Back to Journals » Hepatic Medicine: Evidence and Research » Volume 10
Comparison of extracorporeal cellular therapy (ELAD®) vs standard of care in a randomized controlled clinical trial in treating Chinese subjects with acute-on-chronic liver failure
Authors Duan Z, Xin S, Zhang J, You S, Chen Y, Liu H, Zheng S, Li Z, Ashley R , Millis M
Received 14 July 2018
Accepted for publication 3 October 2018
Published 16 November 2018 Volume 2018:10 Pages 139—152
DOI https://doi.org/10.2147/HMER.S180246
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gerry Lake-Bakaar
Zhongping Duan,1,* Shaojie Xin,2,* Jing Zhang,1 Shaoli You,2 Yu Chen,1 Hongling Liu,2 Sujun Zheng,1 Zheng Li,3 Robert Ashley,3 Michael Millis4
1Artificial Liver Center, Beijing You’an Hospital of Capital Medical University, Beijing, China; 2Department of Infectious Disease, Division III, 302 Military Hospital of China, Beijing, China; 3Research and Development, Vital Therapies, Inc, San Diego, CA, USA; 4Department of Surgery, University of Chicago, Chicago, IL, USA
*These authors contributed equally to this work
Background: Preliminary evidence of safety and efficacy of an extracorporeal cellular therapy (ELAD®) has been demonstrated in subjects with acute forms of liver failure. This study compared ELAD with standard of care in Chinese subjects with acute-on-chronic liver failure (ACLF), predominantly secondary to chronic viral hepatitis.
Subjects and methods: Subjects meeting eligibility criteria were randomized to either the ELAD group or the control group. All subjects received plasma exchange and venovenous hemofiltration and either ELAD treatment for 3–5 days, unless terminated early, along with standard of care or standard of care alone (control) and were then followed up for 12 weeks.
Results: Forty-nine subjects (ELAD subjects, 32; controls, 17) were randomized under this protocol. Kaplan–Meier analysis of transplant-free survival (TFS) revealed a significant difference in favor of ELAD vs control (P=0.049, Wilcoxon signed-rank test). There was a significant difference in TFS on day 28 in ELAD vs control (P=0.022). In a multiple regression model, the relationship between group assignment and outcome was significant (P=0.031) when changes in food intake and Model for End-Stage Liver Disease (MELD) scores at screening were included as additional independent variables. The duration of ELAD treatment alone was a significant predictor of TFS (P=0.043). Median time to a 5-point increase in MELD, transplant, or death was longer than 72 days with ELAD vs 26 days for control (P=0.036). Total bilirubin level decreased by 25% during ELAD treatment vs 37% increase in the control group (P<0.001) over an equivalent period. Adverse events attributed to the ELAD system were expected and could be managed conservatively. Intergroup differences in certain vital signs and laboratory parameters were noted during treatment and generally resolved posttreatment.
Conclusion: ELAD treatment was well tolerated by Chinese subjects with ACLF, predominately secondary to chronic viral hepatitis. Results demonstrate a significant improvement in TFS in ELAD vs control groups in association with significant improvements in serum bilirubin levels presumably related to improvement in hepatic function.
Keywords: acute-on-chronic liver failure, ELAD, bioartificial liver support, ACLF, C3A cells, cellular therapy
Introduction
Of ~130 million patients with chronic hepatitis B and C infections in China, about 2 million each year experience acute hepatocellular dysfunction resulting in hepatic insufficiency that can progress to complete hepatic failure, termed “chronic severe hepatitis” in China and “acute-on-chronic liver failure” (ACLF) elsewhere. This is one of the most common types of hepatic failures in China, with pathogenesis, treatment, and prognosis quite different from those of drug- and alcohol-induced acute liver failure, which is a major cause of hepatic failure in Western countries.1–3 ACLF is difficult to treat with high mortality and poor prognosis.4 The current primary treatment strategy for ACLF with chronic viral hepatitis as the underlying liver disease combines physical therapy, administration of antiviral medicines, and traditional Chinese medicines with plasma exchange, which successfully bridges ~30%–40% of patients to spontaneous recovery. In China, liver transplant is uncommon, with a rate of 1.5 million/year vs 19 million/year in USA in 2009.
ELAD® is an investigational extracorporeal, human cell-based liver support system consisting of a dialysis-type pump and four hollow-fiber cartridges populated with a total of over 100 billion cells from the continuous VTL C3A hepatoma cell line. Prior to this study, 49 subjects with fulminant hepatic failure had been treated with previous versions of ELAD in four clinical trials (16 sites) in USA and Europe. ELAD was well tolerated in those trials,5–7 with preliminary results suggesting that ELAD is an effective bridge to liver transplantation or recovery. This study was the first randomized, controlled trial of ELAD in a group of Chinese subjects with ACLF and was designed to compare ELAD to the standard of care in China and to evaluate the effects of ELAD on transplant-free survival (TFS).
Subjects and methods
This was a randomized, controlled, open-label study at two centers in Beijing, China: the Beijing You’an Hospital of Capital Medical University and 302 Military Hospital of China. The study was approved by a designated institutional review board and was conducted in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2000,8 and in accordance with China Food and Drug Administration (CFDA) guidelines for the conduct of studies on class III medical devices. The registration number was ChiCTR-TRC-13004117.
