Back to Journals » Patient Related Outcome Measures » Volume 14
Content Validation of Patient-Reported Sleep Measures and Development of a Conceptual Model of Sleep Disturbance in Patients with Moderate-to-Severe, Uncontrolled Asthma
Authors Khan AH , Kosa K, De Prado Gomez L , Whalley D, Kamat S , Clark M
Received 8 October 2022
Accepted for publication 18 February 2023
Published 23 March 2023 Volume 2023:14 Pages 57—71
DOI https://doi.org/10.2147/PROM.S392666
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lynne Nemeth
Asif H Khan,1 Katherine Kosa,2 Lucia De Prado Gomez,3 Diane Whalley,4 Siddhesh Kamat,5 Marci Clark6
1Sanofi, Chilly-Mazarin, France; 2Patient-Centered Outcomes Assessment, RTI Health Solutions, Research Triangle Park, NC, USA; 3Sanofi, Reading, UK; 4Patient-Centered Outcomes Assessment, RTI Health Solutions, Manchester, UK; 5Regeneron Pharmaceuticals, Inc, Tarrytown, NY, USA; 6Patient-Centered Outcomes Assessment, RTI Health Solutions, Ann Arbor, MI, USA
Correspondence: Asif H Khan, Sanofi, Chilly-Mazarin, France, Tel +33 1 60 49 50 76, Email [email protected]
Purpose: Sleep disturbance is common in patients with asthma and can lead to subsequent impacts on health-related quality of life (HRQOL). Fit-for-purpose patient-reported outcome measures (PROMs) assessing asthma-related sleep disturbance and next-day HRQOL impact (next-day impact) are needed to evaluate disease burden and treatment effects.
Patients and Methods: Adults (18– 65 years) from three US clinics were recruited for semistructured interviews. Concept elicitation (CE) identified how asthma affects participants’ sleep and how asthma-related sleep disturbances impact their daily lives, which informed conceptual model development. Cognitive debriefing (CD) of the Asthma Sleep Disturbance Questionnaire (ASDQ), Sleep Diary, and Patient-Reported Outcomes Measurement Information System Sleep-Related Impairment Short Form 8a (PROMIS SRI SF8a) was completed to assess each measure’s content validity.
Results: Twelve individuals participated in two interview rounds (6 individuals per round). Participants most frequently reported asthma-related nighttime awakening and decreased sleep quality and duration. Negative impacts of a poor night’s sleep due to asthma symptoms included feeling tired/fatigue/lack of energy and subsequent negative impacts on physical functioning, emotions and mood, mental functioning, work or volunteerism, and social functioning. Across both rounds of CD interviews, participants generally found the Sleep Diary and PROMIS SRI SF8a items relevant and easy to complete with no modifications. The ASDQ was modified for clarity and consistency.
Conclusion: As described in the conceptual model, asthma affects multiple aspects of sleep that can cause next-day fatigue and other subsequent negative HRQOL impacts. This study demonstrates that the ASDQ, Sleep Diary, and PROMIS SRI SF8a items are comprehensive, relevant, and appropriate for patients with moderate-to-severe, uncontrolled asthma. Evaluation of psychometric properties for the ASDQ, Sleep Diary, and PROMIS SRI SF8a based on clinical trial data in patients with moderate-to-severe, uncontrolled asthma will further support their use.
Keywords: asthma, sleep disturbance, patient-reported sleep measures
Introduction
Asthma affected approximately 260 million individuals and caused approximately 460,000 deaths worldwide in 2019.1 Moderate-to-severe asthma that remains uncontrolled despite individuals receiving standard of care presents an unmet medical need. Uncontrolled asthma is predominately a chronic type 2 inflammatory disease characterized by impaired lung function, severe exacerbations, recurrent symptoms, sleep disruption, and poor health-related quality of life (HRQOL).2,3 Asthma symptoms—including wheeze, difficulty breathing, and chest tightness—can appear suddenly as potentially dangerous exacerbations.2 Exacerbations also reduce sleep quality and can impair quality of life by increasing distress, discomfort, and functional limitations.4 Achieving symptom control and minimizing future risk of exacerbations is critical to the treatment of asthma.2
Sleep impairments commonly occur in individuals with asthma and are associated with poorly controlled asthma and subsequent impacts to HRQOL.5 Individuals who have poorly controlled or uncontrolled asthma are especially prone to sleep disturbance, with a reported prevalence rate as high as 82%.3 Adults with severe asthma may experience clinically significant insomnia at a rate approximately three times the general population, with resulting interference in daily functioning.6 Among individuals with moderate-to-severe asthma, reduced control of symptoms has been associated with greater burden of sleep disturbance. Long-term disruption of sleeping patterns may affect physical functioning, emotional functioning, and quality of life,7,10 while increasing the risk of adverse health outcomes and psychological distress.11,12 Accordingly, consideration of nighttime symptoms is important in the evaluation of control of asthma.2
Patient-reported outcome measures (PROMs) can be used to assess the impact of general or disease-specific symptoms on patients’ HRQOL, disease burdens, and mental states.13 Some existing asthma-specific PROMs (eg, the Asthma Quality of Life Questionnaire and Asthma Control Questionnaire) collect very limited data about asthma-related sleep disturbance.14,15 Other PROMs, including the Jenkins Sleep Questionnaire,16 Pittsburgh Sleep Quality Index (PSQI),17 and Epworth Sleepiness Scale (ESS),18 have been used in respiratory conditions but were not developed or validated for asthma patients. Fit-for-purpose PROMs that meet current standards for reliability, validity, interpretability, and ability to detect change are needed to assess asthma-related sleep disturbance and resulting impacts on HRQOL.19,21 Such PROMs are desired for evaluating disease burden and for evaluating the effects of treatment on patients with moderate-to-severe, uncontrolled asthma.
