Back to Journals » Drug Design, Development and Therapy » Volume 18
Dexamethasone versus Dexmedetomidine as Adjuvants in Ultrasound Popliteal Sciatic Nerve Block for Hallux Valgus Surgery: A Mono-Centric Retrospective Comparative Study
Authors Coviello A , Iacovazzo C, Cirillo D , Bernasconi A , Marra A, Squillacioti F, Martone M, Garone E, Coppola F, de Siena AU , Vargas M, Servillo G
Received 21 October 2023
Accepted for publication 9 March 2024
Published 17 April 2024 Volume 2024:18 Pages 1231—1245
DOI https://doi.org/10.2147/DDDT.S442808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Antonio Coviello,1,* Carmine Iacovazzo,1,* Dario Cirillo,1 Alessio Bernasconi,2 Annachiara Marra,1 Francesco Squillacioti,1 Marco Martone,1 Eleonora Garone,1 Filomena Coppola,1 Andrea Uriel de Siena,1 Maria Vargas,1 Giuseppe Servillo1
1Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples “Federico II”, Naples, 80131, Italy; 2Department of Public Health, School of Medicine, University of Naples “Federico II”, Unit of Orthopedics and Traumatology, Naples, Italy
*These authors contributed equally to this work
Correspondence: Antonio Coviello, Department of Neurosciences, Reproductive and Odontostomatological Sciences, University of Naples “Federico II”, Via Sergio Pansini, 5, Napoli NA, Naples, 80131, Italy, Tel +39 3497013533, Fax +39 0817462281, Email [email protected]
Background and Aim: Ultrasound popliteal sciatic nerve block (UPSNB) is commonly performed in foot and ankle surgery. This study aims to assess the use of dexmedetomidine and dexamethasone as adjuvants in UPSNB for hallux valgus (HV) surgery, comparing their efficacy in producing motor and sensory block and controlling postoperative pain. The adverse event rate was also evaluated.
Methods: This mono-centric retrospective study included 62 adult patients undergoing HV surgery: 30 patients received lidocaine 2% 200 mg, ropivacaine 0.5% 50 mg and dexamethasone 4 mg (Group 1), whereas 32 patients received lidocaine 2% 200 mg, ropivacaine 0.5% 50 mg, and dexmedetomidine 1 mcg/Kg (Group 2). At first, the visual analogue scale (VAS) was evaluated after 48 hours. The other outcomes were time to motor block regression, evaluation of the first analgesic drug intake, analgesic effect, adverse effects (hemodynamic disorders, postoperative nausea and vomiting (PONV)) and patient satisfaction. The continuous data were analyzed with student’s t-test and the continuous one with χ2. Statistical significance was set at a p-value lower than 0.05.
Results: No significant difference was found in VAS after 48 hours (4.5 ± 1.6 vs 4.7 ± 1.7, p = 0.621) to motor block regression (18.9 ± 6.0 vs 18.7 ± 6, p = 0.922). The number of patients that took their first analgesic drug in the first 48 h (p = 0.947 at 6 hours; p = 0.421 at 12 hours; p = 0.122 at 24 hours and p = 0.333 at 48 hours) were not significant. A low and similar incidence of intraoperative hemodynamic disorders was recorded in both groups (hypotension p = 0.593; bradycardia p = 0.881). Neither PONV nor other complication was found. Patients in Group 1 reported a lower degree of interference with sleep (p = 0.001), less interference with daily activities (P = 0.002) and with the affective sphere (P = 0.015) along with a more satisfactory postoperative pain management (p < 0.001) as compared to Group 2.
Conclusion: No significant differences were observed in the duration of motor and sensory blockade between patients in both groups. Additionally, both groups showed good pain control with a low rate of adverse effects, even if there was no clinical difference between the groups. However, patients who received dexamethasone reported experiencing less interference with their sleep, daily activities and overall emotional well-being, and overall pain control.
