Back to Journals » Journal of Inflammation Research » Volume 17
Correlation Analysis of IL-17, IL-21, IL-23 with Non-Alcoholic Liver Fibrosis and Cirrhosis
Authors Yang X, Liao L, Liang Z, Yu S, Guo Z
Received 27 November 2023
Accepted for publication 26 March 2024
Published 17 April 2024 Volume 2024:17 Pages 2327—2335
DOI https://doi.org/10.2147/JIR.S452061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Xiaoyan Yang,1 Liyin Liao,1 Zizhen Liang,1 Shenglong Yu,2 Zhonghui Guo1
1Department of Laboratory Medicine, The Affiliated Panyu Central Hospital of Guangzhou Medical University, Guangzhou, 511400, People’s Republic of China; 2Institute of Cardiovascular Medicine, The Affiliated Panyu Central Hospital of Guangzhou Medical University, Guangzhou, 511400, People’s Republic of China
Correspondence: Zhonghui Guo, Department of Laboratory Medicine, The Affiliated Panyu Central Hospital of Guangzhou Medical University, No. 8, Fuyu East Road, Qiaonan Street, Panyu District, Guangzhou, 511400, People’s Republic of China, Tel +86 20 3485 8850, Email [email protected]
Objective: This research aimed to explore the involvement of interleukins (IL) - IL-6, IL-17, IL-21, and IL-23 - in the evolution and diagnosis of non-alcoholic liver fibrosis and cirrhosis.
Methods: The study subjects were selected from the patients who visited the Department of Hepatology of X Hospital in Y City from August 2021 to April 2023. Peripheral blood samples were collected. All participants were divided into liver fibrosis, cirrhosis, hepatitis, and healthy subjects four groups. IL-21, IL-17, IL23, IL-6 were detected by double antibody sandwich.
Results: The results showed that there was a significant difference in the levels of IL-17, IL-21, and IL-23 among the 4 groups (P< 0.0001). ROC curve analysis showed that the AUC values of IL-17, IL-21 and liver fiber 4 items were > 0.70, suggesting that the diagnostic efficacy of IL-17, IL-21 was similar to that of liver fiber 4 items. Spearman correlation analysis showed that IL-17 had a positive correlation with collagen type III N-peptide, type IV collagen, and Laminin (P < 0.05), and no correlation with Hyaluronic acid (P > 0.05).
Conclusion: IL-17, IL-21, and IL-23 play a pivotal role in the inflammatory pathways associated with liver injuries, establishing themselves as potent auxiliary diagnostic markers in identifying liver fibrosis and cirrhosis.
Keywords: non-alcoholic liver fibrosis, cirrhosis, diagnostic markers, cytokines
Introduction
Hepatic fibrosis is a reversible pathological process that occurs when liver tissue triggers an immune-inflammatory response. It follows persistent liver injury caused by viral, parasitic infections, pharmacological, alcohol, and other causes, resulting in overproduction of extracellular matrix, hepatocyte damage, and over repair.1 The study of the pathogenesis of liver fibrosis has evolved from the progressive study of pathological histology to the study of cytology, cytokines, and their molecular level.2 Activation of hepatic stellate cells (HSCs) is central to hepatic fibrosis, and various factors (cytokines, growth factors, enzymes, etc.) act on hepatic HSCs and fibroblasts in different ways and pathways. HSCs lead to changes in their biology and functionality, causing hepatic fibrosis, which can further develop into irreversible pathological changes to cirrhosis until it progresses to hepatocellular carcinoma.3
The gold standard for the diagnosis of liver fibrosis and cirrhosis is liver histopathological biopsy, but liver puncture is an invasive procedure with high risk and is not suitable for routine screening and monitoring of liver fibrosis and cirrhosis.4 Diagnostic imaging and assessment are now limited to the identification of fatty liver, cirrhosis, and its complications, focusing on small structural changes in the liver, with high diagnostic accuracy for cirrhosis and low diagnostic performance for liver fibrosis.5,6 Collagen type III N-peptide (PIIINP), type IV collagen (CIV), Laminin (LN), and Hyaluronic acid (HA) have been used as direct serum markers for the diagnosis of liver fibrosis and cirrhosis.7 Studies have analyzed the normal reference intervals of PIIIP N-P in Northern European children and adults.8
Helper T cell 17 (Th17) is a newly discovered subpopulation of T cells capable of secreting interleukin 17 (IL-17), secreting cytokines such as interleukin 17 (IL-17), interleukin 21 (IL-21), interleukin 22 (IL-22), and interleukin 23 (IL-23) and other cytokines. Interleukin family members are important in autoimmune diseases and the body’s defense response.9 A study based on experiments in the SSC mouse bleomycin model has shown that Th17 cells can be involved in the pathogenesis of skin and lung fibrosis by enhancing the proliferation of fibroblasts and cytokine production. The study also found that they were important for transforming growth factor β (TGF-β) and interleukin 6 (IL-6) (both cytokines present in large quantities in the damaged liver) preferentially differentiate, making it likely that Th17 cells contribute to the development of liver inflammation.10 From this, it is not difficult to speculate that Th17 cells may have an integral role in the progression of hepatic fibrosis in the clinic that their role in clinical liver fibrosis is worth understanding and discovering. They may represent a potential target for the diagnosis and treatment of hepatic fibrosis and cirrhosis.
