Back to Journals » Drug Design, Development and Therapy » Volume 18
Comparative Study Between Dexmedetomidine with Bupivacaine and Bupivacaine Alone in Erector Spinae Plane Block for Postoperative Pain Control of Posterior Lumbosacral Spine Fixation Surgeries: A Randomized Controlled Trial
Authors Abu El Hassan SH, Wahsh EA , Mousa AM, Ibrahim ARN, Mohammed EL
Received 12 October 2023
Accepted for publication 30 January 2024
Published 7 February 2024 Volume 2024:18 Pages 351—363
DOI https://doi.org/10.2147/DDDT.S444485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Sawsan HA Abu El Hassan,1 Engy A Wahsh,2 Abdelmaksod Mohammed Mousa,3 Ahmed RN Ibrahim,4 Emad Lotfy Mohammed5
1Anesthesiology, Intensive Care and Pain Therapy, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 2Department of Clinical Pharmacy, Faculty of Pharmacy, October 6 University, Giza, 12585, Egypt; 3Neurological and Spine Surgery Department, Faculty of Medicine, October 6 University, Giza, 12585, Egypt; 4Department of Clinical Pharmacy, College of Pharmacy, King Khalid University, Abha, 61421, Saudi Arabia; 5Anesthesiology, Intensive Care and Pain Therapy, Faculty of Medicine, Misr University for Science and Technology, Giza, Egypt
Correspondence: Engy A Wahsh; Ahmed RN Ibrahim, Email [email protected]; [email protected]
Background: As posterior lumbosacral spine fixation surgeries are common spine procedures done nowadays due to different causes and mostly accompanied with moderate-to-severe postoperative pain, so should find effective postoperative analgesia for these patients. This study aimed to observe analgesic effect of dexmedetomidine combined with bupivacaine versus bupivacaine alone for erector spinae plane block ESPB for postoperative pain control of posterior lumbosacral spine fixation surgeries.
Methods: Double-blind randomized controlled study including 90 patients who were randomly allocated into 3 groups (30 patients for each): Dexmedetomidine combined with bupivacaine (DB group), bupivacaine (B group), and saline (control) (S group). US-guided ESPB was performed preoperatively bilaterally in all patients of the 3 groups. All patients received intravenous patient-controlled postoperative analgesia with morphine and 1 gm intravenous paracetamol every 8 hours. Primary clinical outcomes were active (while mobilization) and passive (at rest) visual analog scale (VAS) pain score at first 24 hours measured every 2 hours, opioid consumption (number of PCA presses), and need for rescue analgesia. Other clinical outcomes included active and passive VAS pain score at second 24 hours, measured every 4 hours, opioid consumption, need for rescue analgesia, postoperative opioid side effects, and intraoperative dexmedetomidine side effects as bradycardia and hypotension.
Results: Active and passive VAS pain scores, postoperative opioid consumption, need for rescue analgesia, and postoperative opioid side effects were significantly lower in DB group when compared to other groups (B and S groups). There were no additional intraoperative dexmedetomidine side effects as bradycardia and hypotension. The estimated effect-size r was − 0.58 and Cohen’s d was − 1.46.
Conclusion: Addition of dexmedetomidine to bupivacaine 0.25% in ESPB for postoperative pain control in patients of posterior lumbosacral spine fixation surgeries resulted in lower active and passive VAS pain scores, decreased postoperative opioid consumption, need for rescue analgesia and postoperative opioid side effects without additional intraoperative dexmedetomidine side effects.
Clinicaltrials.gov Identifier: NCT05590234.