Keywords: ultrasound popliteal sciatic nerve block, dexamethasone, dexmedetomidine, Hallux valgus, adjuvants in peripheral nerve blocks
Introduction
Hallux valgus (HV) is a complex valgus deformity of the first ray that can cause impaired joint mechanics, dysfunction, and progressive pain at the medial eminence of the first metatarsophalangeal (MTP) joint. Its estimated prevalence stands at 23% in adults aged 18–65 years and 35.7% in those older than 65 years, with a higher prevalence in females.1,2 Surgery is indicated in case of persistent pain and difficulty wearing shoes despite the adoption of conservative treatments (ie, oral analgesics and shoe modification).3 Out of over 130 procedures described to correct HV, Scarf/Akin (SA) osteotomy is the most used, which is performed through a medial approach at the first MTP joint with bony fragments used employing small-diameter screws.4 In this setting, inadequate pain control was associated with a prolonged stay in the post-anesthesia recovery room, an extended hospitalization and a delayed return to normal daily activities.5 On the other side, multimodal analgesia generally results in better pain control, earlier mobilization and fewer side effects or complications with reduced need for opioid assumptions.6,7
Several prospective randomized controlled trials have shown that peripheral nerve blocks represent a safe and effective choice for foot and ankle surgery, including hallux valgus correction.5,8–10 In particular, some studies have shown that Ultrasound Popliteal Sciatic Nerve Block (UPSNB) is a valid postoperative analgesia strategy for hindfoot and forefoot surgery,11 being associated with fewer complications as compared to other type of nerve blocks (such as neuraxial block or ankle block).11 Over the years, different medications, such as opioids, epinephrine, sodium bicarbonate, magnesium sulfate, dexamethasone, ketamine, neostigmine, midazolam, clonidine, and dexmedetomidine have been combined with local anesthetics (LA) to increase the duration of anesthesia or analgesia.12 These combinations have been found to be effective and safe for many types of patients, including pregnant women undergoing caesarean section.13 More specifically, a few studies showed that dexamethasone used as adjuvant prolongs the duration of pain relief after minor foot and ankle surgery.14 On the other side, dexmedetomidine used in peripheral nerve blocks seems to reduce the onset time, prolong the sensory and motor blocks and provide a sedative effect.15,16 To the best of our knowledge, no clear evidence has been provided so far about the superiority of one adjuvant as compared to another.
The aim of this study was to compare the use of dexmedetomidine and dexamethasone as adjuvants in UPSNB during HV surgery, evaluating the pain score after surgery, time to motor block regression, time of first rescue dose intraoperative, common adverse effects and patient satisfaction.
Materials and Methods
This was a Level III monocentric before-and-after17 retrospective comparative study performed at the “Department of Neurosciences, Reproductive, and Odontostomatological Sciences” of the “Federico II” University of Naples (Naples, Italy). Federico II University’s ethics committee did not consider approval necessary for this type of study; all patients who signed the informed consent for anesthesia also consented for the personal data to be used for scientific purposes anonymously. Data regarding patients undergoing HV correction between March 2022 and June 2023 at our institution (recorded as part of daily clinical practice) was obtained from the archive of the department, anonymized and stored in a password-protected computerized database using MS Office Excel 2007 (Microsoft, Redmond, WA, USA). During the time frame taken into consideration, the senior anesthetist modified the anesthetic protocol by replacing the dexamethasone (as adjuvant combined in a LA mixture) with a novel adjuvant drug (dexmedetomidine). All procedures performed were in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was followed.
Inclusion/Exclusion Criteria
The inclusion criteria were as follows:
- adult patients (≥18 years old);
- day-care surgery;
- ASA (American Society of Anesthesiologists) physical status of I–III;
- patients undergone elective open Scarf/Akin osteotomy for HV by the same senior orthopaedic surgeon;
- patients undergone to standardized Ultrasound Popliteal Sciatic Nerve Block (UPSNB) by the same anesthetist;
- patients whose medical records were fully accessible.
The exclusion criteria were as follows:
● patients with neuropathic disease of the affected limb;
● patients with mental disorders;
● patients undergoing general or neuraxial anesthesia;
● patients undergone a different Peripheral Nerve Block (PNB) technique or with contraindications to PNB (allergy);
● patients where the treatment protocol could not be fully applied for different reasons.
Study Population
After application of the above mentioned criteria, 10 patients out of 72 were excluded (another type of PNB or a different type of surgery was performed or data in medical records were not accessible), leaving 62 patients eligible for the study (Figure 1).
 |
Figure 1 Flowchart of the study. |
Anesthesiologic Management
In the operating room, venous access was placed (18–16 G), pantoprazole 40 mg and antibiotic prophylaxis intravenous were administered (cefazolin 1 or 2 g IV or, in case of allergy, clindamycin 600 mg iv) 30 min before skin incision. Electrocardiogram (ECG), pulse oximetry (SpO2), body temperature (TC), and continuous non-invasive blood pressure (NIBP) were monitored every 5 min. Standard premedication was administered using intravenous midazolam (0.01–0.03 mg/Kg) and fentanyl (1 mcg/Kg) in order to improve patient compliance and comfort. We proceeded to perform an intraoperative fluid administration of 3 mL/kg/hour of IV crystalloids.18 Surgical time, intraoperative hemodynamic instability such as hypotension (MAP <60 mmHg, SAP <90 mmHg, or <20% of the initial values), or bradycardia (HR < 60 bpm) were recorded.