The objectives of this study were to characterize the sleep experience among adults with moderate-to-severe, uncontrolled asthma; determine how this experience impacts their lives; develop a conceptual model of the impact of asthma on sleep based on patient-experience data; and evaluate the content validity of the Asthma Sleep Disturbance Questionnaire (ASDQ), Sleep Diary, and Patient-Reported Outcomes Measurement Information System Sleep-Related Impairment Short Form 8a (PROMIS SRI SF8a) in adults with moderate-to-severe, uncontrolled asthma.
Materials and Methods
Study Design
A qualitative, observational study was conducted using two iterative rounds of semistructured interviews. The RTI Institutional Review Board (Federal-Wide Assurance #3331) determined that the study was exempt from review because participation posed little to no risk to individuals. Participants were recruited from three asthma, allergy, and pulmonology specialty clinics in the US. Clinic staff recruited and screened adult patients (18–65 years old) with moderate-to-severe, uncontrolled asthma. Eligibility criteria included having moderate-to-severe asthma, having sleep issues due to asthma during the week before screening, being treated with medium- to high-dose inhaled corticosteroids plus a second controller, having a history of ≥1 asthma exacerbation(s) during the year prior to screening, and having uncontrolled asthma, as determined by a score ≥2.5 on the Asthma Control Questionnaire,22 5-item version (ACQ-5) (Table 1).
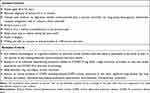 |
Table 1 Inclusion/Exclusion Criteria |
A qualitative research facility scheduled telephone and web-based interviews with eligible patients and two interviewers experienced in qualitative research. Verbal informed consent was obtained from all participants and documented by the researchers before beginning each interview. In accordance with Health and Human Services (HHS) regulation 45 CFR 46.117,23 consent was obtained verbally from participants and not written since “the only record linking the subject and the research would be the informed consent form and the principal risk would be potential harm resulting from a breach of confidentiality” [section (c)(1)(i)] and “the research presents no more than minimal risk of harm to subjects and involves no procedures for which written consent is normally required outside of the research context” [section (c) (1) (ii)].
Semistructured Interviews
All interviews were approximately 90 minutes and followed a semistructured interview guide to ensure that data were collected in a systematic and consistent way and that interview objectives were met while also encouraging spontaneity of responses and a conversational tone. For each interview, the study team followed best practices as described in US Food and Drug Administration (FDA) guidance.24,25 All interviews began with concept elicitation (CE) followed by cognitive debriefing (CD). The CE process is used to explore the most relevant and important concepts from the patient’s perspective and provide the primary evidence for a measure’s content validity, while the CD process is used to evaluate the patient’s understanding of a measure and provide information about its comprehensiveness.19,26,27
At the beginning of each interview, researchers asked participants to start with a brief discussion of the effects the coronavirus disease 2019 (COVID-19) pandemic has had on their daily life and their experience with asthma. Interviewers then commenced with CE to identify how asthma affects participants’ sleep and how their asthma-related sleep disturbances impact their daily lives. The CE component of the interview involved asking a series of open-ended questions designed to obtain spontaneous reports of asthma symptoms experienced at night and how these symptoms impact different aspects of sleep (eg, reduced sleep quality, difficulty falling asleep, nighttime awakenings), as well as specific next-day impacts (eg, daytime tiredness, difficulty concentrating). Participants were asked more targeted questions if certain aspects of sleep were not raised spontaneously.