Keywords: lumbosacral spine fixation surgeries, erector spinae plane block, ESPB, dexmedetomidine, postoperative analgesia, opioid consumption
Introduction
Lumbosacral spine fixation surgeries are among the most common spinal surgical operations done by neurosurgeons and spine surgeons nowadays. These procedures are done due to different causes, which may be congenital, degenerative, neoplastic, or spine trauma. The underlying purpose of this surgery is to stabilize and fuse the lumbosacral spine, reduce the patients’ lower back pain, improve their quality of life, and return them to daily life activities in a short time.1,2
The spine instrumentations in this surgery lead to severe postoperative pain that annoys most patients and may lengthen the hospital stay and prolong the rehabilitation time.3 The postoperative pain seen immediately after lumbosacral fixation surgery is either musculoskeletal pain or acute inflammatory pain that starts with the inflammatory tissue response and decreases over time with wound healing, with a risk of becoming chronic low back pain if not treated early and adequately.4
Multimodal analgesia techniques are applied to provide adequate postoperative analgesia and pain control. The traditional use of opioid-based analgesia techniques is usually accompanied by some annoying adverse side effects, such as nausea, vomiting, pruritus, and respiratory depression. Although epidural analgesia is considered the gold standard approach for control of postoperative pain and proper postoperative analgesia, in the lumbosacral spine fixation surgery, the insertion of epidural catheter preoperatively or intraoperatively is not applicable as this will interfere with surgical field, dural tear may occur during surgery leading to leakage of the local anesthetic and also still the epidural catheter carry the risk of infections, hematomas, and other adverse effects.5–8
Ultrasound-guided plane blocks using local anesthetics are standard multimodal analgesia techniques due to their low complication rate, ease of application, and adequate postoperative analgesia.9 Followed by the first documentation by Forero et al in 2016, the erector spine plane block [ESPB] has been used to provide postoperative analgesia in thoracic and thoracoabdominal surgeries, bariatric surgery, and recently, ESPB has also been used to provide adequate postoperative analgesia in spine surgery. Ultrasound-guided ESPB is considered a novel interfascial plane block technique in which local anesthetic is injected into the fascial plane, which is localized anatomically between the transverse process of the spinal vertebrae and the erector spinal muscles, and it is considered a safe, simple procedure used to perform adequate postoperative analgesia.10,11
The mechanism of action of ultrasound guided ESPB is still not understood and unclear, but it can block the sensory dorsal ramus of the spinal nerve and provide a paraspinal effect by diffusing the local anesthetic into the back muscles.12
Many studies and case reports have reported that ultrasound-guided ESPB leads to adequate and effective postoperative analgesia management in spinal surgeries. However, ESPB makes short postoperative analgesia and pain control not exceeding 6 or 8 hours, even using medium or long-acting local anesthetics.13–15
Dexmedetomidine is a highly selective alpha 2-adrenoceptor agonist with sedative, anxiolytic, sympatholytic, and analgesic-sparing effects and minimal depression of respiratory function.16 Dexmedetomidine acts on pre-, and post-synaptic sympathetic nerve terminals and the central nervous system, decreasing the sympathetic outflow and noradrenaline release and causing sedation, anxiolytic, analgesic, and sympatholytic effects. It lacks opioid-like properties, so opioid-related adverse side effects are not found.17
Recently, the use of dexmedetomidine as a local anesthetic adjuvant raised the interest issue of regional anesthesia and analgesia due to many causes. It shortens the onset time of anesthesia, extending the duration of peripheral nerve block, decreasing the postoperative pain with maximal analgesic effect, and with acceptable adverse effects.18–23
Study Hypothesis and Aim
As posterior lumbosacral spine fixation surgeries are common spine procedures done nowadays due to different causes and as this procedure is mostly accompanied by moderate to severe postoperative pain, it is necessary to find effective and efficient postoperative analgesia for the patients. This study aimed to compare the analgesic effect of dexmedetomidine combined with bupivacaine versus bupivacaine alone for ultrasound-guided ESPB (as a part of a multimodal analgesic approach) for postoperative pain control of posterior lumbosacral spine fixation surgeries.
Materials and Methods
Participants
Patients above 18 years old of either sex submitted for elective posterior lumbosacral spine fixation and fusion surgery were eligible to participate if they were classified as ASA I–III according to the American Society of Anesthesiologists (ASA) classification. The surgery was done on all the patients by the same neurosurgical team and using the same surgical techniques. The exclusion criteria included patient refusal, hypersensitivity to the drugs used in the study, patients with any contraindication to regional anesthesia, such as skin infections at the site of the block, and patients with a history of bleeding disorders or receiving anticoagulant medications. All patients were screened for eligibility criteria. Eligible patients were asked for voluntary informed consent to participate in the trial.
Study Design
This double-blind, randomized controlled trial was conducted on 90 patients on October 6 University Hospitals. It was conducted from November 2022 to July 2023. The study was approved by the Clinical Research Ethical Committee of October 6 University Hospitals (approval number PRC-Me-2210035). It was registered online at ClinicalTrials.gov Identifier: NCT05590234. All patients who participated in this study had to sign a detailed, informed, written anesthesia and surgery consent.