Ultrasound Popliteal Sciatic Nerve Block (UPSNB)
All patients were placed in supine position with the knee slightly flexed to facilitate the transducer placement as for an in-plane approach (Figure 2). An ultrasound (US) linear transducer (Sonosite HLF38x 13–6 MHz, Fujifilm Sonosite Europe, Amsterdam, Netherlands) was used for scanning transversely the popliteal fossa and visualize the popliteal vessels, the biceps femoris muscle placed laterally, the semitendinosus and semimembranosus muscles placed medially, and one of the two branches of the sciatic nerve (usually the tibial nerve (TN)). Once this latter was visualized, the procedure involved using a transducer to track the nerves. The tracker was moved upwards (cranially) until two nerves were visible. These were the TN nerve, located towards the middle, and the CPN nerve, located towards the side. Both nerves were seen to converge at a common paraneural sheath, usually found 5–12 cm above the popliteal crease (Figure 3).19 At this level, from the lateral side of the transducer (lateral-to-medial approach) an 80 or 95-mm long 21-Gauge needle with a 20° or 30° tip (peripheral nerve block kit AV Medical S.R.L., Italy) was inserted in-plane under real-time US guidance. The needle tip insertion point corresponded to the space between the vastus lateralis muscle and the biceps femoris muscle. Its correct position was double-checked through the progressive injection of 3 mL saline solution. The patients then received ultrasound popliteal sciatic nerve block with lidocaine 2% 10 mL (200 mg) plus ropivacaine 0.5% 10 mL (50 mg) and dexamethasone 4 mg as adjuvant (performed as standard procedure until October 2022) or lidocaine 2% 10 mL (200 mg) plus ropivacaine 0.5%10 mL (50 mg) and dexmedetomidine 1 mcg/Kg as adjuvant (performed as standard procedure from November 2022) (Figure 4). The risk factors of postoperative nausea and vomiting (PONV) were analyzed and an Apfel score was assessed for each patient. Intraoperative and postoperative antiemetic treatment was performed in accordance with the 2020 Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting.20 In the postoperative period, the patients were monitored in the Post-Anesthesia Care Unit (PACU) for an average of 30 minutes, before being transferred to the ward. Patients at discharge were given and explained a preprinted template where they could report pain, motility recovery, adverse effects, and treatment. Patients were discharged even with no fully regressed motor block. In addition, the patients were contacted by telephone for the first 48 postoperative hours by the same clinicians that followed them during hospitalization time.
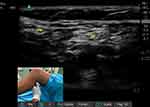 |
Figure 2 Ultrasound popliteal sciatic nerve block (UPSNB): patient and transducer positioning. Abbreviations: CMP, common peroneal nerve; TN, tibial nerve. |
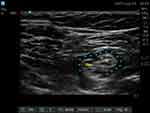 |
Figure 4 Diffusion of the local anesthetic around the nerve target during injection. *Local anesthetic spread. Abbreviations: CMP, common peroneal nerve; TN, tibial nerve. |
Data Extraction
The following data were obtained from medical records: demographics (age, sex, body mass index (BMI), ASA, comorbidities), operative time, sensory and motor blocks efficacy and duration, time to first analgesic rescue drug, analgesic effect, intraoperative hemodynamic instability, PONV and patient satisfaction.
The time to the first analgesic rescue dose (diclofenac 150 mg per os) was assessed at 6, 12, 24 and 48 hours after surgery and the number of patients who took it were recorded. VAS assessment was carried out using a 10-cm long line with verbal anchors at both extremities (“no pain” on the far left and “the most intense experienced pain” on the far right). The patient marked a point on the line corresponding to the rating of pain intensity.21 Pain control was considered satisfactory in case of VAS score less than or equal to 4.
The sensory block was evaluated through pinprick testing in the area of the leg innervated by the sciatic nerve ipsilateral to the block. Patients were classified according to the Hollman scale (Grade 1 = Full sensation; Grade 2 = Weak sensation; Grade 3 = Recognized as light touch; Grade 4 = Loss of sensation).22 For the sensory assessment, the tip of the needle (22 Gauge short bevel) was applied to the skin with a force that was adequate to indent the skin but not enough to puncture it, in order to produce a consistent painful sensation when applied to the areas with normal sensation.
The motor block was evaluated using the modified Bromage scale (Score 0 = Normal motor functions with full flexion and extension of the ankle, foot, and toes; Score 1 = Decreased motor strength with the ability to move toes only; Score 2 = Complete motor blockade with the inability to move toes).23 Only if Hollman grade was 4 and Bromage score was 2 the anesthesia was judged adequate to proceed to surgery.
Postoperatively (within 48 hours after the procedure), time to motor block regression, analgesic effect (Visual Analog Scale or VAS), the incidence of intraoperative and postoperative complications related to anesthesia (intraoperative hemodynamic instability, PONV) were recorded.
Time to motor block regression (duration of motor block), defined as the time between complete block (score 2) after local anesthetic injection (T0) and no motor block (score 0) on the modified Bromage scale was evaluated.22 Motor block was assessed before and 10, 15, and 20 minutes after T0, and thereafter, every 30 minutes during surgery, and every hour in the postoperative period until its complete regression. Pharmacological therapy was based on patient response. After surgery, we administered paracetamol 1 gr orally 3 times a day.