The CD portion began immediately following CE in both interview rounds. In the first round of interviews, participants were asked to review and provide feedback on the ASDQ, Sleep Diary, and PROMIS SRI SF8a items using a “think-aloud” process. Feedback obtained from participants included overall impressions of the PROMs; relevance of the concepts to participants; clarity of the questions and response scales; comprehensiveness of the measure; relative importance of the concepts captured by these PROMs; and whether there were any missing, asthma-related sleep concepts of importance not captured by these questionnaires. Modifications to items were made based on feedback from the first round. The second round of CD interviews explored the importance and relevance of the concepts captured in the PROMs to participants, tested the adequacy of any item modifications made based on the first interview set, and gathered additional information on the understandability, relevance, and comprehensiveness of the PROMs. Interviews were audio recorded, and deidentified transcripts were produced from the recordings. Excel-based field notes were also captured during each interview.
Measures
The ASDQ, Sleep Diary, and PROMIS SRI SF8a are PROMs that evaluate improvement in sleep from the patient’s perspective (Table 2). The ASDQ (also referred to as the AM symptom score)28,29 is a single-item measure to assess the degree of sleep disturbance caused by nighttime asthma symptoms, with a recall period of “last night”, and is scored using a 5-level verbal rating scale (VRS). The ASDQ response options range from 1 (Slept through the night, no asthma symptoms) to 5 (Bad night, awake most of the night because of asthma). The Sleep Diary is a daily diary that includes a total of seven items that, either alone or in combination with additional items, allow for the assessment of sleep quality, nighttime awakenings, difficulty falling asleep, sleep duration, and feeling rested upon awakening (restorative sleep). A recall period of “last night” is included for five items and “today” for two items.30 Two items are scored using a 0–10 numeric rating scale. Participants enter the time, number of times, or total amount of time awake for the remaining items. A key difference between the ASDQ and the Sleep Diary is that the ASDQ evaluates overall sleep disturbance using a single item, whereas the Sleep Diary assesses individual aspects of sleep disturbance using 7 items. Both of these PROMs were developed internally by sponsors of this study and not previously tested with patients with moderate-to-severe, uncontrolled asthma during qualitative interviews. The PROMIS SRI SF8a is an 8-item, general, self-reported measure that assesses alertness, sleepiness, tiredness, and functional impairments connected to sleep impairment using a recall period of the “past 7 days” and a 5-category VRS.31 The PROMIS SRI SF8a was selected for content validity evaluation in patients with asthma because of its focus on sleep-related impairment and its having greater measurement precision than the PSQI and ESS.32
 |
Table 2 Overview of Patient-Reported Outcome Measures Evauated |
Analysis
A thematic analysis approach was used to analyze the results of the interviews, aided by field notes and interview transcripts.33 Results of the first round of interviews were compared with those from subsequent interviews to identify themes or patterns in the data collected during the CE portion of the interview. The occurrence and frequency of participant-reported nocturnal asthma symptoms and the key areas of asthma-related impacts on sleep were summarized in a tabular format. Additionally, concept saturation (ie, the point at which no new aspects of sleep affected by asthma were reported during the interviews) was documented using a saturation grid.19 A conceptual model of the hypothesized impact of asthma on sleep was developed based on the CE data. Conceptual models provide a method for visualizing the patient population and potential treatment effects based on known or hypothesized relationships.19
Feedback collected during the CD portion of the interview pertaining to each of the PROMs tested (including the instructions, item stem, response options, and recall period, as well as concept relevance and any reports of important missing asthma sleep-related concepts) was collated and summarized after each round of interviews. An item-tracking matrix was created to document revisions made to any PROMs following each round of interviews, including the rationale for item modifications. Participant quotes with the interview round number (RD #) and in-depth interview participant number (IDI #) are presented in italicized text to support study results.
Results
Participants
The study sample included a total of 12 participants, with 6 individuals participating in each of the two interview rounds. Characteristics of the 12 participants are presented in Table 3. Most participants were female (66.7%) and White (75.0%), with a mean age of 46 years (range, 26–65). As required in the study inclusion criteria, all participants’ asthma was uncontrolled at the time of screening, with a mean ACQ-5 score of 3.4 (range, 2.6–4.6).
 |
Table 3 Characteristics of Participants Reported at Screening |
Concept Elicitation
Participant feedback from both interview rounds was consistent across all 12 participants and, as such, results from both rounds of interviews are presented together.
Coronavirus Disease, 2019 Pandemic Experience
Most participants (n=10, 83.3%) reported that the COVID-19 outbreak impacted their experiences with asthma. Because of their asthma, 7 participants mentioned they generally stayed at home, and if they do go out in public, they reported being extra cautious to limit exposure to others. Four of the 10 participants also mentioned that it is hard for them to breathe when wearing a face mask.