Randomization, Allocation, and Concealment
The study was conducted in a double-blind fashion where the attending anesthetist who performed the ESPB and the neurosurgical team were blind entirely to the content of the injected solution composition. The responsible anesthetist in this study was the only one not blind to the injected solution composition but was not involved in the postoperative patient assessment.
Patients were randomly allocated using a random allocation sequence by a web-based program. Participants were randomized to three groups in a 1:1:1 ratio. Each group consisted of 30 patients. Dexmedetomidine Bupivacaine ESPB group (DB group) receive 0.5 ug/kg dexmedetomidine plus 20 mL of bupivacaine 0.25%, Bupivacaine ESPB group (B group) receive 20 mL of bupivacaine 0.25%, and Saline ESPB (S group) which is the Control group receive 20 mL of normal saline 0.9%. The block procedure was done bilaterally in the three groups. Participants were randomly allocated to groups using opaque envelopes. These envelopes were opened right before the nerve block by the responsible anesthetist. Neither patients nor investigators knew the group in which the patients were placed and the type of intervention received.
Preoperative Management
All patients were assessed clinically before surgery, including full history, thorough clinical examination, and laboratory investigations. On arrival at the operating suite, patients were fully monitored (5 leads ECG, noninvasive blood pressure, and pulse oximetry). Basal readings of vital data were recorded. Intravenous access was established.
Anesthetic Management
Midazolam 3 mg IV was given for sedation. Induction of anesthesia was performed with IV propofol (2mg/kg), fentanyl (1.5–2 µg/kg), and rocuronium bromide (0.6 mg/kg). Maintenance of anesthesia was done by isoflurane. The prone position was established immediately after the intubation. Intraoperatively, the data (peripheral oxygen saturation, heart rate, noninvasive arterial blood pressure, and end-tidal carbon dioxide level) were recorded every five minutes throughout the operation.
Any decrease in heart rate below 50 beats per minute was treated with intravenous atropine, according to the response. A reduction in mean blood pressure below 20% of the basal reading or systolic BP below 90 mmHg was treated with 5 mg increments of intravenous ephedrine. After the induction of anesthesia and putting the patients in the prone position, the US-guided ESPB was performed in the three groups.
ESPB Technique
Under strict aseptic technique, the ESPB was done in the prone position for all patients of the three groups. A sterile ultrasound curved probe (Philips ultrasound machine HD7 XE C153160013, Philips and Neusoft Medical Systems Co., Ltd. China.) was used for the technique. The ultrasound probe was placed on the third lumbar vertebral body level in the parasagittal plane. When the spinous process was first seen, the probe was moved laterally from the midline, and then the transverse process (TP) of L3 and erector spinae muscle was observed at about 2 to 3 cm from the midline (Figure 1).
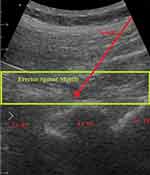 |
Figure 1 Ultrasound image of ESPB. |
Using the in-plane technique, a 22 gauge/8 cm ultrasound-visible needle (Stimuplex, Braun AG, Melsungen, Germany) was used to make a puncture. The direction of the needle was craniocaudal, and the proper position of the needle was confirmed by injecting 2 mL of saline solution. After ensuring the position of the needle, 20 mL of 0.25% bupivacaine plus 0.5 ug/kg dexmedetomidine was administered to the DB group. The same ESPB procedure was done on the other side with the same drugs and volume. In total, 40 mL of 0.25% bupivacaine plus 1 ug/kg dexmedetomidine was administered.
The same block procedure was done in the B group with only 20 mL of 0.25% bupivacaine on both sides (with a total volume of 40 mL). In the S group (control group), the same block procedure was done with 20 mL of 0.9% normal saline on both sides (40 mL total volume).
At the end of surgery, 1 gm of paracetamol IV and 30 mg of ketorolac IV infusion were given to all the patients of the three groups. The patients were extubated after efficient spontaneous breathing and transported to the post-anesthesia care unit (PACU). Patients were discharged from the PACU to the ward with a modified Aldrete score of 12.
Postoperative Pain Management
The postoperative analgesic management was done using the classical protocol of our department to all patients of the three groups, which included a PCA (patient-controlled analgesia) device, and an IV of one gm paracetamol was given every eight hours for the first 48 hours postoperatively. The PCA device (Accufuser Plus ®REF, manufactured by Woo Young Medical Co., Ltd. Korea) has a silicon balloon infuser with a total volume of 300 mL, basal rate of 5 mL per hour, bolus 1 mL, and lockout interval every 15 minutes.
