Back to Journals » International Medical Case Reports Journal » Volume 17
Diagnosis of Thyroid Neoplasm-Associated Dermatomyositis in Ethiopian Woman
Authors Legese GL , Tesfaye YA, Ayele E, Ayalew DG , Abebaw AG, Gurji TB, Tadesse A
Received 4 November 2023
Accepted for publication 12 March 2024
Published 22 March 2024 Volume 2024:17 Pages 201—207
DOI https://doi.org/10.2147/IMCRJ.S448187
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Xudong Zhu
Gebrehiwot Lema Legese,1 Yeabsira Aklilu Tesfaye,1 Eleni Ayele,1 Desalew Getahun Ayalew,1 Aron Girma Abebaw,1 Tiruzer Bekele Gurji,2 Abilo Tadesse1
1Department of Internal Medicine, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Pathology, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Desalew Getahun Ayalew, Email [email protected]
Background: Dermatomyositis is an inflammatory myopathies causing proximal muscle weakness accompanied by muscular inflammation and skin rash. Dermatomyositis patients have a higher incidence of malignancy as compared to the general population.
Case Report: A 52-year-old known female patient with a toxic multi-nodular goiter presented with insidious onset of itchy erythematous rash on her neck and bilateral hands and progressive muscle weakness of 4 months’ duration. Associated with this, she had photosensitivity, and periorbital edema of 2 months’ duration. On physical examination, she had an anterior neck mass, proximal muscle weakness, periorbital edema, and violaceous skin rash on her bilateral arms, shoulders and neck. Thyroid function tests were normal, creatinine kinase was elevated, and muscle biopsy revealed inflammatory myositis. Ultrasound of the anterior neck mass and analysis of fine needle aspiration suggested thyroid cancer.
Conclusion: A high index of clinical suspicion is usually required for early diagnosis of dermatomyositis in resource-limited settings in order to prevent adverse outcomes and identify associated malignancies.
Keywords: dermatomyositis, thyroid neoplasm, para-neoplastic syndrome, Ethiopia, Gondar
Introduction
Dermatomyositis (DM) is a rare autoimmune disease (inflammatory muscle disease) characterized by inflammatory injury of skeletal muscles and progressive symmetrical, predominantly proximal muscle weakness and skin rash.1 Patients with dermatomyositis usually present with violaceus or dusky skin rash on faces and knuckles and proximal, symmetrical muscle weakness.2 Males and females are equally affected, with a prevalence of 1 in 100,000 for both.3 Around 30% of dermatomyositis cases are associated with malignancy. It can sometimes present as para-neoplastic manifestation of malignancy.4 Different case series and population-based studies have estimated that diagnosis of concomitant malignancy among DM patients varies from 15% to 27%, with increasing percentages as age increases.4,5 This paper aimed to present a case of thyroid neoplasm-associated dermatomyositis with a significant clinical response, particularly in the skin, following treatment with prednisolone and methotrexate.