Patient satisfaction was assessed regularly 24 hours after surgery using the Revised American Pain Society Patient Outcome Questionnaire (ASP-POQ-R) (Figures 5 and 6).24–26 The questionnaire was administered to patients over the phone by the trainee physician who had been following the same patient throughout the day of surgery. The following domains were considered: “pain severity” (questions n. 1, n. 2, n. 3, and n. 4), “sleep interference” (questions n. 5c and n. 5d), “pain severity and sleep interference” (elaborated through “pain severity” and “sleep interference” questions), “activity interference” (questions n. 5a and n. 5b), “affective sphere” (questions n. 6a, n. 6b, n. 6c, and n. 6d), “adverse effects” (questions n. 7a, n. 7b, n. 7c, n. 7d), “perception of care” (through questions on “adverse effects”, n. 8, and n. 9) and “quality of postoperative management” (by all previous domains) as suggested by Gordon et al24
 |
Figure 5 Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R). Notes: Adapted from The Journal of Pain, 11/11, Debra B. Gordon, Rosemary C. Polomano, Teresa A. Pellino, Dennis C. Turk, Lance M. McCracken, Gwen Sherwood, Judith A. Paice, Mark S. Wallace, Scott A. Strassels, John T. Farrar, Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for Quality Improvement of Pain Management in Hospitalized Adults: Preliminary Psychometric Evaluation, 1172–1186, Copyright 2010, with permission from Elsevier.24 |
 |
Figure 6 Revised American Pain Society Patient Outcome Questionnaire APS-POQ-R (Continued). Notes: Adapted from The Journal of Pain, 11/11, Debra B. Gordon, Rosemary C. Polomano, Teresa A. Pellino, Dennis C. Turk, Lance M. McCracken, Gwen Sherwood, Judith A. Paice, Mark S. Wallace, Scott A. Strassels, John T. Farrar, Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for Quality Improvement of Pain Management in Hospitalized Adults: Preliminary Psychometric Evaluation, 1172–1186, Copyright 2010, with permission from Elsevier.24 |
Statistical Analysis
The calculation of the sample size was performed assuming the following variables: a standard deviation (SD) equal between the two samples (SD = 1.5); an error α=0.05; a power of 80% (1-β, β=0.2); a Cohen’s d of 1, based on the effect size of the VAS at 48 hours considering a two-tails t-student test for unpaired samples; and a N2;/N1 ratio equal to 1. The calculation of the sample sizes was performed using “G*power” program: the calculated total sample size was 54 patients (27 patients for each group).
Finally, two groups were identified: Group 1 (G1): lidocaine 2% 10 mL (200 mg) ropivacaine 0.5% 10 mL (50 mg) and dexamethasone 4 mg; Group 2 (G2): lidocaine 2% 10 mL (200 mg), ropivacaine 0.5% 10 mL (50 mg) and dexmedetomidine 1 mcg/Kg. Continuous data were reported as mean and SD for parametric data or median and interquartile range (IQR) for non-parametric data. A two-tailed t student was used to compare parametric data, while U-Mann–Whitney test was applied for non-parametric data. Dichotomous variables were presented as number and relative frequencies; the comparison was performed using a χ2 test. A p value <0.05 was considered statistically significant. All statistical analyses were performed using RStudio Team (version 2020, Integrated Development for R. RStudio, PBC, Boston).
Results
Demographics
Overall, 30 patients were included in Group 1 and 32 patients in Group 2. The two groups were comparable according to demographic characteristics (Table 1).
 |
Table 1 Patients Characteristics |
Postoperative Course
There was no statistically significant difference in terms of postoperative pain control (VAS at 48 h 4.5 ± 1.6 for Group 1 and 4.7 ± 1.7 for Group 2 (P = 0.621)). Recovery time from the motor block was superimposable in the two groups (18.9 ± 6 h in Group 1 and 18.7 ± 6 h in Group 2, P = 0.922). The first request for analgesics in the postoperative period occurred within 48 hours for most patients for both groups, although the difference was not statistically significant (6.7% in G1 vs 6.3% in G2 at 6 h, p = 0.947; 16.7% in G1 vs 25% in G2 at 12 h, p = 0.421; 19% in G1 vs 14% in G2 at 24 h, p = 0.122; 0% in G1 vs 12.5% in G2 at 48 h, p = 0.333). In the G1 group 4 patients never requested the analgesic rescue dose, while in the G2 group only 2 patients never requested it (Table 2).
 |
Table 2 Records |
Intra and Postoperative Side Effects
All patients were discharged at home on the same day without adverse effects, postoperative complications, and refusal. There was no significant difference between the two groups in the duration of the operation and the intraoperative complication rate. The intraoperative hemodynamic instability was also similar in the two groups (Table 3).
