Back to Journals » International Medical Case Reports Journal » Volume 17
Clozapine-Induced Severe Toxicity: Exploring the Pharmacokinetic Profile of Clozapine and Its Significance in Hemodynamic Instability – A Case Report
Authors Yu ZX, Pi Y, Chen MK, Dong DJ, Gu Q
Received 14 October 2023
Accepted for publication 1 February 2024
Published 8 February 2024 Volume 2024:17 Pages 111—120
DOI https://doi.org/10.2147/IMCRJ.S444685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Zhu-Xi Yu, Yang Pi, Mei-Kai Chen, Dan-Jiang Dong, Qin Gu
Department of Critical Care Medicine, the Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, Jiangsu, 21008, People’s Republic of China
Correspondence: Qin Gu, Department of Critical Care Medicine, the Affiliated Drum Tower Hospital of Nanjing University Medical school, Nanjing, Jiangsu, 21008, People’s Republic of China, Tel +86-13705150348, Email [email protected]
Abstract: Hemodynamic instability in patients with clozapine intoxication can indirectly reflect the serum concentration of clozapine.We have described a case of a 32-year-old pregnant woman who developed life-threatening clozapine toxicity at 28 weeks of gestation. The levels of clozapine and norclozapine in the serum were high. We initiated hemoperfusion(HP) and other detoxification therapies to remove the drug. The patient had severely dilated peripheral blood vessels, which led to cardiac symptoms such as fatal hypotension and uncontrollable tachycardia, resulting in very high cardiac output and elevated Central venous oxygen saturation (ScvO2). Pharmacological intervention significantly improved the hemodynamics.In light of our observations in the ongoing case, we posit that evaluating hemodynamic parameters before and after blood detoxification could serve as a valuable means to gauge effectiveness and provide guidance for treatment.
Keywords: clozapine, toxicity, pulse index continuous cardiac output, PICCO, hemoperfusion, HP
Background
Clozapine is an atypical antipsychotic used for the treatment of schizophrenia. One an average, two cases of clozapine intoxication are reported each year, of which some have fatal outcome. The common symptoms of clozapine poisoning are seizures, ileus, somnolence, hypotension and cardiac arrhythmia.1 In this report, we present the case of a patient who ingested 5 g clozapine, resulting in maximum measured concentration of 5930.75ng/mL, and exhibited significant hemodynamic disorders. To gain a deeper understanding of clozapine poisoning and its clinical management, it is important to consider the pharmacokinetics of clozapine. This includes examining the processes of absorption, metabolism, and excretion that occur after ingestion, as well as their correlation with hemodynamic instability. By discussing these aspects, we can enhance our knowledge of clozapine poisoning and improve its clinical management.
Case Presentation
History of Presenting Complaint
A 32-year-old pregnant woman diagnosed with paranoid schizophrenia was admitted to the emergency room at a hospital in Anhui province, China after ingesting a toxic dose of 5 g (200*25 mg) of clozapine. The drug was taken approximately 4 hours before admission. The patient was febrile (38.5°C) and in coma on admission, and had an elevated respiratory rate (26 per minute) and tachycardia (146 per minute). The patient was treated with endotracheal intubation, gastric lavage, fluid resuscitation, etc. She was transferred to our hospital 7 hours after clozapine ingestion, and transferred to the intensive care unit (ICU) 3 hours later.
Past Psychiatric History
The patient had a 14-years-long history of schizophrenia, and had been taking clozapine 100mg/d, aripiprazole 20mg/d and lithium carbonate (the dose is unknown).She has not lost weight, and the dosage has not been adjusted with the help of a doctor. She is 28 weeks pregnant at present and has been prescribed clozapine 50mg/d and aripiprazole 20mg/d. The patient had overdosed on clozapine twice in the past.