A PCA device with morphine was attached to all the patients. The PCA infusion (300mL) consisted of 60 mg of morphine with 2 mg of granisetron and 180 mg of ketorolac. The concentration of morphine was set to 0.2 mg/mL, the loading dose was 1 mg (5 mL), the lockout interval was 15 min (bolus of 1mL/15 min), and a 1 mg/h (5mL/hr) continuous infusion was maintained for 48 hrs. IV one gm paracetamol was given every eight hours postoperatively.
A nurse blind to the study used the visual analog scale (VAS; zero, no pain, ten = the most severe pain) to evaluate and record the pain scores and recorded the opioid consumption of the patients. The nurse recorded passive (at rest) and active (while mobilization) VAS scores at intervals of every two hours in the first 24 hours and every 4 hours in the second 24 hours and total opioid consumption for the first 48 hours postoperatively. Rescue analgesic medication was done using pethidine 50 mg IM when passive VAS pain score >6.
Patients who experienced pruritus, nausea, vomiting, or respiratory depression (opioid-related adverse effects) were recorded. On the first day postoperative, all patients were encouraged to ambulate in the ward after wearing lumbosacral support. The subcutaneous closed suction drainage system was removed after 36 hours. All patients were discharged home after 48 hours.
Outcome Measurements
The primary clinical outcome measures at the first 24 hours were active (while mobilization) and passive (at rest) visual analog scale (VAS) pain score, measured every 2 hours, opioid consumption (the number of PCA presses), and the need for rescue analgesia. Other clinical outcomes included active and passive visual analog scale (VAS) pain score at the second 24 hours, measured every 4 hours, opioid consumption (the number of PCA presses), the need for rescue analgesia, postoperative opioid side effects, and intraoperative dexmedetomidine side effects as bradycardia and hypotension. VAS pain score was classified into two severity categories: mild\moderate (VAS 0–6) and severe (VAS>6)24
Sample Size Calculation
As we have three treatment groups (S, B, DB), we performed a one-way analysis of variance (ANOVA) to assess effect size and the required number of patients per group from previous study based on the overall difference of opioid consumption (µg) means25 Given the following values: α=0.05, β=0.05 (implying a power of 0.95). Then, the required sample size per group is 17 Considering 25% dropout, each group should include 22 patients. In addition, Cohen’s d was −1.46 and effect-size r was −0.58
Statistical Analysis
Data were analyzed using the statistical package for social sciences, version 20.0 (SPSS Inc., Chicago, Illinois, USA). We conducted the Shapiro–Wilk test to assess whether the numeric data followed a normal distribution. Quantitative normally distributed data were expressed as mean± standard deviation (SD). Qualitative data were expressed as frequency and percentage. The following tests were used: A one-way analysis of variance (ANOVA) when comparing between more than two means, Post Hoc test: Least Significant Difference (LSD) was used for multiple comparisons between different variables, and Chi-square (X2) test of significance was used to compare proportions between two qualitative parameters. Furthermore, we performed linear and binary regression analyses to elucidate the relationship between the primary outcomes and the intervention groups (S, D, DB).
The confidence interval was set to 95%, and the margin of error accepted was set to 5%. So, the p-value was considered non-significant when the P-value > 0.05, significant if < 0.05, and highly significant if <0.01.
Results
A total of 95 patients were assessed for eligibility criteria. Five patients were excluded; the remaining 90 patients were randomized equally into the DB group (30), B group (30), and S group, which is the control group (30), as shown in Figure 2. The three studied groups were comparable regarding the demographic data (age and sex) without any significant difference between the three groups. No patients in any of the studied groups had a history of drug abuse or addiction (Table 1).
 |
Table 1 Sociodemographic Data of the Studied Groups |
 |
Figure 2 Flow diagram of patient’s enrollment. |
As regards the passive VAS score in the first 24 hours, there was no statistically significant difference between the three groups in the first two hours (Hr 2) postoperatively. In the second and third two hours (Hr 4 and Hr 6) postoperatively, there was no statistically significant difference between the DB group and the B group. However, there was a statistically significant difference when comparing the DB group with the S group in (Hr 4) with P-value 0.01 and a highly statistically significant difference in (Hr 6) with P-value 0.003.