 |
Table 3 Intra and Postoperative Details |
Patient Satisfaction
Patients in Group 1 performed better in the pain intensity domain of the APSPOQ-R questionnaire at 24 h from surgery (least pain 1.2 vs 1.9, P = 0.011) with a lower degree of interference with sleep (1 ± 2.8 vs 5 ± 6.5, P = 0.001) compared with Group 2. The interference with daily activities (0.8 ± 10.7 vs 3.6 ± 4.6, P = 0.002) and with the affective sphere (0.0 ± 0.0 vs 3.03 ± 6.58, P = 0.015) were in favor of dexamethasone as compared to dexmedetomidine (Tables 4 and 5); however, the patients felt more anxious (0.00 ± 0.00 vs 0.77 ± 1.72, P = 0.018) and helpless (0.00 ± 0.00 vs 1.09 ± 2.45, P = 0.018). The overall quality of postoperative pain management was greater in Group 1 than in Group 2 as well (82.1 ± 28.3 vs 107.8 ± 26.4, P < 0.001). Other differences have been reported in Tables 4 and 5.
 |
Table 4 Analysis of Questionnaire APS-POQ-R Domains |
 |
Table 5 Analysis of Questions N° 10, N° 11, N° 12, and N°13 of APS-POQ-R Questionnaire |
Discussion
In this study, comparing dexamethasone and dexmedetomidine as adjuvant drugs to ropivacaine during ultrasound popliteal sciatic nerve block for patients undergoing Scarf/Akin osteotomy for HV correction, we found no significant difference in terms of analgesia, motor block, sensory block and adverse effects. In both cases, a total regression of the motor block was recorded at an average time of approximately 18 h. Interestingly, the satisfaction questionnaire revealed that patients who received dexamethasone as adjuvant medication perceived a better control of postoperative pain, with less pain intensity and less interference with sleep, with daily activities and with the affective sphere as compared to the dexmedetomidine group. To the best of our knowledge, this is the first study that assesses the role of dexamethasone and dexmedetomidine as adjuvant drugs in this setting.
Peripheral nerve blocks may also be considered as a viable alternative in frail patients where different anesthesia techniques (at higher risk) cannot be performed.27 Recent studies have consistently demonstrated a prolongation of peripheral nerve blocks using perineural buprenorphine, clonidine, dexamethasone, dexmedetomidine, and magnesium.28 According to some authors,29–32 a perineural catheter might be very effective at prolonging postoperative analgesia, although it may result technically demanding since an incorrect positioning of the catheter may lead to its dislocation33–35
The use of adjuvants in loco-regional anesthesia is widely discussed in current literature. In the orthopedic field, the value of dexamethasone as an adjuvant has been reported in patients undergoing shoulder surgery. In an RCT by Cummings et al, it was showed that, while 8 mg dexamethasone prolonged the duration of analgesia when added to both 30 mL bupivacaine 0.5% and 30 mL ropivacaine 0.5%, a better synergy could be found coupling dexamethasone to ropivacaine (11 additional hours of analgesia) as compared to dexamethasone and bupivacaine (8 additional hours of analgesia). The total duration of analgesia for both local mixtures was about 22 hours, which is similar to our findings (18 hours). The higher dosage of dexamethasone used by Cummings as compared to our study (ie, 8 mg vs 4 mg) might explain the slight difference between the two cohorts.36 In another RCT, Desmet et al, evaluated the interscalene nerve block in patients undergoing arthroscopic shoulder surgery, comparing the analgesic duration achieved using ropivacaine 0.5% 30 mL alone vs ropivacaine 0.5% 30 mL with the addition of perineural dexamethasone 10 mg vs ropivacaine 0.5% 30 mL plus intravenous dexamethasone 10 mg. They found that the addition of dexamethasone doubled the duration of analgesia when used as a perineural adjuvant compared to the use of local anesthetic alone (12 hours vs 24 hours, respectively). They also concluded that the use of dexamethasone intravenously or perineural was comparable in terms of prolongation of analgesia (21 hours vs 24 hours respectively). Although even in this case the dosage of dexamethasone was higher than in our cohort, we would like to underline that the proportion of patients not requiring a rescue dose was not dissimilar (4/30 patients in our study vs 4/49 in Desmet’s study). These data suggest that 4 mg dexamethasone administered peripherally as adjuvant may be sufficient in this setting.37
Some other studies have reported on the efficacy of dexmedetomidine as adjuvant medication in peripheral nerve blocks. In a study on rats, Brummett et al, reported no damage to the peripheral nervous system (on histopathological examination) even after administering very high dosages (28–40 mcg/kg) of dexmedetomidine, demonstrating its safety of perineural blocks.38 Among clinical studies, Ammar et al investigated the incidence of adverse effects in patients undergoing peripheral nerve block using perineural dexmedetomidine at 0.75 mcg/kg, reporting a 13% incidence of PONV compared with the control group.39 In our study, the dosage choice of perineural dexmedetomidine for nerve block was 1 mcg/kg which was based on a meta-analysis of randomized controlled trials conducted by Vorobeichik et al.40 As compared to the study by Ammar, we did not record any PONV or similar complications, which could be related to the use of opioids for postoperative pain management may be related to this difference.39 It is worth highlighting that the mechanism of action of α2-adrenoceptor agonists in peripheral nerve blocks is not fully understood. While proposed mechanisms include central analgesia, vasoconstriction, and anti-inflammatory effects, none of these mechanisms can fully explain the synergistic effect of α2-adrenoceptor agonists when added to a local anesthetic in peripheral nerve blocks.41,42 Some have hypothesized that the direct action of α2-adrenoceptors on the peripheral nerve may be mediated through an increase in hyperpolarization of the after-potential that follows a single compound action potential,43 but further studies in the field are warranted in order to confirm or disprove this theory.