Case Presentation
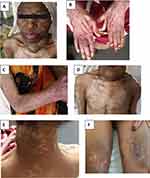
A 52-year-old female patient from Wolkiet (Northwest Ethiopia) was apparently healthy two years ago but then started experiencing irritability, heat intolerance and diaphoresis. She had an anterior neck swelling of 30 years’ duration. For this, she was evaluated at Haider referral hospital (North Ethiopia), diagnosed with a toxic multinodular goiter and managed with propylthiouracil 100 mg orally (po) three times per day. Six months following initiation of the medication, her symptoms improved, and she was offered surgical treatment, which she refused. Four months prior to her current presentation, she developed generalized body fatigue which worsened during activities like climbing stairs, combing her hair and standing from a squatting position. Concomitantly, she noticed violaceous rash over both hands and neck, which later involved the anterior part of her bilateral thighs and shoulders, hair loss and facial puffiness, but no morning stiffness. One week preceding her current visit, she had difficulty swallowing food, and was unable to support her head. On physical examination, she was acutely sick looking, conscious and oriented. Vital signs were all in normal ranges (blood pressure [BP] = 110/80 mmHg; pulse rate [PR] = 88 beats/minute; respiratory rate [RR] = 24 breaths/minute; temperature [Toc] = 36.7°C; Sa02 = 94% while breathing in room air). She had pink conjunctivae and non-icteric sclera. The anterior neck mass measured 4 by 5 cm, and was non-tender, mobile, multi-nodular, hard in consistency, not attached to overlying skin and showed no palpable lymphadenopathy. The chest was resonant on percussion note and clear on auscultation. Heart sounds were well heard, and there was no murmur or gallop. Abdominal examination revealed no enlarged organ or any sign of fluid collection and she had no visible deformity or limitation of movement on locomotor examination. As shown in Figure 1, on integumentary examination, there was an erythematous hyper-pigmented lesion on her face with periorbital edema (heliotrope rashes) (A); confluent violaceous and erythematous macular rashes on the knuckles of the hands (Gottron’s sign) (B) and macular rashes on the forearms with a salt and pepper appearance (C); and violaceous macular rashes on the neck line (V-shaped rashes) (D), shoulder and back (shawl’s sign) (E) and upper thighs, laterally (holster sign) (F). Additionally, she had a hypopigmented area on her nails, hands, anterior chest and bilateral thigh. On neurologic examination, she was alert and oriented to time, place and person, and cranial nerves were all intact. She had difficulty holding objects against gravity and standing from a squatting position. Sensory examination was normal and meningeal irritation signs were absent. Her laboratory test results (see Table 1) showed that she had moderate anemia (hemoglobin = 10.9g/dl, MCV = 78.0 Fl, and RDW: 13.5%), with normal other blood cell lines and erythrocyte sedimentation rate. Liver enzyme was mildly elevated (SGOT/AST: 52.97IU/L SGPT/ALT: 52.99IU/L), and serum albumin was low (2.6g/dl) with a normal bilirubin level. Renal function tests and serum electrolytes were normal. Thyroid function tests were within normal range. Urine analysis and serology test results were unremarkable. Serum creatine kinase before treatment was mildly elevated = 232u/l (normal range =20 – 198). The results of serology tests, such as the ANA, RF and anti-HIV antibody were non-revealing. Another immunological panel was not done due to limited clinical set up (Table 1).
 |
Table 1 Laboratory Profile of Dermatomyositis in a Thyroid Cancer Patient at University of Gondar Hospital |
Thyroid FNAC smears showed cellular aspirate composed of moderately pleomorphic round to polygonal cells admixed with scant colloid suggestive of thyroid follicular neoplasm. Thyroid gland biopsy was not possible since excision is required. Thyroid ultrasound showed multiple hyper-echoic masses on the left side of the thyroid measuring 4.5×2.4 cm, with calcification and multiple ipsilateral neck lymphadenopathy, the largest measuring 1.2 cm on the short axis dimension suggestive of thyroid malignancy. Chest x-ray was normal. Muscle biopsy showed increased nuclear internalization, scattered lymphocyte infiltration and atrophy, which was suggestive of inflammatory myopathy (see Figure 2).
 |
Figure 2 Muscle biopsy of dermatomyositis in thyroid cancer patient at University of Gondar Hospital. |
The diagnosis of thyroid neoplasm-associated dermatomyositis was considered based on ACR-EULAR classification criteria6 and the patient was put on prednisolone 40mg daily, methotrexate 7.5 mg po weekly, folic acid 5 mg po daily and cotrimoxazole 960 mg po 3 times per week. Broad spectrum sunscreen and liquid paraffin (moisturizer) were applied on a daily basis. The patient was advised on regular exercise, avoidance of direct sunlight exposure and was linked to chronic follow-up clinic. After 2 weeks of steroid treatment, the patient showed remarkable improvement in muscle strength, and the steroid dose was tapered every week thereafter. After 2 months of treatment, she showed dermatological improvement and her physical exercise endurance was also significantly better, as shown in Figure 3.