Progress During ICU Admission
The patient was in coma upon arrival with fluctuating consciousness, and was placed under sedation. Initial examination in the ICU revealed Glasgow score of 5, blood pressure 113/60 mmHg, heart rate 129 beats per minute (bpm), respiratory rate 16 breaths per minute, and rectal temperature 38.5°C. Physical examination did not yield any substantial information. Laboratory tests revealed high leukocyte count (10.4*10^9/L) and neutrophilia (9.4*10^9/L), anemia (hemoglobin 110g/L), hyponatremia (129.4mmol/L), hypokalemia (3.42mmol/L), hypoproteinemia (34g/L), and elevated procalcitonin (PCT) (0.956ng/mL), interleukin-6 (IL-6) (459.48pg/mL), D-dimer (11.55mg/L) and troponin T (TNT) (0.082ug/L). Arterial blood gas analysis indicated hypoxemia and metabolic acidosis with pH 7.441, PCO2 25.3 mmHg, PO2 71.6 mmHg, Lac 4mmol/L, HCO3 17.4mmol/L and BE 4.7mmol/L. The electroencephalogram (ECG) showed sinus tachycardia, with heart rate 137 bpm and QT interval 421 ms (Figure 1).
 |
Figure 1 Electrocardiography of the patient on admission. |
The patient remained unconscious with minimal sedation throughout the first day of her stay in the ICU, and was put on mechanical ventilation. Routine supportive measures were taken, such as continuous ECG and respiratory monitoring, maintenance of fluid and electrolyte balance, diuretics, and protection of liver and kidney function. The patient was hemodynamically stable and required modest vasopressors, including esmolol, norepinephrine, and terlipressin, to maintain blood pressure. The first hemoperfusion (HP) was performed 2 hours after admission (about 12 hours after taking clozapine). Blood samples were drawn to measure serum levels of clozapine and its metabolite norclozapine, which were 5930.75ng/mL and 1048.47ng/mL respectively.
A febrile episode of 39.1°C was recorded the following day (about 24 hours after taking clozapine). The patient also developed fatal hemodynamic instability with blood pressure 85–93/39-43 mmHg, heart rate 145–160 bpm, and respiratory rate 26 breaths per minute. Arterial blood gas analysis revealed pH 7.411, PCO2 21.5 mmHg, PO2 94.4 mmHg, Na+ 140mmol/L, K+ 3.07mmol/L, Lac 1mmol/L and BE 8.9mmol/L, and ScvO2 measurement showed pH 7.394, PCO2 25.6 mmHg, PO2 46.5 mmHg, SO2 81%, Na+ 140.1mmol/L, K+ 3.66mmol/L, Lac1.0mmol/L, BE-7.3mmol/L. The ECG showed sinus tachycardia, with heart rate of 127bpm and QT interval 406ms (Figure 2). Pulse index continuous cardiac output (PICCO) monitoring is useful for determining the fluid infusion volume for patients with shock, and is evaluated in terms of cardiac index (CI), central venous pressure (CVP), global end diastolic volume index (GEDI), global ejection fraction (GEF), intrapleural blood volume index (ITBVI), extravascular lung water index (EVLWI) and systemic vascular resistance index (SVRI). The first PICCO hemodynamic monitoring showed that CI was significantly higher, SVRI was significantly lower, and ITBVI and EVLWI were higher (Table 1). Accordingly, the patient was given esmolol (0.1mg/kg/min) to control heart rate, and the combination of norepinephrine (2µg/kg/min) and terlipressin (0.03u/min) to increase peripheral vascular resistance. The dosage of the vasopressors drugs was adjusted according to PICCO results (Table 2).
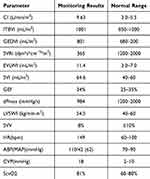 |
Table 1 The First PICCO Hemodynamic Monitoring Results |
 |
Table 2 The Patients Were Monitored by PICCO Hemodynamics and the Dosage of the Vasopressors Drugs |
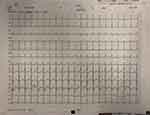 |
Figure 2 Electrocardiography of the patient showing sinus tachycardia about 24 hours after taking clozapine. |
The serum concentrations of clozapine and norclozapine were continuously monitored during hospitalization to evaluate the treatment effect (Figure 3). HP was performed on the 2nd, 3rd, 4th, 8th, 9th and 11th days after admission, and plasma exchange was performed on the 4th and 5th days to remove clozapine and norclozapine from the blood. HP decreased the serum concentration of clozapine and norclozapine. Furthermore, continuous veno-venous hemodiafiltration was performed during the first week of hospitalization to remove any inflammatory mediators and prevent an inflammatory reaction. Symptomatic treatment was also given, such as protection of gastric mucosa, nutritional support and prevention of deep vein thrombosis.