In the foot and ankle area, a few previous studies have investigated the role of the ultrasound popliteal sciatic nerve block to perform surgical procedures.44–46 A double-blind RCT conducted by Vermeylen et al (in which saline, clonidine 100 mcg and dexamethasone 5 mg as adjuvants to ropivacaine 0.75% were compared) showed that the addition of dexamethasone to ropivacaine significantly prolonged the duration of analgesia (about 9 hours) and the time to motor block regression (about 13 hours) in HV surgery. In Vermeylen et al study the duration of the motor blockade was 32 h, whereas in ours it was 18 h. The increase in the duration of motor blockade was to be expected as higher concentrations of local anesthetic and higher doses of dexamethasone were used.47 The effects of perineural dexamethasone administration may be multiple. It could act directly on nociceptive impulse transmission along unmyelinated C-fibers, increasing the expression of inhibitory K+ channels and indirectly increasing the duration of anesthesia by reducing the rate of systemic absorption of anesthetic solution through local vasoconstriction. Its effects could also be mediated by a systemic anti-inflammatory action secondary to absorption by the vessels.48
This study has some limitations. First, some biases related to the retrospective framework of the study should be considered. Second, the small number of patients included, which might generate a type II error and does not allow to highlight differences between the two groups. This could encourage further analyses on larger cohorts of patients in order to confirm our findings. Third, patients who were followed-up by different teams of clinicians were not assisted by the same teams in the intraoperative and postoperative periods. Finally, we are aware that there are a number of variables (ie, anxiety) not considered in this study which might have influenced patients in reporting pain or in triggering reactions like vomiting or nausea and shivers.
Conclusion
This study suggested that dexmedetomidine (1 µg/kg) and dexamethasone (4 mg) as adjuvants to the local anesthetic mixture in the ultrasound popliteal sciatic nerve block following Scarf/Akin osteotomy for HV seem to be comparable in terms of effectiveness, safety, pain management and length of anesthesia. The satisfaction questionnaire showed that the patients who received dexamethasone as adjuvant reported less pain intensity, less interference with sleep and a minor impact on the affective sphere as compared to patients who received dexmedetomidine. Further studies, including randomized controlled trials, are necessary to confirm our findings and establish differences between dexamethasone and dexmedetomidine in UPSNB.
Acknowledgments
The authors would like to thank the reviewer, Anna Onza, for the effort and the time spent in the linguistic revision of the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nery C, Coughlin MJ, Baumfeld D, Ballerini FJ, Kobata S. Hallux valgus in males—part 1: demographics, etiology, and comparative radiology. Foot Ankle Int. 2013;34(5):629–635. doi:10.1177/1071100713475350
2. Nix S, Smith M, Vicenzino B. Prevalence of hallux valgus in the general population: a systematic review and meta-analysis. J Foot Ankle Res. 2010;3(1):21. doi:10.1186/1757-1146-3-21
3. Lee KM, Ahn S, Chung CY, et al. Reliability and relationship of radiographic measurements in Hallux Valgus. Clin Orthop Relat Res. 2012;470(9):2613–2621. doi:10.1007/s11999-012-2368-6
4. Trnka HJ, Zembsch A, Easley ME, Salzer M, Ritschl P, Myerson MS. The chevron osteotomy for correction of hallux valgus. Comparison of findings after two and five years of follow-up. J Bone Jt Surg Am. 2000;82-A(10):1373–1378. doi:10.2106/00004623-200010000-00002
5. Pearce CJ, Hamilton PD. Current concepts review: regional anesthesia for foot and ankle surgery. Foot Ankle Int. 2010;31(8):732–739. doi:10.3113/FAI.2010.0732
6. Wang J, Liu GT, Mayo HG, et al. Pain management for elective foot and ankle surgery: a systematic review of randomized controlled trials. J Foot Ankle Surg. 2015;54(4):625–635. doi:10.1053/j.jfas.2014.05.003
7. Saini S, McDonald EL, Shakked R, et al. Prospective evaluation of utilization patterns and prescribing guidelines of opioid consumption following orthopedic foot and ankle surgery. Foot Ankle Int. 2018;39(11):1257–1265. doi:10.1177/1071100718790243
8. Vadivelu N, Kai AM, Maslin B, et al. Role of regional anesthesia in foot and ankle surgery. Foot Ankle Spec. 2015;8(3):212–219. doi:10.1177/1938640015569769
9. Karaarslan S, Tekgül ZT, Şimşek E, et al. Comparison between ultrasonography-guided popliteal sciatic nerve block and spinal anesthesia for Hallux valgus repair. Foot Ankle Int. 2016;37(1):85–89. doi:10.1177/1071100715600285
10. Loh B, Padki A, Thong S, et al. An evaluation of peripheral sciatic nerve block in patients undergoing Hallux valgus surgery. J Foot Ankle Surg. 2022;9(S–1):S157–S161.