The primary objective of this study was to evaluate the safety and efficacy of ELAD in the treatment of ACLF. Diagnostic criteria for ACLF were based on Chinese guidelines.1 Key eligibility criteria are listed in Table 1. The primary efficacy variable for the study was initially prothrombin activity (PTA; defined as control’s prothrombin time [PT; in seconds] divided by the subject’s PT expressed as a percentage) at baseline vs 14-day PTA, but this was changed to TFS during the study because PTA is less clinically relevant than TFS, and survival alone as a primary outcome measure presupposes that surviving with an allotransplant is equivalent to survival with a functional native liver. TFS, therefore, would clearly be the most desirable outcome for the patients in this study, all of whom were either at risk of death or need a new liver due to their underlying hepatopathologic condition.
  | Table 1 Key inclusion and exclusion criteria Notes: Based on “Guidelines for the treatment and prevention of viral hepatitis.”1 Age, PTA, and platelet count were changed in Amendment 2 to expand the population and to allow for enrollment of subjects with slightly severe baseline disease characteristics. Abbreviations: ACLF, acute-on-chronic liver failure; GI, gastrointestinal; PTA, prothrombin time activity. |
After written informed consent had been obtained (a parent or a legal guardian provided written informed consent for any subject who was younger than 18 years) and eligibility criteria established, subjects were randomized at each center to either ELAD or control treatment based on a central block randomization schedule that was managed by a third-party clinical research organization (Excel PharmaStudies, Inc., Beijing, China), and the “baseline” time point was established. The randomization list was produced using a computer program by the data management department of Excel before the clinical trial began. On enrollment, each subject, who met all the inclusion and exclusion criteria, was assigned a unique number (subject randomization number) corresponding to the chronological order of the study entry. Once assigned, subject randomization numbers were not reused. The original randomization list and computer file were kept securely at Excel, and no one could access the randomization list except for authorized persons who were not involved in the study. The first block of six subjects at one center was randomized in a 1:1 ratio (ELAD subjects:control), and the remaining subjects at both centers were randomized in a 2:1 ratio. In accordance with Chinese guidelines and standard of care in China for the treatment of ACLF, all subjects first underwent plasma exchange of ~2,500–3,000 mL at a blood flow rate of 100 mL/min, a plasma flow rate of 30 mL/min, and a treatment duration of 2–3 hours and continuous venovenous hemofiltration (CVVH) with a post-dilution blood flow rate of 120 mL/min, a substitute fluid flow rate of 2 L/h, and a treatment duration of ~4 hours, with the ultrafiltration volume dependent on the subject’s medical condition. Subjects assigned to the ELAD group received ELAD treatment for a minimum of 3 days up to a maximum of 5 days unless they deteriorated and treatment became futile or the subject withdrew consent. Subjects were followed up for 12 weeks after enrollment.
The active ingredient within the ELAD cartridge is the continuous VTL C3A cell line, which is a subclone of the human hepatoma cell line HepG2. VTL C3A cells have been shown to exhibit certain characteristics of normal liver cells (eg, an inducible cytochrome P450 system)9 and to produce macromolecules and other cellular products such as albumin, factor V, transferrin, antithrombin III, C3 complement, α-1-antitrypsin, and α-fetoprotein (AFP).10 In addition, in vitro cell-based models have demonstrated several potential effects of VTL C3A cell-secreted factors on causal pathways of inflammatory liver diseases, including the production of anti-inflammatory molecules such as interleukin-1 receptor antagonist (IL-1Ra), factors that block hepatocyte apoptosis, and proteins such as vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and angiopoietin 2 (ANG2), which may facilitate a hepatic regenerative response.11
The cartridge that houses the VTL C3A cell line comprises thousands of hollow fibers made up of a semipermeable polysulfone material with a nominal pore size of 0.2 μm, permitting a bidirectional flow between the VTL C3A cells and the ultrafiltrate. During ELAD treatment, the subject’s ultrafiltrate (ultrafiltrated plasma generated from whole blood using a dialysis-type cartridge with a nominal molecular weight cutoff of ~120,000 Da) is pumped through the fibers of the hollow-fiber cartridges allowing toxins, nutrients, and dissolved oxygen from the ultrafiltrate to diffuse across the fiber membrane into the space between the fibers, where they are metabolized by the VTL C3A cells, which are localized in the space between the fibers. Metabolites, along with albumin, transferrin, and other proteins, such as IL-1Ra, VEGF, PlGF, ANG2, and amphiregulin, produced by the VTL C3A cells, can then diffuse back across the membrane into the ultrafiltrate for return to the subject.
ELAD cartridges were provided by Vital Therapies, Inc. (San Diego, CA, USA). Cartridges were packaged in an insulated shipping container at 2°C–8°C and shipped to the clinical site within 24 hours of notification of randomization to ELAD. Cartridges were maintained at 2°C–8°C for no more than 48 hours (including time during shipment) before being used. All subjects randomized to ELAD began treatment within this 48-hour period. The ELAD system was run continuously during the treatment, and the performance of the system was tracked during therapy.