 |
Figure 3 The serum concentrations of clozapine and norclozapine during hospitalization. |
The vasopressor dependency index (VDI)2,3 was used to evaluate the relationship between the vasopressor infusion dose and MAP using the following equation: 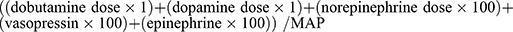 . Epinephrine, norepinephrine, dobutamine, and dopamine are expressed as µg/kg/min and vasopressin as units/min. Consistent with the decline in clozapine, the VDI index gradually decreased during the course of the disease. During drug redistribution however, the clozapine levels recovered and the VDI index increased simultaneously (Table 3 and Figure 4). Thus, there was a positive correlation between VDI index and serum concentration of clozapine (Figure 5).
. Epinephrine, norepinephrine, dobutamine, and dopamine are expressed as µg/kg/min and vasopressin as units/min. Consistent with the decline in clozapine, the VDI index gradually decreased during the course of the disease. During drug redistribution however, the clozapine levels recovered and the VDI index increased simultaneously (Table 3 and Figure 4). Thus, there was a positive correlation between VDI index and serum concentration of clozapine (Figure 5).
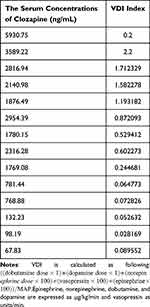 |
Table 3 The Clozapine Levels and the VDI Index During Hospitalization |
 |
Figure 4 The clozapine levels and the VDI index during hospitalization. |
 |
Figure 5 A positive correlation between VDI index and serum concentration of clozapine. |
Progress and Outcome
The patient regained consciousness after three days in the ICU, and was able to obey instructions. In addition, the patient was hemodynamically stable and did not require any vasopressors drugs to maintain her blood pressure after one week. She was extubated on day 7 of ICU admission, and no further respiratory sequelae were observed. At this stage, the serum levels of clozapine and norclozapine decreased to 781.44ng/mL and 1021.32ng/mL respectively. She was discharged after 14 days in the hospital, and the clozapine and norclozapine levels were 67.83 ng/mL and 209.11 ng/mL respectively. Incredibly, her fetus was still alive and there were no signs of labor.
Discussion
Clozapine is a second-generation, antipsychotic, neuroleptic dibenzodiazepine drug that is prescribed for schizophrenia patients who are unresponsive to frequently used antipsychotics or have other psychotic disorders.4 It is the most frequently used antipsychotic drug in mainland China, although its prescription rate seems to have decreased recently.5 In some regions of China, clozapine is prescribed to almost 76.1% of the schizophrenia patients.6 Acute clozapine intoxication is a serious condition with a mortality rate of 12%.3 However, no systematic study so far has focused on the characteristics and outcomes of acute clozapine intoxication.
Clozapine can bind to a multitude of receptors and pharmacologically active metabolites. However, the exact mechanism underlying its antipsychotic effects is unknown. Clozapine has a minor affinity for the dopamine D2 receptor but relatively higher affinity for both D1 and D4 receptors. In addition, clozapine also has a high affinity for the 5-HT2A receptor, along with partial agonistic action, which likely contributes to the low risk of EPS. The strong binding affinity of clozapine for muscarinic receptors, as well as the partial and full agonistic activity of its metabolite desmethylclozapine on some muscarinic receptor subtypes, may contribute its the unique mechanism. Furthermore, clozapine is an agonist of the NMDA receptor glycine site and improves glutamatergic homeostasis through different pathways.7–9
The acceptable serum concentration of clozapine in schizophrenia patients is 350–400ng/mL. However, considerable variations have been observed in the symptomatic responses and side effects of individual patients. Some cases have demonstrated that in other instances of clozapine toxicity, altered consciousness and cardiac arrhythmias may be the predominant manifestations. Although the definite serum concentration of clozapine that is associated with toxicity remains unclear, adverse effects are more likely to occur when the serum levels are above 600–1000ng/mL.10 However, a peak serum level of 24,000ng/mL? is the highest reported dose of clozapine with a non-fatal outcome. Our patient was pregnant and had been taking clozapine 100mg/d over a long period of time. She was brought to the emergency department four hours after ingesting 200 clozapine 25 mg tablets. After hospitalization, the levels of clozapine and norclozapine levels were 5930.75ng/mL and 1048.47ng/mL respectively. Although it is controversial whether prolonged clozapine intake increases the tolerance of patients to acute intoxication, it is reasonable to hypothesize that pre-treatment with clozapine will affect the severity of intoxication. However, a retrospective study of 73 cases with acute clozapine mono-intoxication did not confirm this hypothesis, and showed no significant correlation between clozapine pre-treatment and the severity of intoxication.11