In this study, we attempted to investigate the effects of changes in the levels of IL-6, IL-17, IL-21, and IL-23 in the peripheral blood of patients with liver fibrosis on the progression of liver fibrosis, and at the same time to observe their diagnostic efficacy and compare them with those of the commonly used diagnostic indexes for liver fibrosis, namely, PIIIPN-P, CIV, LN, and HA, to assess their diagnostic efficacy in the diagnosis of liver fibrosis, to provide better diagnostic and therapeutic methods for the clinic.
Materials and Methods
Study Subjects
The study subjects were selected from the patients who visited the Department of Hepatology of X Hospital in Y City from August 2021 to April 2023. The inclusion criteria were: (1) patients with moderate or severe liver fibrosis who met the diagnostic criteria for liver fibrosis according to the “Guidelines for the Integrated diagnosis and treatment of liver fibrosis by traditional Chinese and western medicine (2019 Edition)” including the evidence of image and serum liver fibrosis markers (such as hyaluronic acid HA, laminin LN, type III procollagen peptide, etc.) and related biochemical indicators (such as aspartate aminotransferase);11 (2) patients who were diagnosed as cirrhosis and untreated during the same period according to “Diagnostic criteria for liver cirrhosis and treatment protocols” including the clinical symptoms, biochemical markers and image.12 (3) patients with hepatitis untreated in the first diagnosis with the presence of inflammatory changes on imaging, but not to the extent of cirrhosis or fibrosis.; (4) healthy people who underwent physical examination in the same period. The exclusion criteria were: (1) normal liver function and biochemical tests, but long-term instability or abnormal elevation of serum liver fibrosis markers, etc., which may be suspected of liver fibrosis; (2) the combination of primary or metastatic hepatocellular carcinoma; (3) fatty liver induced by alcoholic and drug hepatitis; (4) chronic liver diseases caused by liver hemangiomas and other etiological factors; (5) patients with concomitant cardio-cerebral, pulmonary, and renal diseases or insufficiency; (6) patients with abnormal mental consciousness who are unable to cooperate with the study of various tests.
According to the above criteria, a total of 358 subjects were enrolled in this study, including 78 patients with moderate or severe liver fibrosis (group A), 80 patients with cirrhosis (group B), 100 patients with hepatitis (group C), and 100 healthy people (group D). The distribution of age, gender, and etiology of the subjects are shown in Table 1.
 |
Table 1 Comparison of Liver Function-Related Biochemical Indexes and Liver Fiber Detection Indexes Among Groups of Patients |
Instruments and Reagents
The biochemical indexes related to liver function included alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transferase (GGT), total bilirubin (TB), indirect bilirubin (DB), bile acid (TBA), alkaline phosphatase (ALP), was detected by Beckman 5821, USA.The determination of PIIINP, CIV, LN, HA adapted Chemiluminescence and was analyzed by Maglumi X8 automatic chemiluminescence detector. The instrument and reagents are produced by Snibe Diagnostic, China. 3mL of venous blood was drawn from the subjects, left for 20 minutes, then centrifuged at centrifugal force 2359g/min for 5 minutes, and the serum was separated for testing, and the test method was strictly based on the instructions of the ELISA kit.