Discussion
Dermatomyositis is an idiopathic inflammatory myopathy typically characterized by differential muscle weakness (predominantly proximal muscles) and skin rashes. The degree of muscle and skin involvement varies from patient to patient. Some patients may have no muscle weakness while others may have minimal or diffuse muscle weakness.7 More than 85% of dermatomyositis patients with muscle weakness have elevated serum creatinine kinase levels.8 In a dermatomyositis suspected patient, detailed evaluation of their history and a physical examination emphasizing skin inspection and assessment of muscle strength are necessary. Patients should be assessed for indicators of severe disease, including cardiorespiratory symptoms and dysphagia requiring early airway support.9 Most dermatomyositis patients present at clinics with a complaint of proximal muscle weakness, which includes symptoms like difficulty arising from a chair, climbing stairs, lifting objects, or washing hair. Symptoms of distal muscle weakness like difficulty holding and manipulating objects may occur later. In most severe forms of the disease the extensor neck muscles and proximal esophagus muscles can be involved, and patients can present with an inability to support their head, dysphagia, dysphonia, and weakness in respiratory muscles, as seen in our patient.10 The peculiar skin manifestations of dermatomyositis can occur before, alongside or shortly after muscle weakness. The commonest cutaneous manifestations of dermatomyositis include the heliotrope eruption, midfacial erythema that involves the nasolabial folds, Gottron papules, Gottron sign, poikilodermatous changes, dilated capillaries, and dropout of capillaries within the proximal nail folds, cuticular hypertrophy, and nonscarring alopecia often with diffuse erythema and scaling.11 The prevalence of malignancy in dermatomyositis patients is as high as 17.2%.12 The commonest malignancies associated with dermatomyositis include nasopharyngeal, lung, breast, ovarian, and hematologic cancers.13,14 There are also case reports of dermatomyositis associated with thyroid cancer patients, of which papillary thyroid cancer is the commonest.15,16 Though there are data discrepancies, it is reported that underlying malignancy is higher in those who have constitutional symptoms, absence of Raynaud's phenomenon, elevated erythrocyte sedimentation rate, and severe form of disease.17 In dermatomyositis patients, screening for cancer is controversial, and requires shared decision-making methods in order to determine whether malignancy screening is valuable.4,12
Although there is no cure for dermatomyositis, there are treatment options that can improve skin manifestations and muscle strength. Prednisone 1 mg/kg per day with a broad spectrum sunscreen is the initial treatment choice, improving symptoms within 4 weeks of treatment initiation.10,18 Following clinical response, the steriod dose should be tapered by 1mg/kg every other day for 10 weeks.19 If prednisolone is not clinically suitable, either methotrexate or azathioprine can suffice. Biological agents like rituximab, and intravenous immunoglobulin (IVIG) are options for those who are unresponsive to standard therapy.20
Conclusion
A high index of clinical suspicion is required for early diagnosis of dermatomyositis in resource-limited settings to improve patient outcomes and identify associated co-morbidities.
Ethical Approval
Our institution does not demand ethical approval for reporting individual case reports.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor in-chief of this journal.
Acknowledgments
We are grateful to all medical personnel who looked after the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors did not receive any financial support from any source.
Disclosure
The authors declare no potential conflicts of interest in this work.