Nocturnal Asthma Symptoms
Asthma symptoms typically experienced at night by participants included shortness of breath (often described as difficulty breathing) (n=11; 91.7%), chest tightness (n=8; 66.7%), wheezing (n=5; 41.7%), coughing (n=4; 33.3%), nasal congestion (n=2; 16.7%), and headache (n=1; 8.3%). All participants reported that their asthma symptoms impact their sleep. Participants were able to clearly distinguish that their sleep disturbance was due to asthma symptoms and not other factors.
Aspects of Sleep Impacted by Asthma
Key aspects of sleep impacted by asthma that emerged from the participants included nighttime awakening, sleep quality, sleep duration, early awakening, and difficulty falling asleep (Table 4). No new aspects of sleep were reported by participants as being affected by their asthma after the first round of interviews; thus, saturation was achieved. Nighttime awakening, reported by all participants, was the most prominent aspect of sleep impacted by asthma symptoms, particularly due to shortness of breath and/or chest tightness. Most participants (n=9; 81.8%) reported that their asthma symptoms impacted their ability to stay asleep regardless of whether they are experiencing an asthma exacerbation (referred to by participants as an asthma “flare” or “attack”). On average, participants reported awakening two times per night (range, 1–5 times per night), three nights per week (range, 1–5 nights per week); participants were typically awake anywhere from a couple of minutes to 2 hours each time (range, 1–120 minutes). During asthma exacerbations, participants reported awakening more frequently than on a typical night, with many participants awakening three times per night and a few participants awakening five times per night (range, 1–5 times per night), 5 nights per week (range, 2–7 times per week); participants were typically awake 30 minutes to 2 hours each time (range, 30 minutes to 5 hours).
 |
Table 4 Aspects of Sleep Affected by Asthma |
Nearly all participants (n=11; 91.7%) reported that asthma symptoms affect their sleep quality. Of these, 8 participants (72.7%) reported that their asthma symptoms impact their sleep quality during a typical night and nights when experiencing an asthma exacerbation. The remaining 3 participants reported that they experience poor sleep quality only during asthma flares. Poor sleep quality was generally defined by participants as not sleeping through the night and not feeling rested in the morning. On average, during a typical night, participants reported that their sleep quality was affected about three times per week (range, 1–5 nights per week); in addition, 6 participants (54.5%) reported that their sleep quality was affected 2–5 nights per week, and 5 participants (45.5%) reported that their sleep quality was affected 7 nights per week during an asthma exacerbation.
Among all sleep impacts due to asthma, the inability to stay asleep (ie, nighttime awakening) was most frequently reported as the most bothersome (n=7, 58.3%), followed by poor sleep quality (n=3, 25.0%) and decreased sleep duration (n=2, 16.7%). Many participants (n=8; 66.7%) reported they had difficulty falling asleep initially (increased time to sleep onset) due to asthma. Of these, three participants reported that their asthma symptoms impact the time it takes to fall asleep regardless of whether they were experiencing an asthma exacerbation. During a typical night, two of the three participants reported that it can take them between 60 and 90 extra minutes to fall asleep up to two times per week. Five participants reported that their asthma symptoms impact the time it takes to fall asleep only during an asthma exacerbation. During an exacerbation, most participants reported it commonly takes them between 30 and 90 additional minutes to fall asleep, but one participant reported that it can take her up to 3 hours to fall asleep. Most participants (n=10; 83.3%) reported that their sleep duration was affected by asthma. The impact of asthma on sleep duration was primarily related to causing participants to wake earlier than normal, as well as increasing the time needed to fall asleep. Many participants (n=9; 75.0%) reported their asthma symptoms sometimes caused them to awaken earlier than normal in the morning. Of these, eight participants (88.9%) reported awakening, on average, 2 hours earlier (range, 45 minutes to 3 hours) during a typical week and during an asthma exacerbation.
Next-Day Impacts of Asthma-Related Poor Sleep
Eleven of the 12 participants were asked how difficulty with sleep (or poor sleep) due to asthma affects them during the next day. The twelfth participant was not probed because the participant reported not being impacted by nighttime symptoms. Table 5 presents participants’ reports on next-day impacts due to difficulties with sleep caused by their asthma. Participants reported tiredness/fatigue/lack of energy, physical functioning (eg, ability to complete housework, exercise), emotions/mood (eg, irritability, crankiness), mental functioning (eg, effects on alertness, concentration, memory), work/volunteerism (eg, ability to go to work, be productive), and social functioning (eg, ability or desire to participate in social gatherings with friends and family) as next-day impacts due to difficulties with sleep caused by asthma (Table 5).
 |
Table 5 Next-Day Impacts Due to Difficulties with Sleep Caused by Asthma |
All 11 participants reported experiencing one or more next-day impacts because of difficulties with sleep due to asthma. Based on spontaneous reports, when asked about how they feel the next day after a poor night’s sleep due to asthma, participants described feeling “tired”, “groggy”, “grouchy”, “cranky”, “foggier”, “crabby”, or “off-kilter.” Tiredness (also described as fatigue or lack of energy), reported by all 11 participants, was the most frequently reported spontaneous next-day impact. Participants also spontaneously reported that they are more impatient, less enthusiastic or engaged, less motivated or productive, and have less energy “to do the things I normally do.”