The enzyme labelling instrument was Biotek EXL800 from Biotek, USA. The cytokine IL-21, IL-17, IL-23, IL-6 ELISA double antibody sandwich kit was produced by Elabscience.
Statistical Analysis
The sample size calculation was based on the formula n = Zα2σ2/δ2, where n is the sample size, Zα is the standard normal deviate corresponding to a significance level of α = 0.05 (Zα = 1.96), σ is the standard deviation of the population estimated, and δ is the margin of error set at 10%. According to this formula, the minimum sample size required for each group was 61. Considering a possible dropout rate of 20%, we increased the sample size to 78 for group A and group B, and 100 for group C and group D. The results were statistically analyzed using SPSS 26.0 statistical software. Gender was expressed as the number of (male/female) cases, 4 inter group comparisons were made using the chi-square test, normally distributed measurements were expressed as the mean ± standard deviation(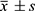 ), multiple comparisons were made using the ANOVA test, and two-group comparisons were made using the t-test; non-normally distributed measurements were expressed as the interrogative spacing M (P25, P75), inter group comparisons were made using the rank-sum test, and multi-group comparisons were made using the Kruskal–Wallis (KW) test for multiple comparisons. Correlations were analyzed using Spearman correlation analysis, and P < 0.05 was considered a statistically significant difference. The results showed that all the variables met the assumptions of normality and homogeneity of variance, except for HA, which was log-transformed to meet the normality assumption. The GraphPad prism 9.5 software was used to calculate subjects’ work characteristic curves (ROC), and the area under the curve (AUC) was calculated by comparing the potential efficacy of the index tests; the joint diagnosis was analyzed by binary logistic regression analysis using SPSS statistical software.
), multiple comparisons were made using the ANOVA test, and two-group comparisons were made using the t-test; non-normally distributed measurements were expressed as the interrogative spacing M (P25, P75), inter group comparisons were made using the rank-sum test, and multi-group comparisons were made using the Kruskal–Wallis (KW) test for multiple comparisons. Correlations were analyzed using Spearman correlation analysis, and P < 0.05 was considered a statistically significant difference. The results showed that all the variables met the assumptions of normality and homogeneity of variance, except for HA, which was log-transformed to meet the normality assumption. The GraphPad prism 9.5 software was used to calculate subjects’ work characteristic curves (ROC), and the area under the curve (AUC) was calculated by comparing the potential efficacy of the index tests; the joint diagnosis was analyzed by binary logistic regression analysis using SPSS statistical software.
Results
Comparison of the General Data of the Subjects in the 4 Groups
Calculated by Kolmogorov–Smirnov test, except for the age of the patients in each group, which was normally distributed, all other data indexes were non-normally distributed. The difference in age of patients in each group was statistically significant (P<0.0001, χ2=8.386), but the difference in gender ratio was not statistically significant (P=0.902). The biochemical indexes related to liver function and liver fibrosis indexes of liver fiber 4 items (PIIINP, CIV, LN, HA) were significantly different in each group (P<0.0001). In this study, the liver function-related indicators between groups A, B and C were significantly different (P<0.0001), except for ALT, which was not significantly different (P=0.123), and other related biochemical indicators were significantly different (P<0.0001) when compared among groups A, B and C. Comparison of the 4 indicators of hepatic fibrosis among groups A and B showed that there were significant differences in the liver fiber 4 items between the groups (P<0.0001) (Table 1).
Comparison of Cytokine Levels of IL-6, IL-17, IL-21 and IL-23 in Peripheral Blood of 4 Groups of Subjects
The cytokine levels in the peripheral blood of the 4 groups of subjects were normally distributed by the Kolmogorov–Smirnov test. There was no significant difference in the levels of IL-6 among the 4 groups (P=0.755), and there was a significant difference in the levels of IL-17, IL-21, and IL-23 among the 4 groups (P<0.0001, Table 2). With the aggravation of liver lesions IL-17 and IL-23 levels gradually increased significantly; while IL-21 levels increased significantly in group A and decreased significantly in group B compared with group D. The differences in IL-6 levels were not statistically significant between groups A and B (P=0.3620, t=0.914), IL-17 levels were significantly different (P=0.0003, t=3.692), significant difference in IL-21 content (P<0.0001, t=10.67), and significant difference in IL-23 content (P<0.0001, t=6.683).