References
1. Cardelli C, Zanframundo G, Cometi L, et al. Idiopathic inflammatory myopathies: one year in review 2021. Clin Exp Rheumatol. 2022;40(2):199–209. doi:10.55563/clinexprheumatol/vskjxi
2. Rashidian S, Falahati M, Kordbacheh P, et al. A study on etiologic agents and clinical manifestations of dermatophytosis in Yazd, Iran. Curr Med Mycol. 2015;1(4):20–25. doi:10.18869/acadpub.cmm.1.4.20
3. Kronzer VL, Kimbrough BA, Crowson CS, Davis JM, Holmqvist M, Ernste FC. Incidence, Prevalence, and Mortality of Dermatomyositis: a Population-Based Cohort Study. Arthritis Care Res. 2023;75(2):348–355. doi:10.1002/acr.24786
4. Khanna U, Galimberti F, Li Y, Fernandez AP. Dermatomyositis and malignancy: should all patients with dermatomyositis undergo malignancy screening? Ann Translat Med. 2021;9(5):432. doi:10.21037/atm-20-5215
5. Qiang JK, Kim WB, Baibergenova A, Alhusayen R. Risk of Malignancy in Dermatomyositis and Polymyositis. J Cutaneous Med Surgery. 2017;21(2):131–136. doi:10.1177/1203475416665601
6. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheumatic Dis. 2017;76(12):1955–1964. doi:10.1136/annrheumdis-2017-211468
7. Didona D, Solimani F, Caposiena Caro RD, et al. Dermatomyositis: a comprehensive review of clinical manifestations, serological features, and therapeutic approaches. Ital J Dermatol Venereol. 2023;158(2):84–98. doi:10.23736/S2784-8671.23.07458-3
8. Oddis CV, Medsger TA. Relationship between serum creatine kinase level and corticosteroid therapy in polymyositis-dermatomyositis. J Rheumatol. 1988;15(5):807–811.
9. Wang S, Keaton R, Kendrick Z. Severe Cutaneous Findings in a Woman with Dermatomyositis. Clin practice cases em med. 2019;3(3):222–225. doi:10.5811/cpcem.2019.3.41058
10. Tanboon J, Nishino I. Update on dermatomyositis. Curr Opinion Neurol. 2022;35(5):611–621. doi:10.1097/WCO.0000000000001091
11. Sun Y, Li DF, Zhang YL, Liang X, Li TF. Characterisation of Disease Patterns of Dermatomyositis with Different Initial Manifestations. Int J Gene Med. 2022;15:6519–6528. doi:10.2147/IJGM.S372658
12. Fang YF, Wu YJ, Kuo CF, Luo SF, Yu KH. Malignancy in dermatomyositis and polymyositis: analysis of 192 patients. Clin Rheumatol. 2016;35(8):1977–1984. doi:10.1007/s10067-016-3296-8
13. Opinc AH, Makowska JS. Update on Malignancy in Myositis-Well-Established Association with Unmet Needs. Biomolecules. 2022;12(1):111. doi:10.3390/biom12010111
14. Ungprasert P, Leeaphorn N, Hosiriluck N, Chaiwatcharayut W, Ammannagari N, Raddatz DA. Clinical features of inflammatory myopathies and their association with malignancy: a systematic review in asian population. ISRN Rheumatol. 2013;2013:509354. doi:10.1155/2013/509354
15. Yilmaz U, Ugurlu S. Dermatomyositis Associated With Papillary Thyroid Cancer: systematic Review of the Literature and a Case Report. J clin Rheumatol. 2021;27(4):168–171. doi:10.1097/RHU.0000000000001048
16. Shah M, Shah NB, Moder KG, Dean D. Three cases of dermatomyositis associated with papillary thyroid cancer. Endocrine Practice. 2013;19(6):e154–7. doi:10.4158/EP13145.CR
17. Shao C, Li S, Sun Y, et al. Clinical characteristics and prognostic analysis of Chinese dermatomyositis patients with malignancies. Medicine. 2020;99(34):e21899. doi:10.1097/MD.0000000000021899
18. Hengstman GJ, van den Hoogen FH, van Engelen BG. Treatment of the inflammatory myopathies: update and practical recommendations. Exp Opinion Pharmacother. 2009;10(7):1183–1190. doi:10.1517/14656560902913815
19. Waldman R, DeWane ME, Lu J. Dermatomyositis: diagnosis and treatment. J Am Acad Dermatol. 2020;82(2):283–296. doi:10.1016/j.jaad.2019.05.105
20. Marrani E, Abu-Rumeileh S, Mastrolia MV, Maccora I, Pagnini I, Simonini G. A systematic review on biological therapies in juvenile idiopathic inflammatory myopathies: an evidence gap in precision medicine. Clin exp Rheumatol. 2022;40(2):457–470. doi:10.55563/clinexprheumatol/ltrj4l
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.