In addition to drug–drug interactions, smoking, caffeine consumption, infection and inflammation contribute to clozapine intoxication.12,13 During pregnancy, the cardiovascular, hematological, digestive and urinary systems undergo considerable changes, which affect drug pharmacokinetics. After 12 weeks of pregnancy, the serum levels of clozapine and norclozapine gradually increase due to the decrease in the concentration of drug binding proteins in the plasma.14 Clozapine use during pregnancy may increase the risk of obesity, hypertension and gestational diabetes.However, the data on common pregnancy and birth complications, such as miscarriage, stillbirth, preterm birth, small for gestational age, neonatal adaption and childhood neurodevelopment, are overall insufficient to provide confident estimates of the effect of taking clozapine during pregnancy.15 Our patient was diagnosed with gestational diabetes, which is consistent with previous studies.16
Patients with clozapine poisoning typically present with altered consciousness ranging from somnolence to coma or agitation, along with delirium, tachycardia, blood pressure abnormalities and seizures. Fatal intoxication may manifest as cardiorespiratory arrest or severe respiratory depression, or may be the consequence of aspiration pneumonia. Cardiac side-effects such as hypotension, tachycardia, myocarditis and prolonged QT interval are also observed with clozapine, which is an atypical neuroleptic.17,18 Blood pressure abnormalities due to clozapine intake are presumably due to its antagonistic effect on alpha-adrenergic receptor and calcium ions, inhibition of the central pressor reflex and weakened myocardial contractility. Peripheral vasodilation and the anticholinergic effect of clozapine can lead to tachycardia. In addition, clozapine affects cardiac electrical activity by blocking the ion channels of myocardial cells, including fast sodium influx channel (FINA), slow calcium influx channel (ICA) and potassium outflow channel (IK), and blocks the fast activation delayed integration potassium (IKr).19,20 Our patient presented with severe tachycardia, fatal hypotension and significantly decreased peripheral vascular resistance, all of which are consistent with clozapine-associated cardiotoxicity. On the basis of hemodynamic monitoring, we gave the patient esmolol, a short acting and highly selective β1 receptor blocker, to reduce heart rate. In addition, the a2 receptor agonist norepinephrine and the selective V1 receptor agonist terlipressin were also given in combination to constrict the blood vessels and increase peripheral vascular resistance.
The current guidelines for treating clozapine intoxication recommend treatment selection based on the clinical symptoms.21 There is currently no specific antidote for acute clozapine intoxication. A combination of symptomatic treatment and blood purification can alleviate the effects of poisoning by reducing drug absorption and accelerating drug clearance.22 HP is an extracorporeal drug removal intervention wherein blood is passed over an adsorbent structure to directly remove the toxic substances. It is particularly effective against drugs with high protein binding capacity, high molecular weight, lipid solubility and small volume of distribution. Clozapine is a lipid-soluble compound of molecular mass 326.83d and protein binding rate > 90%. Therefore, it meets 3 criteria for HP, with the exception for its large volume distribution (2–5 L/kg).23 In a retrospective chart review, patients with clozapine poisoning who underwent HP regained consciousness significantly faster than their counterparts with the same plasma level of clozapine (>2000 ng/mL) who did not undergo the procedure. A combination of HP and symptomatic treatment is the best therapeutic option when plasma clozapine concentration is high. Blood samples were drawn regularly during hospitalization to measure serum levels of clozapine/norclozapine continuously and dynamically. Both decreased significantly before and after HP. Once HP removes a large amount of clozapine from the plasma, it is redistributed to maintain its concentration, resulting in decreased levels in the vital organs.24
The patient also underwent plasma exchange, an extracorporeal process wherein a large volume of whole venous blood is drawn, and the plasma is separated. The remaining blood components are infused back into the patient. Replacement fluids such as albumin or fresh-frozen plasma may be used. Plasma exchange can remove toxins that bind to plasma proteins, reduce the toxicological effects of any residual drug and prevent drug redistribution.25 In order to remove other small and medium-sized toxins and inflammatory mediators, we also performed continuous veno-venous hemofiltration (CVVH). If it is not possible to monitor the blood concentration of clozapine, we can indirectly assess its drug concentration through its hemodynamic instability and administer corresponding blood purification therapy.