 |
Table 2 Comparison of Cytokine Content Between the 4 Groups |
Analysis of the efficacy of each index of IL-17, IL-21, IL-23, and liver fiber alone and in combination in diagnosing liver fibrosis and cirrhosis. In this study, the results of each index were analyzed by ROC curve analysis, the diagnostic value of each index for diagnosing liver fibrosis cirrhosis was distributed and evaluated. The constant terms and coefficients were obtained by binary logistic regression analysis, and the coefficients were used to derive the joint predictors (Figure 1, Tables 3 and 4), the predicted probability values were used for the ROC curve analysis to evaluate the diagnostic performance of the combined tests. The optimal diagnostic threshold for each item was taken as the calculated or predicted probability value corresponding to the maximum of the Youden index (Y1=sensitivity+specificity-1) when the diagnostic efficacy of the experimental indexes also reached the maximum.
 |
Table 3 Comparison of the Diagnostic Efficacy of Cytokines and the 4 Liver Fiber Diagnostics for the Diagnosis of Liver Fibrosis |
 |
Table 4 Comparison of Diagnostic Efficacy of Cytokines and 4 Liver Fiber Diagnostics for Liver Cirrhosis |
Table 3 shows the comparison of the diagnostic efficacy of cytokines and liver fiber 4 in diagnosing liver fibrosis. The results showed that the AUC values of IL-17, IL-21 and liver fiber 4 items were >0.70, suggesting that the diagnostic efficacy of IL-17, IL-21 was similar to that of liver fiber 4 items. Among the cytokines, IL-21 had the highest AUC value and the best diagnostic efficacy, while PIIINP had the highest AUC value among the 4 liver fiber items, and the AUC value of the 3 cytokines combined was greater than that of the cytokines individually, the sensitivity and specificity of their detection were both greater than 80%. The combined diagnostic efficacy of the 4 liver fiber items was optimal.
Table 4 shows the comparison of the diagnostic efficacy of cytokines and liver fiber 4 items for the diagnosis of cirrhosis. The results showed that the AUC values of IL-17, IL-23, and the 4 liver fiber items were >0.70. The AUC value of IL-17 was the highest in the cytokines when detected alone, while the AUC value of LN was the highest in the 4 liver fiber items when detected alone. The AUC values of the three cytokines combined were greater than the AUC values of each cytokine alone, and the sensitivity and specificity of the test was greater than 80%. The combined diagnostic efficacy of the 4 liver fiber tests was optimal, with sensitivity and specificity greater than 80%.
Correlation Analysis of IL-17, IL-21, IL-23 and 4 Items of Liver Fiber
The results of Spearman correlation analysis showed that IL-17 had a positive correlation with PIIINP, CIV, and LN(P < 0.05), and no correlation with HA (P > 0.05); IL-21 had no correlation with PIIINP, CIV, LN and HA (P > 0.05); IL-23 had a negative correlation with PIIINP (P < 0.05), and no correlation with CIV, LN, and HA (P>0.05), the results are detailed in Table 5.
 |
Table 5 Correlation Analysis of IL-17, IL-21, IL-23 and 4 Items of Liver Fiber |
Discussion
Hepatic fibrosis is caused by repeated inflammation, anti-inflammatory response, and restorative immune response alternately, and usually occurs without obvious symptoms. Only when substantial liver damage occurs, there are obvious clinical manifestations. Due to the limited reversibility of hepatocellular carcinoma, early detection and intervention are necessary. Non-invasive laboratory tests for the diagnosis of liver fibrosis reduce the risk of invasive tests and are readily accepted by patients, but diagnostic assessment tools for the diagnosis of liver fibrosis are complex and cumbersome and have been a medical challenge.