Conceptual Model
The conceptual model (Figure 1) displays the relationships between nighttime asthma symptoms, comorbid conditions, areas of sleep disturbance, and next-day impacts due to difficulties with sleep reported by interview participants, with the most commonly reported concepts bolded.
Cognitive Debriefing
Cognitive debriefing followed the CE portion of the interviews. In round 1, the ASDQ, Sleep Diary, and PROMIS SRI SF8a items were tested with interview participants. Modifications were made to three of the five response options for the ASDQ based on round 1 CD feedback. The original and modified versions of the ASDQ were then both tested in round 2 along with the other PROMs. All participants agreed that these PROMs would provide researchers with a comprehensive assessment of how asthma affects their sleep, with no important aspects of sleep missing. No participants found any measure redundant with another. Table 6 summarizes the aspects of sleep disturbance impacted by nighttime asthma symptoms and next-day impacts due to asthma-related sleep disturbances, as depicted in the conceptual model (Figure 1), that are evaluated by the ASDQ, Sleep Diary, and PROMIS SRI SF8a.
 |
Table 6 Patient-Reported Asthma and Sleep-Related Concepts Captured by Patient-Reported Outcome Measures |
Asthma Sleep Disturbance Questionnaire
All participants who provided feedback on the instructions (n=10) easily understood and consistently interpreted the instructions. Participants easily understood and consistently interpreted the recall period, “since last night”, generally reporting it as “since I went to bed last night until this morning.” Across both rounds of interviews, all participants understood and consistently interpreted the question as asking about their asthma symptoms from the previous night and how these symptoms impacted their sleep. Participants further agreed that the ASDQ captures an aspect of sleep that is important to them.
Most round 1 participants (n=5) initially selected a response easily and reported that the first and fifth response options were clear. However, when further probed about their interpretation of each response option, some participants had some difficulty understanding the second, third, and fourth response options and provided different interpretations of them than what was intended. Based on feedback from round 1 participants, the second, third, and fourth response options were modified for improved clarity and consistency so that each response option was mutually exclusive (Table 7).
 |
Table 7 Example of Item-Tracking Matrix for the Second and Third Response Options in the Asthma Sleep-Disturbance Questionnaire |
In round 2, both the original item response options and modified ASDQ response options were cognitively debriefed with participants. Round 2 participants clearly understood and accurately interpreted the first and fifth response options. All round 2 participants (n=6) correctly interpreted the phrasing of the original second response option; however, all participants agreed that the modified response option was clearer than the original. All round 2 participants (n=6) found the modified third and fourth response options to be more concise and understandable than the original response options, even if they understood the original items. Following review of the number of patient-reported nocturnal awakenings compared with the participant responses on the ASDQ in the Liberty Asthma QUEST trial,34 the modifications to the second and fourth response options were maintained, and the third response option was modified for consistency with the original intent/meaning of this response (Table 7).
Sleep Diary
Participant feedback was consistent across both rounds of CD interviews. Participants agreed that the Sleep Diary captured aspects of sleep that are important to them and agreed that it would give researchers a good idea of how asthma affects their sleep. Participants also agreed that no aspects of sleep impacted by asthma were missing or should be added or removed from the diary. Feedback received indicated that no modifications were needed to the item wording in the Sleep Diary. All interview participants (n=12, 100.0%) found the instructions clear and easy to understand and consistently interpreted the instructions, generally defining “upon awakening” as meaning after waking for the day. The recall period of “last night” was defined consistently and accurately as being from the time participants went to bed the previous night until the time they awoke the morning of the interview. Participants easily understood each item and provided consistent interpretations of the items’ meanings.
PROMIS SRI SF8a
Participant feedback was consistent across both rounds of CD interviews. Participants agreed that the PROMIS SRI SF8a captured the important ways in which they were impaired by difficulties with sleep due to asthma and would provide researchers a good idea of how difficulties with sleep due to asthma affected them during the past 7 days. Feedback received indicated that no modifications were needed to the wording of items in the PROMIS SRI SF8a. All participants found the instructional text for the PROMIS SRI SF8a to be clear and easy to understand, and they interpreted it consistently. Participants understood and consistently interpreted the 7-day recall period and reported that it was easy for them to remember each sleep-related impairment over the past 7 days. While it was not feasible to obtain the participant’s interpretation of each individual item on this questionnaire due to time constraints, all participants reported that the items were clear and easily understood.