Conclusions
To summarize, we have described a case of a 32-year-old pregnant woman who developed life-threatening clozapine toxicity at 28 weeks of gestation. The levels of clozapine and norclozapine in the serum were high due lower concentrations of drug binding proteins. We therefore initiated HP and other detoxification therapies to remove the drug. The patient had severely dilated peripheral blood vessels, which led to cardiac symptoms such as fatal hypotension and uncontrollable tachycardia, resulting in very high cardiac output and elevated ScvO2. According to the hemodynamic parameters monitored by PICCO, we administered the β1 receptor blocker esmolol, a2 receptor agonist norepinephrine, and selective V1 receptor agonist terlipressin. Pharmacological intervention significantly improved the hemodynamics.
Once the clozapine binding sites are saturated, the concentration of the free drug in the plasma increases significantly, which is responsible for recurrent hemodynamic instability. Interestingly, only one study has reported severe hemodynamic instability due to clozapine toxicity. Based on our findings in the current case, we hypothesize that hemodynamic instability in patients with clozapine intoxication can indirectly reflect the serum concentration of clozapine. Hemodynamic monitoring before and after blood detoxification can help assess the efficacy and guide treatment.
Abbreviations
ICU, intensive care unit; ScvO2, Central venous oxygen saturation; PICCO, Pulse index continuous cardiac output; CI, cardiac index; CVP, central venous pressure; GEDI, global end diastolic volume index; GEF, global ejection fraction; ITBVI, intrapleural blood volume index; EVLWI, extravascular lung water index; SVRI, systemic vascular resistance index; VDI, vasopressor dependency index; HP, Hemoperfusion; CVVH, continuous veno-venous hemofiltration; PE, Plasma exchange.
Data Sharing Statement
Patient data supporting the results reported in the article can be obtained from the corresponding author by email.
Ethics Approval and Consent to Participate
The patient ‘s husband (Patient with paranoid schizophrenia) has given his written informed consent for the publication of de-identified case information regarding the patient ‘s medical condition and its management.
Consent for Publication
The patient’s husband, who is the legal representative of the patient with paranoid schizophrenia, has provided written informed consent for the publication of de-identified case information regarding the patient’s medical condition and its management. Institutional approval is not required as the patient is an existing patient and informed consent has been obtained.
Acknowledgments
The authors are grateful to all doctors and nurses who have participated in Department of Critical Care Medicine, the Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing, Jiangsu, P.R.China.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; ZXY took part in drafting, revising or critically reviewing the article; QG gave final approval of the version to be published; DJD have agreed on the journal to which the article has been submitted; MKC and YP agree to be accountable for all aspects of the work. Additionally, all authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors received no funding to support this publication.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Curto M, Girardi N, Lionetto L, et al. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep. 2016;18(7). doi:10.1007/s11920-016-0704-3
2. Roberts RJ, Miano TA, Hammond DA, et al. Evaluation of vasopressor exposure and mortality in patients with septic shock. Crit Care Med. 2020;48(10):1445–1453. doi:10.1097/CCM.0000000000004476
3. Antal O, Ștefănescu E, Mleșnițe M, et al. Hemodynamic predictors for sepsis-induced acute kidney injury: a preliminary study. J Clin Med. 2020;9(1):151. doi:10.3390/jcm9010151
4. Daskalakis ZJ, George GTP. Clozapine, GABAB, and the treatment of resistant schizophrenia. Clin Pharmacol Ther. 2009;86(4):442Y446. doi:10.1038/clpt.2009.115
5. Farooq S, Choudry A, Cohen D, et al. Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. Br J Psychiatry. 2019;43(1):8–16.