Liver fibrosis occurs when the balance of deposition and removal within the extracellular matrix (ECM) is disrupted by liver injury and inflammation.13 The direct biomarker that can usually be used to diagnose hepatic fibrosis is the ECM secretory protein, the level of which can vary with the degree of hepatic fibrosis.7 In this study, the serum concentrations of PIIINP, CIV, LN, and HA were significantly higher in the cirrhotic group compared to the hepatic fibrosis group, and the change in concentration can effectively distinguish between hepatic fibrosis and cirrhosis. These four indicators are used as direct serum markers for the assessment and diagnosis of liver fibrosis, and they respond to the dynamic onset and development of liver fibrosis, suggesting damage to the hepatocyte matrix for degradation and clearance.14
Given the existence of some clinical applications of PIIINP, CIV, LN, and HA, we analyzed the diagnostic efficacy of these 4 markers for liver fibrosis and cirrhosis. Fewer studies have examined the efficacy of these 4 markers in diagnosing liver fibrosis, and some studies have shown that the specificity and sensitivity of PIIINP, LN, and HA in diagnosing cirrhosis can reach more than 90%.15 CIV reflects the regeneration of hepatocytes, and it can be used to differentiate between the screening of liver fibrosis and the assessment of the degree of liver fibrosis by other indicators.16 In this study, CIV had the highest diagnostic specificity for liver fibrosis and cirrhosis but the lowest diagnostic sensitivity for liver fibrosis, which is consistent with previous studies; PIIINP had superior diagnostic efficacy and similar efficacy for diagnosing liver fibrosis and cirrhosis, and HA had the lowest diagnostic specificity for liver fibrosis. It is possible that PIIINP and HA are non-specific markers for the liver17,18, and can be secreted by other tissues and fibers, but PIIINP is one of the most important components of hepatic ECM, and the blood levels of PIIINP, LN, and HA can be used for the assessment of the activity of hepatitis and the grading and staging of the degree of liver fibrosis.19 The specificity, sensitivity, and AUC values of the four indices for the diagnosis of cirrhosis in this study were similar to those of liver fibrosis, which also suggests that PIIINP, CIV, LN, and HA are all of the clinical values for the diagnosis of liver fibrosis and cirrhosis, but their diagnostic thresholds are similar so that they cannot strictly differentiate between liver fibrosis and cirrhosis. These four indicators reflect different aspects of liver injury development, and the combined test improves the specificity and sensitivity of a single diagnosis, which can be used to comprehensively analyze the characteristics of liver injury and assess the degree and stage of liver injury, and the diagnostic efficacy of the combined test of PIIINP, CIV, LN, and HA is better than that of individual tests.
Elevated serum levels of IL-6 in non-alcoholic fatty liver disease (NASH) patients have been associated with insulin resistance, steatosis, and liver injury,20,21 which activates HSC proliferation and activation, resulting in hepatic collagen deposition and promoting hepatic fibrosis formation. Serum levels of IL-6 and IL-6 receptors are elevated in patients with NASH, and IL-6 levels correlate positively with the number of circulating leukocytes and monocytes;22 IL-6 production is a mechanism macrophage differentiation by hepatic stomatal cells limiting CD9+ and local IL-6 levels were found to be reduced in early human liver disease compared to normal liver tissue, suggesting a protective role of local IL-6 in healthy liver.23 However, there was no significant variability in IL-6 levels in the four groups of patients in this study, and the analysis may be due to the local role of IL-6 in liver tissues, with no change in peripheral serum levels, or differences in the sensitivity of the assay, and the selection of the number of sample cases with the volume change, and therefore the next diagnostic efficacy analyses were not carried out.
IL-17 activates the STAT3 signaling pathway in HSC, producing type 1 collagen and promoting the development of liver fibrosis; the absence of IL-23 or the use of IL-17E (also known as IL-25) attenuates hepatic fibrosis;24 and the level of IL-17 is positively correlated with the degree of liver damage in patients with hepatitis A. In hepatitis B (HBV) and hepatitis C virus (HCV) infections, IL-23 promoted chronic inflammation, IL-23 may enhance HCV infection mediated by dendritic cells and macrophages,25 and IL-23 concentrations were significantly elevated in patients with cirrhosis caused by HBV and HCV infections,26 there is a close correlation between IL-17 and IL-23 expression levels, and IL-23 may play a role in the pathogenesis of hepatitis B cirrhosis by inducing the production of IL-17 and the latter. In the present study, IL-17 and IL-23 concentrations increased significantly with increasing hepatic inflammation in patients and reached the highest concentrations in cirrhotic patients, similar to previous studies. We attempted to calculate the diagnostic efficacy of IL-17 and IL-23 for the diagnosis of liver fibrosis and cirrhosis and found that the diagnostic efficacy of IL-17 was similar to the diagnostic efficacy of the 4 liver fiber items in the diagnosis of liver fibrosis, while the diagnostic efficacy of IL-23 was slightly worse, but IL-23 had the highest specificity, and the diagnostic efficacy of both IL-17 and IL-23 was better in the diagnosis of cirrhosis than that of liver fibrosis.