Discussion
The findings from this study, the first qualitative data on this topic that we are aware of, provide evidence that the ASDQ, Sleep Diary, and PROMIS SRI SF8a items are relevant and appropriate as outcome measures for assessment of sleep disturbances and related next-day impacts in patients with moderate-to-severe, uncontrolled asthma and have been demonstrated to be comprehensive for this purpose. While other measures assessing nighttime awakening from asthma focus on a single aspect of sleep disruption,14,15 this study shows that the concept of sleep disruption goes beyond a single aspect. Concept elicitation interview findings across all study participants support the negative impact of nocturnal asthma symptoms on sleep, especially shortness of breath and chest tightness. Participant interviews confirmed that nighttime asthma symptoms impacted their sleep in some way, with participants most frequently reporting disturbances in nighttime awakening, sleep quality, and sleep duration. These specific sleep disturbances were also reported as most bothersome by participants. Nighttime awakening, reported by all participants, was the most prominent aspect of sleep impacted by asthma.
Difficulties with sleep lead to other impacts that need to be captured. Most participants (11 of 12) reported feeling tired/fatigue/lack of energy and having subsequent negative impacts on physical functioning, emotions and mood, mental functioning, work or volunteerism, and social functioning because of difficulties with sleep due to asthma. Clinicians treating patients with asthma need to recognize that these detrimental next-day impacts are linked to asthma through sleep disturbance. Poor emotional health in a patient with asthma may be caused or aggravated by sleep disturbance. The effects of these distal impacts could be reduced by including improved sleep as a goal of asthma treatment. Thus, studies assessing the effect of therapeutic interventions on improving sleep-related outcomes are needed.
The next-day impacts of sleep disturbance due to asthma need to be captured; however, there have been gaps in either the ability of PROMs to fully capture these or in the validation of sleep-related PROMs for patients with asthma. The ASDQ and Sleep Diary were able to capture the full aspects of sleep in patients with asthma and have demonstrated content validity for use in this population. Across both rounds of CD interviews, participants generally found the Sleep Diary items relevant and easy to complete, and no changes to any of the items were indicated. Although participants were initially able to respond to the ASDQ, when probed, some had difficulty understanding and providing consistent interpretations of the middle three response options. After the options were modified, all round 2 participants agreed that the modified response options were clearer and more understandable than the original response options. All final questionnaires were relevant, easy to complete, and appropriate for patients with moderate-to-severe, uncontrolled asthma.
The CE, conceptual model, and in-depth CD of all aspects of the ASDQ and the Sleep Diary provide support for the content validity of these measures in patients with moderate-to-severe, uncontrolled asthma. As such, these measures are suitable for use in future studies of the impact of nocturnal asthma symptoms on sleep, including clinical trials evaluating the effects of therapeutics on sleep in adult patients with moderate-to-severe, uncontrolled asthma. We would estimate participants in future clinical trials needing approximately < 1 minute to complete the ASDQ, 2 minutes for the Sleep Diary, and 2 minutes for the PROMIS SRI SF8a.35 A previous version of the ASDQ (AM Symptom Score) has been used before in the phase 3 QUEST trial,28 as well as a phase 2b trial29 to assess symptoms in patients with moderate-to-severe, uncontrolled asthma. Another trial, MORPHEO (NCT04502862), is an ongoing, phase 4, randomized controlled trial with the key objective of evaluating the effect of a therapeutic on sleep disturbance in patients with uncontrolled, persistent asthma.36 This trial uses the ASDQ, Sleep Diary, and PROMIS SRI SF8a to measure sleep disturbance, and data from the trial will facilitate the development of clinically relevant change scores. Furthermore, the use of these patient-reported sleep measures in additional large-scale studies will enable a comprehensive, standardized assessment of asthma-related sleep disturbance and next-day impacts from the patient’s perspective. In clinical trials, these measures will capture issues that are clinically relevant and meaningful to trial participants and allow for comparative efficacy assessment of different interventions, providing valuable information regarding the impact of trial interventions.
A limitation of this study is the amount of time that was available to complete both CE and CD of all sleep-related PROMs with each participant. While the instructions, items, and response options for the PROMIS SRI SF8a were reviewed for acceptability, comprehension, and relevance, there was insufficient time to obtain patients’ interpretation of each individual item and response options for this measure. However, the primary findings of this article focus on the ASDQ and Sleep Diary measures. Another limitation is that this research was conducted during the height of the COVID-19 pandemic, and individual experiences of COVID-19 may have initiated a response shift, defined as the phenomena “by which an individual’s self-evaluation of a construct changes due to internal standards of measure, change in values or priorities or a personal definition of a target construct”.37,38 This response shift may have affected participants’ responses, including psychosocial and HRQOL constructs. Given that COVID-19 is a respiratory virus, key signs and symptoms of asthma are susceptible to influence, and heightened levels of anxiety and fear may further influence responses. To help mitigate this potential confounding influence on study results, the study team followed best practices as described in FDA emergent guidance.24 The brief discussion of the effects that the pandemic had on participants’ daily lives and their experiences with asthma at the beginning of each interview highlighted the effects that the pandemic had on participants’ experiences with asthma.