The subject was connected to ELAD by a standard dual-lumen hemodialysis catheter inserted in a large vein for central access. Blood was drawn from the subject at a rate of 100–150 mL/min. The system was heparinized to achieve a PTT of 60–80 seconds. Ultrafiltrated plasma was generated from whole blood at a rate of 20–50 mL/min using a dialysis-type cartridge with a nominal molecular weight cutoff of ~120,000 Da, a larger pore size than is regularly used in dialysis (to allow passage of larger proteins while maintaining immune isolation of the cells from the subject’s blood). Ultrafiltrate was then pumped into a recirculation circuit containing four ELAD cartridges, each containing ~110 g of VTL C3A cells localized between the fibers of the hollow-fiber cartridge, at a rate of 500 mL/min. Glucose was added to the circuit to match the glucose consumption rate of the cartridges reported during treatment. An oxygenator was included in the ELAD cartridge recirculation circuit to provide oxygen to the VTL C3A cells within the cartridges. Oxygen consumption was continuously monitored by an in-line gas analyzer placed proximal and distal to the ELAD cartridges. Oxygen consumption and glucose utilization are used to assess the metabolic activity and viability of the cells. A cell filter (0.2 µm) was placed in the ultrafiltrate return line, which delivered the treated ultrafiltrate to remix with the cellular component of the blood and return the conditioned blood back to the subject.
Standard of care treatment administered alone or with ELAD, where appropriate, was consistent with Chinese guidelines for subjects with acute flare of viral hepatitis and included traditional Chinese medicines; adequate oral/intravenous calorie supplementation; pharmacologic agents (eg, hepatocyte growth promoting factors, antiviral agents, detoxification agents); plasma or albumin for hypoalbuminemia and ascites; plasma, prothrombin complex, fibrinogen, and other hemostatic drugs as needed for bleeding; oxygen inhalation when PaO2 was <80 mmHg; and abdominal paracentesis for abdominal infection or severe abdominal distention affecting respiration and circulation.
Objectives
The primary efficacy endpoint was the Kaplan–Meier analysis of TFS measured by time to failure, defined as death or liver transplant. Criteria for transplantation were identical between the ELAD and control groups. China does not have a “wait list” for liver transplant, and transplants are typically made available only to those subjects in whom rapid disease progression is taking place. In this study, transplantation was carried out in accordance with the guidelines of the Chinese Society of Infectious Diseases and Chinese Society of Hepatology of Chinese Medical Association.2
Secondary efficacy endpoints included the proportion of transplant-free survivors at 14, 28, 56, and 84 days post-baseline. A secondary post hoc time to progression analysis was performed, which included an additional parameter of increase of Model for End-Stage Liver Disease (MELD) by ≥5 points between baseline and at least 3 days post-baseline together with transplant and death as a measure of disease progression.
All analyses were based on the intent-to-treat (ITT) population of all randomized subjects. Kaplan–Meier survival functions were calculated, and the groups were compared using the Wilcoxon signed-rank test. A secondary analysis of TFS was carried out at 14, 28, 56, and 84 days post-baseline and analyzed using Fisher’s exact test. To examine the effects of baseline variables, a proportional hazards regression model containing a factor for treatment (ELAD vs control) as well as two important baseline variables (MELD and change in food intake scores) was used. To account for ELAD treatment duration, a second model used treatment duration (set to 0 for control subjects) rather than treatment group. All statistical analyses were performed using SAS software (SAS®, version 9.1.3; SAS Institute Inc., Cary, NC, USA).
Safety was evaluated by adverse events (AEs), vital signs, and laboratory evaluations. AEs occurring any time after treatment started and during the treatment period (day 1–5) were categorized using a modified Medical Dictionary for Regulatory Activities coding system. The severity of the AEs was graded according to “WHO Toxicity Grading Criteria.” The relationship of an AE to treatment was independently graded by the investigator as unrelated, unlikely, possibly, probably, or definitely related to treatment. Additional analyses were performed on AEs graded as “severe” and at least “possibly” related to treatment.
Laboratory values including bilirubin, albumin, prealbumin, cholinesterase, international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, C3, C4, α-1-acid glycoprotein (AAG), α-1-antitrypsin, apolipoprotein B, transferrin, ceruloplasmin, ammonia, vital signs, MELD scores,12 clinical symptom scores (fatigue, food intake, appetite, nausea, abdominal flatulence, hiccup, nose/gum bleeding, pruritus, hepatoencephalopathy, and ascites; each scored on a 4-point scale), and changes from baseline were analyzed using one-way ANOVA.
On completion of the base study, a new protocol was developed to follow-up subjects who survived the original study in order to learn more about the long-term (3 and 5 years) condition of the subjects, including outcome, transplant, and incidence of liver cancer.
Results
Enrollment began in March 2006 and was completed in December 2006. Subjects were followed up for a total of 12 weeks, with the window for the last visit extended to 125 days. The original statistical plan for the study called for the enrollment of 120 subjects with a 1:1 randomization schedule. Soon after the study began (at the time of enrollment of the 6th subject in the study), it became apparent that it would be impossible to enroll sufficient control subjects to support this plan; so, a revised statistical plan was submitted for review which called for a 2:1 randomization, with the same number of active subjects as originally envisaged (60 – hence a total of 90 subjects, randomized 2:1 ELAD:control).