11. Li Y, Zhang Q, Wang Y, et al. Ultrasound-guided single popliteal sciatic nerve block is an effective postoperative analgesia strategy for calcaneal fracture: a randomized clinical trial. BMC Musculoskelet Disord. 2021;22(1):735. doi:10.1186/s12891-021-04619-5
12. Opperer M, Gerner P, Memtsoudis SG. Additives to local anesthetics for peripheral nerve blocks or local anesthesia: a review of the literature. Pain Manag. 2015;5(2):117–128. doi:10.2217/pmt.15.2
13. Coviello A, Iacovazzo C, D’Abrunzo A, et al. Sufentanil vs. dexmedetomidine as neuraxial adjuvants in cesarean section: a mono-centric retrospective comparative study. J Clin Med. 2022;11(22):6868. doi:10.3390/jcm11226868
14. Zhao WL, Ou XF, Liu J, Zhang WS. Perineural versus intravenous dexamethasone as an adjuvant in regional anesthesia: a systematic review and meta-analysis. J Pain Res. 2017;10:1529–1553. doi:10.2147/JPR.S138212
15. Bilotta F, Pugliese F. The evolving clinical use of dexmedetomidine. Lancet. 2020;396(10245):145–147. doi:10.1016/S0140-6736(20)30902-8
16. Coviello A, Esposito D, Galletta R, Maresca A, Servillo G. Opioid-free anesthesia-dexmedetomidine as adjuvant in erector spinae plane block: a case series. J Med Case Rep. 2021;15(1):276. doi:10.1186/s13256-021-02868-5
17. Sedgwick P. Before and after study designs. BMJ. 2014;349:g5074. doi:10.1136/bmj.g5074
18. Simmons JW, Dobyns JB, Paiste J. Enhanced recovery after surgery: intraoperative fluid management strategies. Surg Clin North Am. 2018;98(6):1185–1200. doi:10.1016/j.suc.2018.07.006
19. Perlas A, Wong P, Abdallah F, Hazrati LN, Tse C, Chan V. Ultrasound-guided popliteal block through a common paraneural sheath versus conventional injection: a prospective, randomized, double-blind study. Reg Anesth Pain Med. 2013;38(3):218–225. doi:10.1097/AAP.0b013e31828db12f
20. Gan TJ, Belani KG, Bergese S, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131(2):411–448.
21. McCormack HM, de L. Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007. doi:10.1017/S0033291700009934
22. Lee R, Kim YM, Choi EM, Choi YR, Chung MH. Effect of warmed ropivacaine solution on onset and duration of axillary block. Korean J Anesthesiol. 2012;62(1):52–56. doi:10.4097/kjae.2012.62.1.52
23. Craig D, Carli F. Bromage motor blockade score – a score that has lasted more than a lifetime. Can J Anesth. 2018;65(7):837–838. doi:10.1007/s12630-018-1101-7
24. Gordon DB, Polomano RC, Pellino TA, et al. Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172–1186. doi:10.1016/j.jpain.2010.02.012
25. Delić N, Kljaković-Gašpić T, Fabris M, et al. ESRA19-0435 Patient satisfaction and pain scores after upper extremity fracture surgery: general vs. regional anaesthesia. Reg Anesth Pain Med. 2019;44:A122.
