraman LN, Coles-Brennan C, Fadli Z, Jones LW. Biological and clinical implications of lysozyme deposition on soft contact lenses. Optom Vis Sci. 2015;92(7):750–757. doi:10.1097/OPX.0000000000000615
17. Soltys-Robitaille CE, Ammon DM Jr, Valint PL Jr, Grobe GL 3rd. The relationship between contact lens surface charge and in-vitro protein deposition levels. Biomaterials. 2001;22(24):3257–3260. doi:10.1016/S0142-9612(01)00163-6
18. Keith D, Hong B, Christensen M. A novel procedure for the extraction of protein deposits from soft hydrophilic contact lenses for analysis. Curr Eye Res. 1997;16(5):503–510. doi:10.1076/ceyr.16.5.503.7049
19. Wright EA, Payne KA, Jowitt TA, et al. Preservation of human tear protein structure and function by a novel contact lens multipurpose solution containing protein-stabilizing agents. Eye Contact Lens. 2012;38(1):36–42. doi:10.1097/ICL.0b013e31823fdb2a
20. McMonnies CW. An amplifying cascade of contact lens-related end-of-day hyperaemia and dryness symptoms. Curr Eye Res. 2018;43(7):839–847. doi:10.1080/02713683.2018.1457163
21. Saliman NH, Morgan PB, MacDonald AS, Maldonado-Codina C. Subclinical inflammation of the ocular surface in soft contact lens wear. Cornea. 2020;39(2):146–154. doi:10.1097/ICO.0000000000002192
22. McCanna DJ, Oh S, Seo J, et al. The effect of denatured lysozyme on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2018;59(5):2006–2014. doi:10.1167/iovs.17-22260
23. Subbaraman LN, Glasier MA, Varikooty J, Srinivasan S, Jones L. Protein deposition and clinical symptoms in daily wear of etafilcon lenses. Optom Vis Sci. 2012;89(10):1450–1459. doi:10.1097/OPX.0b013e318269e583
24. Hall B, Jones L, Forrest JA. Measuring the kinetics and activity of adsorbed proteins: in vitro lysozyme deposited onto hydrogel contact lenses over short time periods. J Biomed Mater Res A. 2013;101(3):755–764. doi:10.1002/jbm.a.34357
25. Babaei Omali N, Heynen M, Subbaraman LN, et al. Impact of lens care solutions on protein deposition on soft contact lenses. Optom Vis Sci. 2016;93(8):963–972. doi:10.1097/OPX.0000000000000928
26. Efron N, Brennan NA, Chalmers RL, et al. Thirty years of ‘quiet eye’ with etafilcon A contact lenses. Cont Lens Anterior Eye. 2020;43(3):285–297. doi:10.1016/j.clae.2020.03.015
27. Barniak VL, Burke SE, Venkatesh S. Comparative evaluation of multi-purpose solutions in the stabilization of tear lysozyme. Cont Lens Anterior Eye. 2010;33(Suppl 1):S7–11. doi:10.1016/j.clae.2010.06.011
28. Zhao Z, Carnt NA, Aliwarga Y, et al. Care regimen and lens material influence on silicone hydrogel contact lens deposition. Optom Vis Sci. 2009;86(3):251–259. doi:10.1097/OPX.0b013e318196a74b
29. Heynen M, Ng A, Martell E, Subbaraman LN, Jones L. Activity of deposited lysozyme on contemporary soft contact lenses exposed to differing lens care systems. Clin Ophthalmol. 2021;15:1727–1733. doi:10.2147/OPTH.S296116
30. Subbaraman LN, Bayer S, Glasier MA, Lorentz H, Senchyna M, Jones L. Rewetting drops containing surface active agents improve the clinical performance of silicone hydrogel contact lenses. Optom Vis Sci. 2006;83(3):143–151. doi:10.1097/01.opx.0000204513.76568.57
31. Alcon Laboratories, Inc. (Fort Worth, TX). FOCUS® DAILIES®, FOCUS® DAILIES® Toric, FOCUS® DAILIES® Progressives, DAILIES® AquaComfort Plus®, DAILIES® AquaComfort Plus® Toric and DAILIES® AquaComfort Plus® Multifocal (nelfilcon A) one-day contact lenses [package insert]; 2013. Available from: https://2.myalcon.com/sites/g/files/rbvwei471/files/2019-04/W92024162_I_NELFILCON_A.pdf.
32. Alcon Laboratories, Inc. (Fort Worth, TX). DAILIES TOTAL1® and DAILIES TOTAL1® Multifocal (delefilcon A) soft contact lenses for daily disposable wear [package insert]; 2019. Available from: http://embed.widencdn.net/pdf/plus/alcon/xe3t4jzpll/W900038292_I_DELFCN_US.pdf.
33. Alcon Laboratories, Inc. (Fort Worth, TX). Package insert for alcon precision1™ (verofilcon A) soft contact lenses [package insert]; 2019. Available from: http://embed.widencdn.net/pdf/plus/alcon/tmgdjw6wwf/W900177326-I-VEROFA-PREC1-US.pdf.