Due to the experimental nature of ELAD treatment, and as the study was open label, safety and efficacy of ELAD were monitored continuously during the course of the study to ensure that a positive benefit–risk ratio was maintained. On completion of 49 subjects, an unplanned interim analysis of the study was carried out by the ethics committee of the You’an Hospital, the principal investigative site in the study. As a result, the ethics committee called for randomization to be stopped because, in their view, the efficacy data favored ELAD, and therefore, it would be unethical to continue to enroll control subjects. They recommended that the study should be terminated and written up for review by the CFDA.
Following enrollment of the 49-subject cohort discussed in this article, the study inclusion criteria and treatment duration were changed, and an additional 20 subjects were enrolled before the study was finally terminated. As there were significant differences in the patient population and the study design for these additional 20 subjects, it was not deemed appropriate by the independent statistical reviewer to include them in the analysis of the first 49 subjects.
At the time of the interim analysis, the study had enrolled 32 subjects in the ELAD group and 17 subjects in the control group. Based on the usual equation for power calculation with unequal sample sizes,13 when terminated at 49 subjects in total, this study had 80% power to detect a difference between groups at least 86% of the size of the SD with a two-sided α level of 0.05.
There were 49 subjects (32 ELAD subjects and 17 controls) enrolled in the study, comprising the ITT analysis population (Figure 1). All subject management for both the ELAD-treated and control subjects took place outside of an intensive care unit (ICU) environment. The treatment groups were generally similar in demographic and baseline characteristics (Table 2). All clinical symptoms were assessed using the grading standards of symptoms used in China Drug Clinical Trials.2
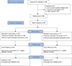  | Figure 1 Clinical trial flowchart. |
Most subjects were diagnosed with chronic hepatitis B alone or in combination with alcoholic liver disease (Table 2). Consistent with the diagnoses, various parameters associated with hepatic dysfunction were elevated, including MELD score, PT, aPTT, INR, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBil), direct bilirubin (DBil), alkaline phosphatase (ALP), C-reactive protein (CRP), and fatigue. Values of other parameters decreased, including PTA, albumin, prealbumin, cholinesterase, uric acid, cholesterol, C3, C4, transferrin, ceruloplasmin, AAG, and food intake. Statistically significant differences between the ELAD and control groups at the screening visit included MELD scores, flatulence, ALP, cholesterol, PT, PTA, and INR (Table 2).
Twenty-two subjects had at least one protocol deviation. The only intergroup difference attaining statistical significance (P=0.043) was for the coagulation status inclusion criterion (PTA, PT, or INR), with one subject assigned to ELAD (3.1%), being outside the acceptable range in contrast to four control subjects (23.5%).
There were no between-group differences in the duration of the initial plasma exchange (2.8 and 2.4 hours on average in the ELAD and control groups, respectively) or subsequent hemofiltration (3.3 and 3.5 hours on average). The mean (± SD) duration of ELAD treatment was 68.1±17.9 hours (range, 4.2–115.5 hours), with two subjects on the system for <35 hours, 27 on the system for 36–84 hours, and three on the system for 108–120 hours. Four subjects discontinued ELAD treatment due to at least one of the following AEs: acute hemolysis, platelet decrease, red blood cell (RBC) decrease, moist sounds in both lungs, respiratory failure, and allergy.
Concomitant therapies were used by 46.9% ELAD and 35.3% control subjects (Table 3). Additional plasma exchange and renal replacement therapy were administered in accordance with Chinese standard of care, as required by the subject, irrespective of group assignment. There was no difference between the groups in screening creatinine levels (Table 2). There was no restriction on subjects being administered both CVVH and ELAD simultaneously.
  | Table 3 Concomitant therapies Note: aP-values determined using Fisher’s exact test.
|
Concomitant blood products were administered to both ELAD and control subjects in accordance with the clinical need. Significantly more subjects in the ELAD group than the control group were administered globulin, platelets, RBCs, and prothrombin complex (P<0.05). This finding is consistent with the consumption of blood components typically associated with the use of extracorporeal therapies.
In accordance with Chinese standard of care, all the subjects in both groups were administered hepatocyte growth factor (a substance extracted from pig livers), reduced glutathione, and vitamin C as detoxification agents.
Loss of appetite and food intake reduction are symptoms of liver failure subjects, and their severity is often related to the severity of liver failure, although not necessarily completely parallel. Food intake is an indicator reflecting the severity of disease and its progression, but not a predictor of outcome. Loss of appetite may be due to cholestasis that affects the digestive function and causes abdominal distension. However, the specific mechanism of loss of appetite in subjects with liver failure is not yet clear. Subjects received enteral feeding according to international guidelines.14 For both ELAD-treated and control subjects, the daily consumption by each subject was recorded in a food chart, including time and type and quantity of food, and used to assess the pertinent clinical parameters. Nutritional support therapy was administered based on the quantity (calories) and composition of food intake. If, in the clinical judgment of the physician, intake was insufficient, enteral or parenteral nutrition was supplemented. The goal of nutritional support therapy was to provide 25–30 kcal/kg from nonprotein calories per day. However, as digestion and fluid intake were restricted in these subjects, it was sometimes not possible to meet this standard. Enteral therapy was generally well tolerated, although some subjects had diarrhea, at which point the therapy was either stopped or the dosage reduced, and some subjects did not like the taste, in which case therapy was stopped.