IL-21 is an effector of Th17 cells in fibrosis, and in cases of systemic scleroderma (SSc), IL-21 promotes the proliferation of fibroblasts and enhances the ability of collagen secretion, which promotes the development of fibrosis in SSc patients.27 IL-21 levels in peripheral blood IL-21+CD4+ T lymphocytes and plasma have been studied in patients with viral infection-related cirrhosis with different degrees of liver fibrosis, and it was found that the more severe the degree of hepatic fibrosis, the more IL-21+CD4+ T lymphocytes were significantly increased, and in vitro experiments have also demonstrated that IL-2I promotes activation of hepatic stellate cells, increases the expression of α-smooth muscle actin(α-SMA), and inhibits hepatic stellate cell LX-2 apoptosis and unregulated collagen production.28 In our study, IL-21 was significantly increased in patients in the liver fibrosis group but decreased in the cirrhosis group. It has been shown that in cirrhotic patient’s follicular helper T cells are enriched in the spleen, which secrete IL-21 and increase the concentration of IL-21 in the spleen of patients.29 Therefore, IL-21 secretion may be increased in the spleen in cirrhotic patients in the present study, whereas IL-21 levels in the peripheral blood did not change significantly or may even decrease due to enrichment in the spleen. In this study, unlike IL-17 and IL-23, the efficacy of IL-21 for the diagnosis of liver fibrosis was superior to the efficacy for the diagnosis of cirrhosis.
This study also had some limitations. First, we did not classify the degree of disease in patients with liver fibrosis and cirrhosis and we did not limit the etiology of these two groups of patients. Considering that our patients mainly had liver fibrosis and cirrhosis caused by parasites and HBV, HCV, and a small amount of fatty liver, it is suggested that Th17 cells may be involved in the development of chronic hepatitis caused by viral hepatitis and parasites. Moreover, the relatively small sample size might cause statistical bias.
Conclusion
Our study found that IL-17, IL-21, and IL-23 play a pivotal role in the inflammatory pathways associated with liver injuries, establishing themselves as potent auxiliary diagnostic markers in identifying liver fibrosis and cirrhosis. The advantages of serum markers for the diagnosis of hepatic fibrosis lie in the ease of sampling, better diagnostic efficacy, and good operability.
Data Sharing Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of The Affiliated Panyu Central Hospital of Guangzhou Medical University. Written informed consent was obtained from all participants.
Funding
This work was supported by Science and Technology Project of Panyu, Guangzhou (2020-Z04-043), Scientific Research project of The Affiliated Panyu Central Hospital of Guangzhou Medical University (No. 2022Y002).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. doi:10.1016/j.mam.2018.09.002
2. Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–166. doi:10.1038/s41575-020-00372-7
3. Yang F, Li H, Li Y, et al. Crosstalk between hepatic stellate cells and surrounding cells in hepatic fibrosis. Int Immunopharmacol. 2021;99:108051. doi:10.1016/j.intimp.2021.108051
4. Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449–1457. doi:10.1016/j.hep.2003.09.022
5. Maybury CM, Samarasekera E, Douiri A, et al. Diagnostic accuracy of noninvasive markers of liver fibrosis in patients with psoriasis taking methotrexate: a systematic review and meta-analysis. Br J Dermatol. 2014;170(6):1237–1247. doi:10.1111/bjd.12905
6. Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41(12):1271–1280. doi:10.1111/apt.13196
7. Aleknaviciute-Valiene G, Banys V. Clinical importance of laboratory biomarkers in liver fibrosis. Biochem Med (Zagreb). 2022;32(3):30501.