In the fourth, fifth, and sixth two hours (Hr 8, Hr 10, and Hr12), there was a statistically significant difference by comparing the DB group with the B group, and there was a highly statistically significant difference between the DB group and S group with P-value <0.001. For the rest of the first 24 hours (Hr14, Hr16, Hr18, Hr 20, Hr 22, and Hr 24), there was a highly statistically significant difference by comparing the DB group with the B group. There was also a highly statistically significant difference when comparing the DB group with the S group with a P-value <0.001 (Figure 3).
As regards the active VAS score in the first 24 hours, in the first two hours (Hr 2) postoperatively, there was no statistically significant difference between the three groups. There was no statistically significant difference between the DB and B groups in the second and third two hours (Hr 4 and Hr 6). However, there was a highly statistically significant difference when comparing the DB group by S group with a P-value <0.001.
In the fourth two hours (Hr 8) postoperatively, there was a statistically significant difference between the DB group and B group and a highly statistically significant difference when comparing the DB group with the S group with a P value of 0.006. For the rest of the first 24 hours (Hr 10, Hr12, Hr14, Hr16, Hr18, Hr 20, Hr 22, and Hr 24), there was a highly statistically significant difference by comparing the DB group with the B group and DB group with S group with P-value <0.001 (Figure 4).
The passive VAS score in the second 24 hours, In the first, second, and third four hours (Hr 4, Hr 8, and Hr 12), there was highly statistically significant difference when comparing the DB group with the B group and when comparing the DB group with S group with P-value 0.002. In the fourth four hours (Hr 16), there was a statistically significant difference when comparing the DB group with the B group and a highly statistically significant difference when comparing the DB group with the S group with a P value of 0.019. In the fifth and sixth four hours (Hr 20 and Hr 24), there was no statistically significant difference when comparing the DB group with the B group and the DB group with the S group with a P-value of 0.207 (Figure 5).
In the first four hours of the active VAS score in the second 24 hours (Hr 4), there was a highly statistically significant difference when comparing the DB group with the B group and the DB group with the S group with P-value 0.002. In the second and third four hours (Hr 8 and Hr 12), there was a statistically significant difference when comparing the DB group with the B group and the DB group with the S group with a P-value <0.05.
In the fourth, fifth, and sixth four hours (Hr 16, Hr 20, and Hr 24), there was no statistically significant difference between the three groups (Figure 6).
Regarding the rescue analgesic medication (Pethidine 50mg IM when VAS pain score > 6), in the first 24 hours, there was a highly statistically significant difference according to the use of rescue analgesia when comparing DB group with B group (p-value 0.003) and when comparing DB group with S group (P-value < 0.00).
In the second 24 hours, there was highly statistically significant difference according to the rescue analgesia by comparing DB group with B group (P-value 0.006) and a statistically significant difference between DB group with S group (p-value 0.01) (Table 2).
 |
Table 2 Rescue Analgesia in 1st and 2nd 24 Hours |
According to the total postoperative opioid (morphine) consumption, which was expressed as a number of PCA presses, there was a highly statistically significant difference when comparing DB group with B group and when comparing DB group with S Group in the first 24 and second 24 hours postoperative (P-value < 0.00) (Table 3).
 |
Table 3 Total Postoperative Opioid Consumption (Number of PCA Presses) |
As regards the intraoperative findings like bradycardia and hypotension between the three groups, there was no statistically significant difference between the three groups according to the intraoperative findings with a p-value > 0.05 (Table 4).
 |
Table 4 Intraoperative and Postoperative Complications |
According to postoperative complications, including nausea, vomiting, and pruritus in the first 48 hours postoperatively, there was a high Statistical significance difference between DB and B group regarding nausea, vomiting, and pruritus (P-value 0.001, 0.001, and 0.003), respectively.
There was a high statistical significance difference between DB and S group regarding nausea, vomiting, and pruritus (P-value < 0.00, 0.00, and 0.001), respectively (Table 4).