34. Bausch & Lomb Incorporated (Rochester, NY). BAUSCH + LOMB INFUSE™ (kalifilcon A) one-day soft (hydrophilic) contact lenses [package insert]; 2020. Available from: https://www.bausch.com/Portals/69/-/m/BL/United%20States/USFiles/Package%20Inserts/Vision%20Care/lenses/INFUSE-PIFG.pdf.
35. CooperVision (Scottsville, NY). CLARITI 1 DAY CONTACT LENS [package insert]; 2019. Available from: https://coopervision.com/sites/coopervision.com/files/pi01092_rev_a_clariti_1_day_family_us_invigor_and_non-invigor.pdf.
36. FDA 510(k) Summary K191763. MyDay (stenfilcon A) soft (hydrophilic) daily disposable contact lens; 2019. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf19/K190965.pdf.
37. Johnson & Johnson Vision Care, Inc. (Jacksonville, FL). 1-DAY ACUVUE® MOIST brand contact lenses. 1-DAY ACUVUE® MOIST brand contact lenses for ASTIGMATISM. 1-DAY ACUVUE® MOIST Brand MULTIFOCAL contact lenses etafilcon A soft (hydrophilic) contact lenses visibility tinted with UV blocker for daily disposable wear [package insert]; 2021. Available from: https://www.acuvue.com/sites/acuvue_us/files/m-07-17-02_-_1_day_acuvue_moist_family_-_package_insert_and_fitting_guide.pdf.
38. Johnson & Johnson Vision Care, Inc. (Jacksonville, FL). 1-DAY ACUVUE® TruEye® brand contact lenses with HYDRACLEAR® 1 technology (narafilcon A) visibility tinted with UV blocker for daily wear single use only lenses [package insert]; 2013. Available from: https://www.acuvue.com/sites/acuvue_us/files/as011303_fig_trueye.pdf.
39. Johnson & Johnson Vision Care, Inc. (Jacksonville, FL). ACUVUE® oasys BRAND CONTACT LENS 1-day with hydraLuxe™ [package insert]; 2016. Available from: https://www.acuvue.com/sites/acuvue_us/files/ao-03-16-13_web_final_pi.pdf.
40. Salton MR. The lysis of micro-organisms by lysozyme and related enzymes. J Gen Microbiol. 1958;18(2):481–490. doi:10.1099/00221287-18-2-481
41. Masschalck B, Van Houdt R, Van Haver EG, Michiels CW. Inactivation of gram-negative bacteria by lysozyme, denatured lysozyme, and lysozyme-derived peptides under high hydrostatic pressure. Appl Environ Microbiol. 2001;67(1):339–344. doi:10.1128/AEM.67.1.339-344.2001
42. Bmrb.io [homepage on the Internet]. BMRB Featured System: Lysozyme. Madison, WI: Biological Magnetic Resonance Data Bank
43. Berman ER. Tears. In: Blakemore C, editor. Biochemistry of the Eye. Perspectives in Vision Research. Boston: Springer; 1991:63–88.
44. Huang TY, Chang CH, Baskaran N, Wei Y. Correlation between surface friction and the hydrophobicity of structure-related side-chain exposure of albumin on contact lens. Colloids Surf B Biointerfaces. 2022;209(Pt 1):112152. doi:10.1016/j.colsurfb.2021.112152
45. Su CY, Yeh LK, Lai CC, Li KY, Tseng CL, Fang HW. Effects of lysosomal deposition on the friction coefficient of hydrogel contact lenses. Cont Lens Anterior Eye. 2020;43(2):144–148. doi:10.1016/j.clae.2019.09.007
46. Wlodarczyk SR, Custódio D, Pessoa A Jr, Monteiro G. Influence and effect of osmolytes in biopharmaceutical formulations. Eur J Pharm Biopharm. 2018;131:92–98. doi:10.1016/j.ejpb.2018.07.019
47. Almeida M, Magalhães M, Veiga F, Figueiras A. Poloxamers, poloxamines and polymeric micelles: definition, structure and therapeutic applications in cancer. J Polym Res. 2018;25:31. doi:10.1007/s10965-017-1426-x
48. Mustafi D, Smith CM, Makinen MW, Lee RC. Multi-block poloxamer surfactants suppress aggregation of denatured proteins. Biochim Biophys Acta. 2008;1780(1):7–15. doi:10.1016/j.bbagen.2007.08.017
49. Chin J, Mustafi D, Poellmann MJ, Lee RC. Amphiphilic copolymers reduce aggregation of unfolded lysozyme more effectively than polyethylene glycol. Phys Biol. 2017;14(1):16003. doi:10.1088/1478-3975/aa5788
50. Poellmann MJ, Sosnick TR, Meredith SC, Lee RC. The pentablock amphiphilic copolymer T1107 prevents aggregation of denatured and reduced lysozyme. Macromol Biosci. 2017;17(2):1600217. doi:10.1002/mabi.201600217
51. Ling MX, Nguyen M, McFaul CA, Lee RC. Peroxidation of tetronic 1107 reduces protein chaperone effect. Phys Biol. 2022;19(4):046008. doi:10.1088/1478-3975/ac6eaf
52. Tonge S, Jones L, Goodall S, Tighe B. The ex vivo wettability of soft contact lenses. Curr Eye Res. 2001;23(1):51–59. doi:10.1076/ceyr.23.1.51.5418
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