8. Larsen JB, Knudsen CS, Parkner T. Procollagen III, N-terminal propeptide (PIIINP): establishment of reference intervals in Northern European adults and children using the MAGLUMI 800 chemiluminescence immunoassay. Scand J Clin Lab Invest. 2021;81(5):389–393. doi:10.1080/00365513.2021.1929444
9. Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–297. doi:10.1007/s00281-019-00733-8
10. Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. doi:10.1155/2011/345803
11. Ping Zhang X, Fei Shang X, Xiu Hui L. Progress in the diagnosis and treatment of hepatic fibrosis by combining traditional Chinese and western medicine. J Clin Hepatobil Dis. 2023;39(02):284–289.
12. Wang SX. Diagnostic criteria and therapeutic options for liver cirrhosis. Chin Clin. 2000;2000(10):25–26.
13. Nallagangula KS, Nagaraj SK, Venkataswamy L, et al. Liver fibrosis: a compilation on the biomarkers status and their significance during disease progression. Future Sci OA. 2018;4(1):FSO250. doi:10.4155/fsoa-2017-0083
14. Qi Di Z, Lun Geng L. Diagnostic value of serum markers for liver fibrosis. Dr Liver. 2022;2022:(02):28–29.
15. Jothimani DKE. Performance of non-invasive markers of liver fibrosis. JCEH. 2018;6(413):s77.
16. El-Mezayen HA, Habib S, Marzok HF, et al. Diagnostic performance of collagen IV and laminin for the prediction of fibrosis and cirrhosis in chronic hepatitis C patients: a multicenter study. Eur J Gastroenterol Hepatol. 2015;27(4):378–385. doi:10.1097/MEG.0000000000000298
17. Lynch M, Higgins E, McCormick PA, et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 2014;150(8):856–862. doi:10.1001/jamadermatol.2013.9336
18. Rostami S, Parsian H. Hyaluronic Acid: from biochemical characteristics to its clinical translation in assessment of liver fibrosis. Hepat Mon. 2013;13(12):e13787. doi:10.5812/hepatmon.13787
19. Gawrieh S, Wilson LA, Yates KP, et al. Relationship of ELF and PIIINP with liver histology and response to vitamin E or pioglitazone in the PIVENS trial. Hepatol Commun. 2021;5(5):786–797. doi:10.1002/hep4.1680
20. Jorge A, Andrade J, Paraiso AF, et al. Body mass index and the visceral adipose tissue expression of IL-6 and TNF-alpha are associated with the morphological severity of non-alcoholic fatty liver disease in individuals with class III obesity. Obes Res Clin Pract. 2018;12(Suppl 2):1–8. doi:10.1016/j.orcp.2016.03.009
21. Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 2016;64(6):1403–1415. doi:10.1016/j.jhep.2016.02.004
22. Buonomo EL, Mei S, Guinn SR, et al. Liver stromal cells restrict macrophage maturation and stromal IL-6 limits the differentiation of cirrhosis-linked macrophages. J Hepatol. 2022;76(5):1127–1137. doi:10.1016/j.jhep.2021.12.036
23. Hou X, Yin S, Ren R, et al. Myeloid-cell-specific IL-6 signaling promotes MicroRNA-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology. 2021;74(1):116–132. doi:10.1002/hep.31658
24. Meng F, Wang K, Aoyama T, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143(3):765–776. doi:10.1053/j.gastro.2012.05.049
25. Schurich A, Pallett LJ, Lubowiecki M, et al. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9(3):e1003208. doi:10.1371/journal.ppat.1003208
26. Rey I, Effendi-Ys R. Association between serum IL-6, IL-10, IL-12, and IL-23 levels and severity of liver cirrhosis. Med Arch. 2021;75(3):199–203. doi:10.5455/medarh.2021.75.199-203
27. Xing X, Li A, Tan H, et al. IFN-γ + IL-17 + Th17 cells regulate fibrosis through secreting IL-21 in systemic scleroderma. J Cell Mol Med. 2020;24(23):13600–13608. doi:10.1111/jcmm.15266
28. Feng G, Zhang JY, Zeng QL, et al. Interleukin-21 mediates hepatitis B virus-associated liver cirrhosis by activating hepatic stellate cells. Hepatol Res. 2014;44(10):E198–E205. doi:10.1111/hepr.12215
29. Zhao J, Shi J, Qu M, et al. Hyperactive follicular helper T cells contribute to dysregulated humoral immunity in patients with liver cirrhosis. Front Immunol. 2019;10:1915. doi:10.3389/fimmu.2019.01915
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