Efficacy results
The primary Kaplan–Meier analysis of transplant-free survival (Figure 2) showed a significant difference in favor of ELAD (P=0.049, Wilcoxon signed-rank test).
  | Figure 2 Kaplan–Meier analysis of transplant-free survival.
|
The proportions of subjects who were alive (no transplant), had died, and had liver transplantation at study days 28 and 84 are presented by the treatment group in Table 4. A statistically significant higher proportion of transplant-free survivors in the ELAD group vs control group was observed on day 28 (P=0.022, Fisher’s exact test). While directionally supportive of ELAD treatment, the difference in the proportion of transplant-free survivors between the ELAD and control groups was not statistically significant on day 14 (93.8% vs 81.6%, respectively, P=0.197), day 56 (71.9% vs 41.2%, P=0.063), or day 84 (P=0.134). No subject required a liver transplant between day 28 and day 84 in either group. Five ELAD-treated and one control subject died during this period. In accordance with Chinese guidelines,2 most subjects who received transplants suffered rapid disease progression between baseline and the time of transplant as measured by the change in MELD score.
  | Table 4 Proportion of survivors on days 28 and 84 Note: aOne subject in each group withdrew consent.
|
An individual regression analysis of the effect of treatment on TFS did not show a significant relationship between group assignment and outcome (P=0.065); however, in a multiple regression model combining treatment with change in food intake and MELD scores at screening as additional independent variables, the relationship between group assignment and outcome became significant (P=0.031; Table 5). MELD score and food intake were selected for inclusion in the multiple regression models in order to adjust for intergroup differences at baseline in disease severity (MELD score) and to incorporate a clinical parameter believed a priori to be strongly correlated with outcome (food intake). The duration of ELAD treatment alone was a significant predictor of TFS on day 84 (P=0.043; Table 5). With treatment effect, the statistical significance increased when adjusting for food intake and MELD scores at screening. It should be noted that baseline MELD and food intake were significantly correlated with outcome on their own, irrespective of whether treatment or treatment duration were included in the multiple regression models. The direction of the relationship between food intake/MELD scores and outcome indicates that subjects with high scores for either of these independent variables were at an increased risk of death or transplantation, as were subjects assigned to the control group (Table 6).
  | Table 5 Proportional hazards regression of treatment group/duration of treatment, MELD score, and food intake on transplant-free survival Abbreviation: MELD, Model for End-Stage Liver Disease. |
An increase >5 points in the MELD score over 30 days has been associated with a threefold increased relative risk of death compared to subjects without such a rapid increase.15 In a retrospective Kaplan–Meier analysis of time to progression, the median time to an increase of MELD by ≥5 points, transplant, or death was >72 days for ELAD-treated subjects vs 26 days for control subjects (P=0.036; Figure 3).
  | Figure 3 Kaplan–Meier analysis of time to progression.
|
Furthermore, of the 28 total subjects who survived without transplantation, none exceeded the threshold of a 5-point increase in MELD between baseline and at least 3 days post-ELAD treatment, whereas 11 of the 21 subjects who died or were transplanted showed evidence of rapid disease progression (change in MELD ≥5 points) prior to the event taking place (P<0.001).
Statistically significant intergroup differences (assessed as change from baseline) following the start of ELAD treatment were observed for fatigue, appetite, pruritus, shifting dullness, and ascites. For some symptoms, such as appetite, pruritus, shifting dullness, and ascites, the net effect of these changes from baseline was simply to reduce or eliminate baseline intergroup differences. In all cases, the magnitude of the within-group mean change during the course of the study was relatively minor in terms of clinical relevance.
Statistically significant intergroup differences in change from baseline were found for TBil and DBil over the entire treatment interval (baseline through days 3–5 for both groups). The overall magnitude of decrease in mean end-of-treatment (days 3–5) TBil and DBil from baseline in the ELAD group was 25.1% (17.5 to 13.1 mg/dL) and 20.4% (9.3 to 7.4 mg/dL), respectively, compared to an increase in the control group of 36.8% (17.1 to 23.4 mg/dL) and 33.7% (9.8 to 13.1 mg/dL). On day 14, the difference between the treatment groups was not statistically significant for TBil or DBil (data on file, Vital Therapies, Inc.). There were no other differences of note in the laboratory or clinical parameters between the ELAD and control groups during the course of ELAD treatment.
In a post hoc analysis, subjects were followed up for up to 5 years after completion of the study in order to assess long-term survival and the incidence of cancer. Of the original 49 subjects, TFS was 14/32 (43.8%) vs 2/17 (11.8%) for ELAD and control subjects, respectively, through 5 years (log-rank P<0.05). These follow-up data suggest that the relative TFS benefit observed in the ELAD group compared with the control group after 3 months is maintained through 5 years. Three subjects (2/32, 6.3% ELAD; 1/17, 5.9% control) were diagnosed with liver cancer during this period, consistent with the expected incidence of liver cancer in patients with chronic hepatitis B infection.
Safety results
Significantly more ELAD subjects than control subjects experienced AEs during the treatment period; 29 ELAD subjects (90.6%) reported 114 AEs, whereas 10 control subjects (58.8%) reported 29 AEs (P=0.021). These differences were observed predominantly in blood and lymphatic disorders and infections. Notable differences were also observed for general disorders, and administration site conditions and respiratory disorders but the intergroup differences did not reach statistical significance.