Conclusion
Sleep disturbance due to asthma affects multiple aspects of sleep, including difficulty falling asleep, nighttime awakening, early awakening, sleep duration, and sleep quality. These nighttime sleep disturbances cause next-day fatigue in patients, with subsequent additional negative impacts on physical functioning, emotions and mood, mental functioning, work or volunteerism, and social functioning. Validated PROMs are needed to capture these impacts from sleep disturbance, and the findings from this study provide evidence of content validity for the ASDQ, Sleep Diary, and PROMIS SRI SF8a items in patients with moderate-to-severe, uncontrolled asthma. To ensure these measures are fit for purpose, an evaluation of their psychometric properties (reliability and validity), ability to detect change and interpretability based on clinical trial data in patients with moderate-to-severe, uncontrolled asthma is needed to further support their future use in this population.
Abbreviations
ACQ-5, Asthma Control Questionnaire 5-item version; ASDQ, Asthma Sleep Disturbance Questionnaire; CD, cognitive debriefing; CE, concept elicitation; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ESS, Epworth Sleepiness Scale; FDA, US Food and Drug Administration; GED, general education development; HRQOL, health-related quality of life; ICS, inhaled corticosteroid; IDI, in-depth interview participant number; IgE, immunoglobulin E; NRS, numeric rating scale; PROM, patient-reported outcome measure; PROMIS SRI SF8a, Patient-Reported Outcomes Measurement Information System Sleep-Related Impairment Short Form 8a; RD, round number; PSQI, Pittsburgh Sleep Quality Index; US, United States; VRS, verbal rating scale.
Data Sharing Statement
Data are primarily in the form of transcripts and cannot be made available in order to protect participant privacy in accordance with the principles of the Belmont Report.
Ethics Approval and Consent to Participate
The RTI Institutional Review Board determined that the study was exempt. Participants provided informed consent to participate.
Consent for Publication
Participants provided consent for publication of anonymized study findings.
Acknowledgments
The authors thank Brian Samsell, PhD of RTI Health Solutions for medical writing assistance. Sanofi provided funding for publication support in the form of manuscript writing, styling, and submission.
Author Contributions
AHK, KK, LPG, DW, SK, and MC substantially contributed to the conception or design of this research. AHK, KK, DW, and MC substantially contributed to the acquisition and analysis of data for this work. All authors substantially contributed to the interpretation of data for this work. AHK, KK, and MC substantially contributed to the drafting of the manuscript. All authors critically revised the manuscript for important intellectual content. All authors provided final approval of the version to be published, agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Sanofi provided the financial support for the study. RTI Health Solutions, an independent nonprofit research organization, received funding from Sanofi to provide publication support in the form of manuscript writing, styling, and submission.
Disclosure
AHK and LPG are employees of Sanofi and may hold shares and/or stock options in the company. MC, KK, and DW are employees of RTI Health Solutions, an independent nonprofit research organization, which received funding to conduct the study presented here. SK is an employee of Regeneron Pharmaceuticals, Inc. and may hold shares and/or stock options in the company. The authors report no other conflicts of interest in this work.
References
1. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222.
2. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2021. Available from: https://ginasthma.org/gina-reports/.
3. Alanazi TM, Alghamdi HS, Alberreet MS, et al. The prevalence of sleep disturbance among asthmatic patients in a tertiary care center. Sci Rep. 2021;11(1):2457. doi:10.1038/s41598-020-79697-x
4. McDonald VM, Hiles SA, Jones KA, Clark VL, Yorke J. Health-related quality of life burden in severe asthma. Med J Aust. 2018;209(S2):S28–S33. doi:10.5694/mja18.00207
5. Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM. American Lung Association Asthma Clinical Research C. Sleep quality in asthma: results of a large prospective clinical trial. J Asthma. 2008;45(3):183–189. doi:10.1080/02770900801890224
6. Luyster FS, Strollo PJ, Holguin F, et al. Association between insomnia and asthma burden in the Severe Asthma Research Program (SARP) III. Chest. 2016;150(6):1242–1250. doi:10.1016/j.chest.2016.09.020
7. Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30(3):274–280.
8. Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. doi:10.1016/j.smrv.2011.03.008
9. Lian Y, Xiao J, Liu Y, et al. Associations between insomnia, sleep duration and poor work ability. J Psychosom Res. 2015;78(1):45–51. doi:10.1016/j.jpsychores.2014.09.009
10. Burgoa V, Rejas J, Ojeda P, investigators of the Coste Asma study. Self-perceived sleep quality and quantity in adults with asthma: findings from the CosteAsma Study. J Investig Allergol Clin Immunol. 2016;26(4):256–262. doi:10.18176/jiaci.0044
11. Stone KL, Xiao Q. Impact of poor sleep on physical and mental health in older women. Sleep Med Clin. 2018;13(3):457–465. doi:10.1016/j.jsmc.2018.04.012
12. Scott D, Paterson JL, Happell B. Poor sleep quality in Australian adults with comorbid psychological distress and physical illness. Behav Sleep Med. 2014;12(4):331–341. doi:10.1080/15402002.2013.819469
13. Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68. doi:10.4137/HSI.S11093
14. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
15. Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76–83. doi:10.1136/thx.47.2.76
16. Ding B, Small M, Bergstrom G, Holmgren U. A cross-sectional survey of night-time symptoms and impact of sleep disturbance on symptoms and health status in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:589–599. doi:10.2147/COPD.S122485
17. Chang CH, Chuang LP, Lin SW, et al. Factors responsible for poor sleep quality in patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2016;16(1):118. doi:10.1186/s12890-016-0281-6
18. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
19. Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1--eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–977. doi:10.1016/j.jval.2011.06.014
20. Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims; 2020. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf.
21. Food and Drug Administration. Patient-focused drug development guidance: methods to identify what is important to patients and select, develop or modify fit-for-purpose clinical outcome assessments; 2020. Available from: https://www.fda.gov/drugs/news-events-human-drugs/patient-focused-drug-development-guidance-methods-identify-what-important-patients-and-select.
22. Juniper EF, Bousquet J, Abetz L, Bateman ED, Committee G. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma Control Questionnaire. Respir Med. 2006;100(4):616–621. doi:10.1016/j.rmed.2005.08.012
23. Department of Health and Human Services. 45 CFR 46.117 Documentation of Informed Consent; 2022. Available from: https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46/subpart-A/section-46.117.
24. Food and Drug Administration. FDA guidance on conduct of clinical trials of medical products during COVID-19 pandemic: guidance for industry, investigators, and institutional review boards; 2020. Available from: https://www.fda.gov/media/136238/download.
25. Food and Drug Administration. Patient-focused drug development: methods to identify what is important to patients. Guidance for industry, Food and Drug Administration staff, and other stakeholders; 2022. Available from: https://www.fda.gov/media/131230/download.
26. Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for Evaluating and Documenting Content Validity for the Use of Existing Instruments and Their Modification PRO Task Force Report. Value Health. 2009;12(8):1075–1083. doi:10.1111/j.1524-4733.2009.00603.x
27. Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity--establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2--assessing respondent understanding. Value Health. 2011;14(8):978–988. doi:10.1016/j.jval.2011.06.013
28. Busse WW, Maspero JF, Rabe KF, et al. Liberty Asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. 2018;35(5):737–748. doi:10.1007/s12325-018-0702-4
29. Corren J, Castro M, Chanez P, et al. Dupilumab improves symptoms, quality of life, and productivity in uncontrolled persistent asthma. Ann Allergy Asthma Immunol. 2019;122(1):41–49 e42. doi:10.1016/j.anai.2018.08.005
30. Kelsay K. Management of sleep disturbance associated with atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):198–201. doi:10.1016/j.jaci.2006.04.038
31. Hanish AE, Lin-Dyken DC, Han JC. PROMIS sleep disturbance and sleep-related impairment in adolescents: examining psychometrics using self-report and actigraphy. Nurs Res. 2017;66(3):246–251. doi:10.1097/NNR.0000000000000217
32. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;10(1):6–24. doi:10.1080/15402002.2012.636266
33. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
34. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092
35. Lei DK, Yousaf M, Janmohamed SR, et al. Validation of patient-reported outcomes information system sleep disturbance and sleep-related impairment in adults with atopic dermatitis. Br J Dermatol. 2020;183(5):875–882. doi:10.1111/bjd.18920
36. ClinicalTrials.gov. Dupilumab asthma sleep study (MORPHEO). ClinicalTrials.gov Identifier: NCT04502862; 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT04502862.
37. Sprangers MAG, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507–1515. doi:10.1016/S0277-9536(99)00045-3
38. Schwartz CE, Andresen EM, Nosek MA, Krahn GL, Measurement R. Response shift theory: important implications for measuring quality of life in people with disability. Arch Phys Med Rehabil. 2007;88(4):529–536. doi:10.1016/j.apmr.2006.12.032
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