6. Nielsen J, Young C, Ifteni P, et al. Worldwide differences in regulations of clozapine use. CNS Drugs. 2016;30(2):149–161. doi:10.1007/s40263-016-0311-1
7. Weiner DM, Meltzer HY, Veinbergs I, et al. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology. 2004;177(1–2):207–216.
8. Seeman P. An update of fast-off dopamine D2 atypical antipsychotics. Am J Psychiatry. 2005;162(10):1984–1985. doi:10.1176/appi.ajp.162.10.1984-a
9. Tauscher J, Hussain T, Agid O, et al. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161(9):1620–1625. doi:10.1176/appi.ajp.161.9.1620
10. Renwick AC, Renwick AG, Flanagan RJ, et al. Monitoring of clozapine and norclozapine plasma concentration-time curves in acute overdose. Clin Toxicol. 2000;38:325Y328.
11. Krämer I, Rauber-Lüthy C, Kupferschmidt H, Krähenbühl S, Ceschi A. Minimal dose for severe poisoning and influencing factors in acute human clozapine intoxication: a 13-year retrospective study. Clin Neuropharmacol. 2010;33(5):230–234. doi:10.1097/WNF.0b013e3181f0ec55
12. Bebawi E, Wakim L, Doré M. Maxime Doré.Clozapine intoxication with severe adverse effects induced by an inflammatory and infectious process: a case report. J Med Case Rep. 2021;15(1):47. doi:10.1186/s13256-020-02660-x
13. Yartsev A, Peisah C. Caffeine-clozapine interaction associated with severe toxicity and multiorgan system failure: a case report. BMC Psychiatry. 2021;21(1):192. doi:10.1186/s12888-021-03199-x
14. Damkier P, Videbech P. The safety of second-generation antipsychotics during pregnancy: a clinically focused review. CNS Drugs. 2018;32(4):351–366. doi:10.1007/s40263-018-0517-5
15. Beex-Oosterhuis MM, Samb A, Heerdink ER, et al. Safety of clozapine use during pregnancy: analysis of international pharmacovigilance data. Pharmacoepidemiol Drug Saf. 2020;29(6):725–735. doi:10.1002/pds.5016
16. Mehta TM, Van Lieshout RJ. A review of the safety of clozapine during pregnancy and lactation. Arch Womens Ment Health. 2017;20(1):1–9. doi:10.1007/s00737-016-0670-0
17. Rohde C, Polcwiartek C, Kragholm K, et al. Adverse cardiac events in outpatients initiating clozapine treatment: a nationwide register-based study. Acta Psychiatr Scand. 2018;137:47–53. doi:10.1111/acps.12827
18. Hollingworth SA, Winckel K, Saiepour N, et al. Clozapine-related neutropenia, myocarditis and cardiomyopathy adverse event reports in Australia 1993–2014. Psychopharmacology. 2018;235:1915–1921. doi:10.1007/s00213-018-4881-0
19. Crumb WJ, Ekins S, Sarazan RD, et al. Effects of antipsychotic drugs on I (to), I (Na), I (sus), I (K1), and hERG: QT prolongation, structure activity relationship, and network analysis. Pharm Res. 2006;23:1133Y1143.
20. Knoph KN, Morgan RJ, Palmer BA, et al. Clozapine-induced cardiomyopathy and myocarditis monitoring: a systematic review. Schizophr Res. 2018;199:17–30. doi:10.1016/j.schres.2018.03.006
21. Schulte PF, Cohen D, Bogers JP, et al. A Dutch guideline for the use of clozapine. Aust N Z J Psychiatry. 2010;44(11):1055–1056.
22. Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE. Goldfrank’s Toxicologic Emergencies. New York: McGraw-Hill Professional; 2011.
23. Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. J Toxicol Clin Toxicol. 2001;39:1–14. doi:10.1081/CLT-100102873
24. Jia-Li H, Xiang Y-T, Li W-B, Cai Z-J, Ungvari GS. Hemoperfusion in the treatment of acute clozapine intoxication in China. J Clin Psychopharmacol. 2007;27(6):667–671. doi:10.1097/jcp.0b013e31815a5881
25. Hanafy Mahmoud S, Buhler J, Chu E, et al. Drug dosing in patients undergoing therapeutic plasma exchange. Neurocrit Care. 2021;34(1):301–311.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