There was only one treatment-emergent serious adverse event (TESAE; acute hemolysis) reported in the ELAD group when the study was actively treating subjects. Since the outcome “death” was listed as TESAE and specific clinical events leading to death were not specified, a retrospective review of the narratives of the subjects who died was conducted in March 2017 by the VTL Medical Monitor to determine the probable cause of death.
Among these 49 subjects, 10 of 32 subjects who underwent ELAD treatment died. Sixty percent of the ELAD-treated subjects died due to multiorgan failure. Twenty percent of subjects died due to hepatic failure, one subject died due to gastrointestinal bleeding, and another died secondary due to acute hemolysis. Five of 17 control subjects died. Sixty percent of subjects died due to multiorgan failure, and 40% of subjects died due to hepatic failure. The number of subjects experiencing at least one TESAE was balanced between the ELAD and control groups (31% vs 29%, respectively).
It should be noted that not all subjects received antibiotics. If the subjects had fever, increased white blood cell (WBC), or other signs of active infection, the treating physician used antibiotics in accordance with standard of care.
The types and severity of AEs recorded were consistent with previous clinical studies of ELAD and not unexpected in subjects undergoing extracorporeal therapy. Relatively few events were thought to be possibly or probably related to ELAD treatment, and they were all in system organ classes of blood and lymphatic disorders; general disorders and administration site conditions; hepatobiliary disorders, and immune system disorders.
Some intergroup differences in vital signs and safety laboratory parameters were noted during treatment, as described below. All of these differences resolved posttreatment, by day 14 if not earlier, with the exception of increased AFP.
Reductions in blood pressure on day 1, reduction in heart rate on days 2–5, and reduction in temperature on days 1 and 2 were noted for the ELAD subjects, while control subjects had slight reductions (diastolic blood pressure) or increases (systolic blood pressure, heart rate, and temperature) during an equivalent period. None of these differences were deemed to be clinically meaningful.
RBC, hematocrit, hemoglobin, and blood platelet values declined to a greater extent in the ELAD group than in the control group during the treatment period, consistent with the administration of extracorporeal therapy. Coagulation factors, albumin, RBC, and other blood products were supplemented in both groups in accordance with the protocol, based on certain laboratory parameters. Overall, WBC counts increased in the ELAD group compared with the control group. These differences resolved following treatment. The proportion of subjects who had lung infection was higher in the ELAD group (15.6%) than in the control group (0%) during days 1–5 (P=0.149). However, except for one ELAD subject, where it was reported that the infection started on day 3 and the subject died on day 4 due to hepatic failure, these lung infections resolved by day 28. There was no subject in which lung infection led to death. AST was elevated to a much higher level in ELAD than control subjects over the entire treatment interval, probably primarily due to AST production by the VTL C3A cells. γ-Glutamyl transpeptidase (GGT) was also elevated but to a lesser degree; ELAD did not have a significant differential effect on ALT levels. Globulin was reduced substantially in the ELAD group during days 1 and 2 of treatment, while a slight increase was observed in the controls, and ALP was similarly affected by ELAD treatment.
Sodium, chloride, and potassium values were significantly decreased relative to baseline in the control group compared to an increase (sodium/chloride) or decrease (potassium) for ELAD subjects (P<0.05). In particular, there was a statistically significant difference in serum sodium concentration with a mean increase in the ELAD-treated subjects compared to a mean decrease in the control subjects during treatment (Table 7). Except for AFP, which was elevated in the ELAD group due to production of AFP by the VTL C3A cells, intergroup differences in change from baseline were not observed for other proteins such as CRP, IgG, IgM, or IgA. There were no differences between the two treatment groups in measurements for blood gases.
  | Table 7 Mean change in serum sodium concentration Notes: aOne ELAD subject was missing serum sodium measurement. bSeven control subjects were missing serum sodium measurement.
|
Discussion
The primary analysis of ELAD treatment in this study demonstrated a significantly increased TFS in the ELAD group compared to the standard of care (control). Secondary analysis showed that the proportion of transplant-free survivors was significantly higher in the ELAD group at 28 days post-baseline, and the treatment effect on day 84 was statistically significant when two other independent variables (change in food intake and baseline MELD score) were included in the multiple regression models. In addition, the time to progression defined as an increase of ≥5 points on the MELD score, transplant, or death was significantly longer with ELAD treatment compared to standard of care treatment.
The difference between the number of transplants in the ELAD vs the control group (3% vs 29%) raises the possibility of bias toward transplant in the control group. At the time of conduct of this study, subjects were not “listed” for transplant in China in the same way that they are in USA. Transplant frequency was much lower in China than in USA, and transplant allocation in China was determined primarily by acute need, as determined by rapid deterioration in liver function. Most of the subjects who received liver transplants during the study exhibited rapid increases in MELD score immediately prior to transplant. This deterioration, coupled with the high cost of transplant, minimizes the possibility of such bias.