26. Chaw SH, Lo YL, Lee JY, et al. Evaluate construct validity of the Revised American Pain Society Patient Outcome Questionnaire in gynecological postoperative patients using confirmatory factor analysis. BMC Anesthesiol. 2021;21(1):20. doi:10.1186/s12871-020-01229-x
27. Coviello A, Iacovazzo C, Cirillo D, et al. Tetra-block: ultrasound femoral, lateral femoral-cutaneous, obturator, and sciatic nerve blocks in lower limb anesthesia: a case series. J Med Case Rep. 2023;17(1):270. doi:10.1186/s13256-023-04017-6
28. Kirksey MA, Haskins SC, Cheng J, Liu SS, Schwentner C. Local Anesthetic Peripheral Nerve Block Adjuvants for Prolongation of Analgesia: a Systematic Qualitative Review. PLoS One. 2015;10(9):e0137312. doi:10.1371/journal.pone.0137312
29. Ilfeld BM. Continuous peripheral nerve blocks: an update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg. 2017;124(1):308–335. doi:10.1213/ANE.0000000000001581
30. Bingham AE, Fu R, Horn JL, Abrahams MS. Continuous peripheral nerve block compared with single-injection peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Reg Anesth Pain Med. 2012;37(6):583–594. doi:10.1097/AAP.0b013e31826c351b
31. Vorobeichik L, Brull R, Bowry R, Laffey JG, Abdallah FW. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br J Anaesth. 2018;120(4):679–692. doi:10.1016/j.bja.2017.11.104
32. Malik T, Mass D, Cohn S. Postoperative analgesia in a prolonged continuous interscalene block versus single-shot block in outpatient arthroscopic rotator cuff repair: a prospective randomized study. Arthroscopy. 2016;32(8):1544–50.e1. doi:10.1016/j.arthro.2016.01.044
33. Coviello A, Spasari E, Ianniello M, et al. Intra-procedural catheter displacement for continuous adductor canal block: catheter-through-needle method vs catheter-through-split-cannula method. Perioperat Care Operat Room Manag. 2022;27:100255. doi:10.1016/j.pcorm.2022.100255
34. Coviello A, Spasari E, Ianniello M, et al. Corrigendum to Intra-procedural catheter displacement for continuous adductor canal block: catheter-through-needle method vs catheter-through-split-cannula method. Perioperat Care Operat Room Manag. 2022;28:100265. doi:10.1016/j.pcorm.2022.100265
35. Coviello A, Bernasconi A, Balato G, et al. Positioning the catheter tip anterior or posterior to the saphenous nerve in continuous adductor canal block: a mono-centric retrospective comparative study. Local Reg Anesth. 2022;15:97–105. doi:10.2147/LRA.S383601
36. Cummings KC 3rd, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. 2011;107(3):446–453. doi:10.1093/bja/aer159
37. Desmet M, Braems H, Reynvoet M, et al. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth. 2013;111(3):445–452. doi:10.1093/bja/aet109
38. Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109(3):502–511. doi:10.1097/ALN.0b013e318182c26b
39. Ammar AS, Mahmoud KM. Ultrasound-guided single injection infraclavicular brachial plexus block using bupivacaine alone or combined with dexmedetomidine for pain control in upper limb surgery: a prospective randomized controlled trial. Saudi J Anaesth. 2012;6(2):109–114. doi:10.4103/1658-354X.97021
40. Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118(2):167–181. doi:10.1093/bja/aew411
41. Iskandar H, Benard A, Ruel-Raymond J, Cochard G, Manaud B. The analgesic effect of interscalene block using clonidine as an analgesic for shoulder arthroscopy. Anesth Analg. 2003;96(1):260–2, table of contents. doi:10.1213/00000539-200301000-00052
42. Giampaolino P, Della Corte L, Mercorio A, et al. Laparoscopic gynecological surgery under minimally invasive anesthesia: a prospective cohort study. Updates Surg. 2022;74(5):1755–1762. PMID: 35759109; PMCID: PMC9244282. doi:10.1007/s13304-022-01310-9
43. Gaumann DM, Brunet PC, Jirounek P. Hyperpolarizing afterpotentials in C fibers and local anesthetic effects of clonidine and lidocaine. Pharmacology. 1994;48(1):21–29. doi:10.1159/000139158
44. Jeon HJ, Park YC, Lee JN, Bae JS. Popliteal sciatic nerve block versus spinal anesthesia in hallux valgus surgery. Korean J Anesthesiol. 2013;64(4):321–326. PMID: 23646241; PMCID: PMC3640164. doi:10.4097/kjae.2013.64.4.321
45. Mendicino RW, Statler TK, Catanzariti AR. Popliteal sciatic nerve blocks after foot and ankle surgery: an adjunct to postoperative analgesia. J Foot Ankle Surg. 2002;41(5):338–341. PMID: 12400720. doi:10.1016/s1067-2516(02)80055-2
46. Rosati Tarulli F, Izzo A, Coviello A, et al. Intramedullary nailing for calcaneal fractures: what are the available techniques? A review of the literature. Lo Scalpello J. 2022;36(3):159–164. doi:10.36149/0390-5276-244
47. Vermeylen K, De Puydt J, Engelen S, et al. A double-blind randomized controlled trial comparing dexamethasone and clonidine as adjuvants to a ropivacaine sciatic popliteal block for foot surgery. Local Reg Anesth. 2016;9:17–24. doi:10.2147/LRA.S96073
48. Desai N, Albrecht E, El-Boghdadly K. Perineural adjuncts for peripheral nerve block. BJA Educ. 2019;19(9):276–282. doi:10.1016/j.bjae.2019.05.001
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