Binary regression analysis did not reveal any significant differences between the B and DB groups compared to the S groups concerning the presence of high levels of active and passive VAS scores (above 6) during the first 24 hours and the second 24 hours (Table 5). However, linear regression analysis indicated that the DB group exhibited a statistically significantly lower score for the number of PCA presses in both the first and second 24 hours, as well as a lower frequency of rescue analgesia administration during the same periods compared to the other groups (Table 5).
 |
Table 5 Regression Analysis Between Primary Outcomes and Studied Groups (S, B, DB) |
Discussion
Lumbosacral spine fixation surgeries are one of the most common spinal surgical procedures done nowadays. It is accompanied by severe annoying postoperative pain that may lengthen the hospital stay and delay the return to normal daily life activities. It was a must to provide adequate, effective, nontraditional postoperative analgesic techniques.1 After the first documentation by Forero et al in 2016, the ESPB has been used to provide postoperative analgesia in thoracic and thoracoabdominal surgeries, bariatric surgery and recently, ESPB has also been used to provide adequate postoperative analgesia in spine surgery.10,11 However, the use of ESPB makes a short postoperative analgesia and pain control not exceeding 6 or 8 hours even with the use of medium or long-acting local anesthetics.13–15
In this study, we studied and evaluated the additional analgesic effects of dexmedetomidine as an adjuvant to local anesthetic (Bupivacaine) to prolong the postoperative analgesic effect of ESPB in lumbosacral spine fixation surgeries.
To our knowledge, there are many previous studies which had revealed that a variety of adjuvants added to local anesthetics in various regional anesthetic techniques had potent added analgesic effects, but there has been only some clinical research about the addition of dexmedetomidine as a local anesthetic adjuvant in ESPB, and also a few have satisfactorily assessed the quality of postoperative recovery.26
Yi-han et al, found that dexmedetomidine added to ropivacaine in ESPB could notably reduce postoperative pain and had a better analgesic effect at 12, 24 and 48 h after surgery and can decrease the opioid consumption in patients undergoing posterior lumbar spine surgery without obvious adverse side effects as a local anesthetic adjuvant.27
Gao et al reported that the ESPB block time could be lengthened by approximately 120% by using dexmedetomidine (1 µg/kg) with 0.5% ropivacaine in combination with video-assisted thoracoscopic lobectomy surgery.28
Wang et al revealed that the addition of 1 μg/kg of dexmedetomidine with 0.33% ropivacaine in ESPB in patients undergoing modified radical mastectomy might better provide postoperative analgesia and pain control than ropivacaine alone, thus improving postoperative analgesia and pain control.29
Wang et al did randomized controlled clinical research to study the effects of the addition of dexmedetomidine as an adjuvant to ropivacaine for ultrasound-guided ESPB for patients with cancer esophagus undergoing open thoracotomy. They discovered that the addition of dexmedetomidine to ropivacaine for ESPB effectively prolonged the postoperative analgesia duration and reduced opioid consumption without additional side effects.15
In addition, the results of our study were analogous to the results of several other clinical researches that studied the effect of adding dexmedetomidine to local anesthetic in other nerve blocks other than ESPB and they all showed that addition of dexmedetomidine to local anesthetic had prolonged the block time and reduced the number of PCA compressions and the need for postoperative rescue analgesia such as Almarakbi et al, who studied the effects of addition of dexmedetomidine to bupivacaine in transversus abdominis plane block in patients undergoing abdominal hysterectomy and they noticed that addition of dexmedetomidine to the block had effectively prolonged the duration of postoperative analgesia without major adverse effects.30 Also, Rancourt et al studied the effect of adding dexmedetomidine to ropivacaine in posterior tibial nerve sensory blockade, and they noted a prolongation of the duration of the block.22
In our study, we noted that a dose of 1µg/kg of dexmedetomidine, when added to bupivacaine 0.25% in ESPB, was safe and not accompanied by significant fluctuations in blood pressure or heart rate. However, there are many studies showed that dexmedetomidine is not safe at all doses and at certain doses can cause systemic side effects as Esmaoglu et al who did a clinical research about the effect of dexmedetomidine added to levobupivacaine in prolonging axillary brachial plexus block, and they found that adding 100 µg of dexmedetomidine to local anesthetic shortened the onset time and prolonged the duration of the block and postoperative analgesia but resulted in significant postoperative bradycardia.31
Also, Hussain et al revealed that dexmedetomidine increase postoperative analgesia and prolonged the brachial plexus block time but significantly increase the possibility of intraoperative bradycardia when dexmedetomidine was injected perineurally at a dose of more than 50µg.32
Limitations
We had a limitation in our study that we studied a single dose of the tested drug (1ug/kg) of dexmedetomidine, and it was limited only to posterior lumbosacral spine fixation procedures. Also, our study encountered a limitation in reporting (VAS) severity scores categorized as mild/moderate (Less or equal to 6) and severe (above 6) rather than continuous scores.