Many of the changes in laboratory data observed were consistent with the behavior of extracorporeal systems in general and the need to replenish blood components during their use, including alterations in vital signs, hematologic and blood coagulation indices, and perhaps even the slight imbalances in electrolytes, and reductions in prealbumin and albumin. The VTL C3A liver cell line used in ELAD treatment produces large quantities of AST and AFP, both of which were markedly increased in the blood from subjects during ELAD treatment, with GGT levels also increased but to a lesser extent. The production of AST by viable VTL C3A cells has been measured in the laboratory (data on file, Vital Therapies, Inc.). Viable VTL C3A cells produce AST during normal cell culture processes. For example, AST production has been measured during the ELAD cartridge (C3A cell growth) production process and when ELAD cartridges are placed on an ELAD bedside unit in the laboratory. It is likely that AST, and perhaps GGT, level(s) in ELAD subjects were increased by the production of these proteins by VTL C3A cells (data on file, Vital Therapies, Inc.), although it is possible that subclinical and transient hemolysis may also arise due to the stress of extracorporeal therapy.
Statistically significant reductions in several laboratory analyses were observed in ELAD subjects during treatment relative to controls, the most notable being the decrease in bilirubin values (44%). Although VTL C3A cells lack the ability to transport active bilirubin into the cells via OATP1A2 and OATP1B1,16 bilirubin has been reported to be present in cell membranes of VTL C3A cells, either bound to proteins other than albumin or present in a conjugated form.17 In addition, short-term improvements in the subject’s native liver function as a result of the anti-inflammatory proteins secreted by the VTL C3A cells may have resulted in improvements in bilirubin secretion. Hyperbilirubinemia, particularly if persistent for longer than 7 days, is strongly prognostic for death.18–22 Therefore, it is possible that the changes in plasma bilirubin levels may be the biomarker most closely linked to the apparently better outcome of ELAD-treated subjects with regard to TFS.
An analysis based on adult candidates for primary liver transplantation found that both the MELD score and the serum sodium concentration were significantly associated with mortality and also identified a significant interaction, indicating that the effect of the serum sodium concentration was greater in subjects with a low MELD score.23 Although a potential interaction between MELD scores and serum sodium concentration was not evaluated in the present study, there was a statistically significant difference in serum sodium concentration with a mean increase in the ELAD-treated subjects compared to a mean decrease in the control subjects during treatment.
Across all safety variables, ELAD was reasonably well tolerated, with AEs attributed to the system being expected and easily treated. Multiorgan failure and hepatic failure are the most common TESAEs in both groups. Overall, except for the subject who died secondary to an acute hemolysis, the causes of death in this ACLF patient population would be expected based on the type and severity of the underlying disease.
It should be noted that 93.8% subjects were on ELAD treatment for 43.3–115.5 hours, and death was not thought to be ELAD related for the 10 subjects who died. Importantly, 22 survivors (one transplant) had an uneventful course after 48–115.5 hours of treatment. During ELAD treatment, there was a transient decrease in platelet and RBC levels, a side effect common to other treatments involving extracorporeal circulation, such as CVVH or dialysis. It is important to consider the balance between treatment time and efficacy in order to maximize patient benefit while controlling the side effects. For subjects with high risk or evidence of infection, antibiotics were administered during ELAD treatment. In addition, antisepsis and isolation were applied to prevent infection, and it is generally advised that the use of ELAD should be confined to the ICU setting.
It should be noted that standard of care in China was differently defined compared with the standard of care in Western countries. Also, the “wait list” for liver transplants was not available during the study. Transplant frequency was much lower in China than in USA, and transplant allocation in China was determined primarily by acute need.
There is a hypothetical potential for breakthrough of VTL C3A cells from the ELAD system that could have the potential to cause the formation of liver tumors in treated subjects. In order to mitigate that risk, the ELAD system uses ELAD cartridges made with 0.2 μm polysulfone fibers that are highly robust, with no evidence of breakage during treatment, and the system incorporates a downstream 0.2 μm cell filter that does not allow the passage of VTL C3A cells. Long-term follow-up of subjects in this study showed that the 5-year incidence of liver cancer (6%) among the treated subjects in this study was completely within the range of normal incidence for this population (5%–10%) in China24 and similar to the incidence among the control subjects.
This study had some limitations. First, we did not carry out liver biopsies due to the poor coagulation function, and, therefore, a histological definition of the subject’s disease is not available and the potential histological impact of ELAD treatment was not established. Second, the primary efficacy variable was changed from PTA at baseline vs 14-day PTA to TFS during the study because TFS is more clinically relevant, and mortality of patients with allotransplant is higher than patients with a functional native liver. Third, the sample size was small due to the early termination. Finally, this study was terminated before it was fully enrolled. Termination took place because, following enrolment of 49 subjects, an unplanned interim analysis by the independent ethics committee responsible for monitoring the conduct of the study at the primary enrollment site revealed that the rate of TFS in the ELAD group significantly exceeded the control group, and it was determined that it would be unethical to continue enrolling subjects in the control arm of the study, leading to early study termination. Despite only enrolling 60% of the planned number of subjects, the treatment effect proved significantly positive.
In conclusion, ELAD treatment was well tolerated by 32 Chinese subjects with ACLF, with efficacy data indicating an increase in TFS relative to standard of care (control) in association with ELAD-mediated reductions in select laboratory parameters related to hepatic function.
Data sharing
Individual participant data will not be available. See Supplementary material for Protocol Amendment 1.