Conclusion
Dexmedetomidine, as an adjuvant to bupivacaine used in ESPB for postoperative pain control of posterior lumbosacral spine fixation procedures had significantly prolonged the efficacy and time of analgesia of ESPB (up to 36 hours), reduced passive and active VAS score, reduced the need for rescue analgesia, reduced numbers of PCA presses (opioid consumption) and decreased postoperative opioid side effects without additional intraoperative dexmedetomidine adverse effects in the first 48 hours.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of October 6 University Hospitals (approval number PRC-Me-2210035). It was registered online at ClinicalTrials.gov Identifier: NCT05590234.
Informed Consent Statement
A written informed consent was obtained from all subjects involved in the study.
Data Sharing Statement
The analyzed data is available at the following link: https://zenodo.org/records/10407181.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number RGP2/145/44
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number RGP2/145/44.
Disclosure
The authors declare no conflicts of interest in this study.
References
1. Lee BH, Park JO, Suk KS, et al. Pre-emptive and multimodal perioperative pain management may improve quality of life in patients undergoing spinal surgery. Pain Phys J. 2013;16:217–226.
2. Derbent A, Yilmaz B, Uyar M, et al. Chronic pain following spine surgery. J Turk Soc Algol. 2012;24:1–9. doi:10.5505/agri.2012.49368
3. Devin CJ, Mcgirt MJ. Best evidence in multimodal pain management in spine surgery and means of assessing postoperative pain and functional outcomes. J Clin Neurosci. 2015;22:930–938. doi:10.1016/j.jocn.2015.01.003
4. Atabey C, Zorlu E, Kurt H, et al.The cheapest way of the pain management after lumbar spinal surgical procedures: cold pack application. Gulhane Med J. 2016;58:33–36. doi:10.5455/gulhane.195850
5. Cohen BE, Hartman MB, Wade JT, et al. Postoperative pain control after lumbar spine fusion: patient-controlled analgesia versus continuous epidural analgesia. Spine. 1997;22:1892–1897. doi:10.1097/00007632-199708150-00016
6. Gottschalk A, Freitag M, Tank S, et al. Quality of postoperative pain using an intraoperatively placed epidural catheter after major lumbar surgery. Anesthesiology. 2004;101:175–180. doi:10.1097/00000542-200407000-00027
7. Naik BI, Nemergut EC, Kazemi A, et al. The effect of dexmedetomidine on postoperative opioid consumption and pain after major spine surgery. Anesth Analg J. 2016;122:1646–1653. doi:10.1213/ANE.0000000000001226
8. Gessler F, Mutlak H, Tizi K, et al. Postoperative patient controlled epidural analgesia in patients with spondylodiscitis and posterior spinal fusion surgery. J Neurosurg Spine. 2016;24:965–970. doi:10.3171/2015.8.SPINE15415
9. Chin KJ, McDonnell JG, Carvalho B, et al. Essentials of our current understanding: abdominal wall blocks. Regional Anesth Pain Med J. 2017;42:133–183. doi:10.1097/AAP.0000000000000545
10. Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med J. 2016;41:621–627. doi:10.1097/AAP.0000000000000451
11. Almeida CR, Oliveira AR, Cunha P, et al. Continuous bilateral erector of spine plane block at T8 for extensive lumbar spine fusion surgery: case report. Pain Pract J. 2019;19:536–540. doi:10.1111/papr.12774
12. Yang HM, Choi YJ, Kwon HJ, et al. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anesthesia. 2018;73:1244–1250. doi:10.1111/anae.14408
13. Melvin JP, Schrot RJ, Chu GM, et al. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: a case series. Can J Anaesth. 2018;65(9):1057. doi:10.1007/s12630-018-1145-8
14. Singh S, Chaudhary NK. Bilateral ultrasound guided erector spinae plane block for postoperative pain management in lumbar spine surgery: a case series. J Neurosurg Anesthesiol. 2019;31(3):354. doi:10.1097/ANA.0000000000000518
15. Wang Q, Li H, Wei S, et al. Dexmedetomidine added to ropivacaine for ultrasound-guided erector spinae plane block prolongs analgesia duration and reduces perioperative opioid consumption after thoracotomy: a randomized controlled clinical study. Clin J Pain. 2021;38(1):8–14. doi:10.1097/AJP.0000000000000992
16. Weerink M, Struys M, Hannivoort L, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Internat J Clin Anesthesiol. 2017;35:15–19.
17. Mishra PR, Mishra SK, Sarkar K, et al. Comparative study of intrathecal dexmedetomidine vs fentanyl as an adjuvant to hyperbaric bupivacaine. Anna Internat Med Dent Res. 2017;3(3):28–31. doi:10.21276/aimdr.2017.3.3.AN5
18. Chen Z, Liu Z, Feng C, Jin Y, Zhao X. Dexmedetomidine as an adjuvant in peripheral nerve block. Drug Des Devel Ther. 2023;17:1463–1484. doi:10.2147/DDDT.S405294
19. Ping Y, Ye Q, Wang W, Ye P, You Z. Dexmedetomidine as an adjuvant to local anesthetics in brachial plexus blocks: a meta-analysis of randomized controlled trials. Medicine. 2017;96(4):e5846. doi:10.1097/MD.0000000000005846
20. Marhofer D, Kettner SC, Marhofer P, et al. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: a volunteer study. Br J Anaesth. 2013;110:438–442. doi:10.1093/bja/aes400
21. Wu HH, Wang HT, Jin JJ, et al. Does dexmedetomidine as a neuraxial adjuvant facilitate better anesthesia and analgesia? A systematic review and meta-analysis. PLoS One. 2014;9:e93114. doi:10.1371/journal.pone.0093114
22. Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115(4):958–962. doi:10.1213/ANE.0b013e318265bab7
23. Singh N, Gupta S, Kathuria S. Dexmedetomidine vs dexamethasone as an adjuvant to 0.5% ropivacaine in ultrasound-guided supraclavicular brachial plexus block. J Anaesthesiol Clin Pharmacol. 2020;36(2):238–243. doi:10.4103/joacp.JOACP_176_19
24. Jones Katherine R, Vojir CP, Hutt E, et al. Determining mild, moderate, and severe pain equivalency across pain-intensity tools in nursing home residents. J Rehabil Res Dev. 2007;44(2):305. doi:10.1682/JRRD.2006.05.0051
25. Alimian M, Imani F, Rahimzadeh P, Faiz SHR, Bahari-Sejahrood L, Hertling A. Adding dexmedetomidine to bupivacaine in ultrasound-guided thoracic paravertebral block for pain management after upper abdominal surgery: a double-blind randomized controlled trial. Anesth Pain Med. 2021;11(6):e120787. doi:10.5812/aapm.120787
26. Heesen M, Klimek M, Imberger G, et al. Co-administration of dexamethasone with peripheral nerve block: intravenous versus perineural application: systematic review, meta-analysis, metaregression and trial-sequential analysis. Brit J Anesth. 2018;120(2):212–227. doi:10.1016/j.bja.2017.11.062
27. Yi-han W, Rong T, Jun L, et al. Dexmedetomidine combined with ropivacaine for erector spinae plane block after posterior lumbar spine surgery: a randomized controlled trial. BMC Musculosk Dis. 2022;23:235. doi:10.1186/s12891-022-05198-9
28. Gao Z, Xiao Y, Wang Q, et al. Comparison of dexmedetomidine and dexamethasone as adjuvant for ropivacaine in ultrasound-guided erector spinae plane block for video-assisted thoracoscopic lobectomy surgery: a randomized, double-blind, placebo-controlled trial. Ann Translat Med. 2019;7(22):668. doi:10.21037/atm.2019.10.74
29. Wang X, Ran G, Chen X, et al. The effect of ultrasound-guided erector spinae plane block combined with dexmedetomidine on postoperative analgesia in patients undergoing modified radical mastectomy: a randomized controlled trial. Pain Therapy. 2021;10(1):475–484. doi:10.1007/s40122-020-00234-9
30. Almarakbi WA, Kaki AM. Addition of dexmedetomidine to bupivacaine in transversus abdominis plane block potentiates post-operative pain relief among abdominal hysterectomy patients: a prospective randomized controlled trial. Saudi J Anaesth. 2014;8(2):161–166. doi:10.4103/1658-354X.130683
31. Esmaoglu A, Yegenoglu F, Akin A, et al. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesthesia Analg. 2010;111(6):1548–1551. doi:10.1213/ANE.0b013e3181fa3095
32. Hussain N, Grzywacz VP, Ferreri CA, et al. Investigating the efficacy of dexmedetomidine as an adjuvant to local anesthesia in brachial plexus block: a systematic review and metanalysis of 18 randomized controlled trials. Reg Anesth Pain Med. 2017;42(2):184–196. doi:10.1097/AAP.0000000000000564
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