Acknowledgments
This study was financially supported in part by the Chinese State Key Projects for Basic Research (no 2007CB512801) and for High-Tech Program (2006AA02A410), the Beijing Municipal Key Project (Z0006264040791), and Vital Therapies Inc. We thank Prof Jumei Chen, Prof Yedong Wang, and Prof Jun Zhao of 302 Military Hospital of China for their valuable suggestions throughout this study. We would also like to thank Vital Therapies, Inc., and its employees Dar He, John Brotherton, Kameron Maxwell, and Terry Winters for their helpful scientific advice, hard work, and unwavering support during the conduct of the study.
Disclosure
Dr Zhongping Duan was a member of the Vital Therapies China Clinical Advisory Board during the clinical trial. Dr Michael Millis is a member of the Vital Therapies Board of Directors and the Vital Therapies’ Scientific and Clinical Advisory Boards. Zheng Li and Robert Ashley are employees of Vital Therapies, Inc. The other authors report no conflicts of interest in this work.
References
Chinese Medical Association-Chinese Society of Infectious and Parasitic diseases in conjunction with Chinese Society of Hepatic Disease. Viral hepatitis prevention and treatment programs. Zhonghua Gan Zang Bing Za Zhi. 2000;8:324–329. | ||
Liver Failure and Artificial Liver Group, Chinese Society of Infectious Diseases, Chinese Medical Association. Severe Liver Diseases and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Diagnostic and treatment guidelines for liver failure. Zhonghua Gan Zang Bing Za Zhi. 2006;14:643–646. | ||
Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3(1):269–282. | ||
Li Q, Yuan GY, Tang KC, Liu GW, Wang R, Cao WK. Prognostic factors for chronic severe hepatitis and construction of a prognostic model. Hepatobiliary Pancreat Dis Int. 2008;7(1):40–44. | ||
Sussman NL, Gislason GT, Conlin CA, Kelly JH. The Hepatix extracorporeal liver assist device: initial clinical experience. Artif Organs. 1994;18(5):390–396. | ||
Ellis AJ, Hughes RD, Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24(6):1446–1451. | ||
Millis JM, Cronin DC, Johnson R, et al. Initial experience with the modified extracorporeal liver-assist device for patients with fulminant hepatic failure: system modifications and clinical impact. Transplantation. 2002;74(12):1735–1746. | ||
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284(23):3043–3045. | ||
Landeen LK, Lapetoda J, van Allen J, Heredia N, Brotherton J, Ashley R. P1104: expression of liver-specific cytochrome P450 isoenzymes and oxygenases in C3A cells prior to and after treatment with the ELAD liver support system. J Hepatol. 2015;62(Suppl 2):S764. | ||
Brotherton J, He D, Asslani S, Millis M. ELAD® cellular and system performance improvements [AASLD abstract 855]. Hepatology. 2007;46(Suppl 1):615A–616A. | ||
Bedard PW, Lapetoda J, van Allen J, Heredia N, Michalopoulos GK, Landeen LK. ELAD VTL C3A cells may impact liver regeneration through secreted factors. In: AASLD – The Liver Meeting; November 13–17, 2015; San Francisco, CA, USA. | ||
Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. | ||
Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2(2):93–113. | ||
Polson J, Lee WM; American Association for the Study of Liver Disease. AASLD position paper: the management of acute liver failure. Hepatology. 2005;41(5):1179–1197. | ||
Foxton MR, Kendrick S, Sizer E, et al. Change in model for end-stage liver disease score on the transplant waiting list predicts survival in patients undergoing liver transplantation. Transpl Int. 2006 Dec;19(12):988–994. | ||
Lapetoda J, Landeen LK, Michalopoulos GK, Bedard PW. Anti-apoptotic effects of cellular therapy: VTL C3A cell-secreted factors reduce in vitro hepatocellular injury via multiple mechanisms. In: 17th International Symposium on Albumin Dialysis (ISAD); September 16–18, 2016; Rostock-Warnemünde, Germany. | ||
Maghzal GJ, Leck MC, Collinson E, Li C, Stocker R. Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase. J Biol Chem. 2009;284(43):29251–29259. | ||
López-Velázquez JA, Chávez-Tapia NC, Ponciano-Rodríguez G, et al. Bilirubin alone as a biomarker for short-term mortality in acute-on-chronic liver failure: an important prognostic indicator. Ann Hepatol. 2014;13(1):98–104. | ||
Mathurin P, Abdelnour M, Ramond MJ, et al. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology. 2003;38(6):1363–1369. | ||
Morris JM, Forrest EH. Bilirubin response to corticosteroids in severe alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2005;17(7):759–762. | ||
Lee M, Kim W, Choi Y, et al. Spontaneous evolution in bilirubin levels predicts liver-related mortality in patients with alcoholic hepatitis. PLoS One. 2014;9(7):e100870. | ||
Li J, Wu J, Liu HX, et al. Early bilirubin response in acute-on-chronic hepatitis B liver failure patients treated with corticosteroids predicates a lower 3-month mortality. Int J Clin Exp Med. 2016;9(6):10364–10373. | ||
Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. European Concerted Action on Viral Hepatitis (EUROHEP. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97(11):2886–2895. | ||
Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–S50. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


