Back to Journals » Clinical and Experimental Gastroenterology » Volume 17
Clinicopathological Characteristics, Treatment and Prognosis in Duodenal Adenocarcinoma with Liver Metastasis: A SEER-Based Study
Received 26 September 2023
Accepted for publication 12 February 2024
Published 26 February 2024 Volume 2024:17 Pages 51—59
DOI https://doi.org/10.2147/CEG.S439275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vipul Yagnik
Zhengchun Zhu,1 Hong Liu,2 Fei Zhong1
1Department of Oncology, Fuyang Hospital of Anhui Medical University, Fuhe Modern Industrial Park, Fuyang, People’s Republic of China; 2Department of Cardiovascular Medicine, Fuyang Hospital of Anhui Medical University, Fuhe Modern Industrial Park, Fuyang, People’s Republic of China
Correspondence: Fei Zhong, Email [email protected]
Background and Objectives: Duodenal adenocarcinoma (DAC) is a rare tumor that is often accompanied by liver metastasis in advanced stages. The aim of this study was to evaluate the correlation between clinicopathological characteristics and survival in DAC patients with liver metastasis, and to explore appropriate treatment options.
Methods: 482 DAC patients with liver metastasis were retrospectively identified from the Surveillance, Epidemiology and End Results (SEER) database (2011– 2020). Univariate and multivariate Cox regression analyses were performed to explore the clinicopathological factors related to survival. The Kaplan-Meier method was used to identify the independent risk factors associated with survival.
Results: The 1-year overall survival (OS) and cancer-specific survival (CSS) rates for the entire cohort were 25.4% and 28.3%, and the 5-year OS and CSS rates were 2.4% and 2.9% respectively. Univariable analysis and multivariate analysis identified chemotherapy and surgery as the independent risk factors for OS and CSS. Patients who underwent chemotherapy and surgery had better CSS and OS rates, whereas radiotherapy failed to improve outcomes.
Conclusion: We identified several prognostic factors of DAC with liver metastasis. Chemotherapy and surgery can prolong the survival of DAC patients with liver metastasis, which lays the foundation for identifying the optimal treatment strategy.
Keywords: duodenal adenocarcinoma, liver metastasis, clinicopathological characteristics, survival, SEER database
Introduction
Although the incidence of duodenal adenocarcinoma (DAC) has increased in recent years, it remains a rare malignancy that accounts for a mere 0.5% of all gastrointestinal tumors, with an incidence of 0.2–0.5 per 100,000 people.1–4 The common symptoms of DAC are abdominal pain, abdominal distension, nausea, vomiting, fatigue, weight loss, gastrointestinal bleeding and anemia, of which abdominal pain is most frequent.5 Jaundice and intestinal obstruction may occur in the late stage of DAC.6 Due to the lack of specific symptoms, most patients are diagnosed with advanced stage, resulting in poor prognosis.7 Among 223 cases of small intestinal adenocarcinoma (including 132 duodenal adenocarcinoma), the most common metastatic sites were peritoneum (50%), liver (47.1%), and lung (14.3%).8 In addition, there is no standardized therapeutic regimen for DAC due to its low incidence rates. Resection is currently the only curative option for early stage DAC; however, the efficacy of palliative care including palliative resection, chemotherapy, and radiotherapy for DAC with distant metastasis remains ambiguous.9 Studies show that tumor size, stage, grade, regional lymph node metastasis and surgery are some of the risk factors associated with the survival of patients with DAC.10–14 However, the survival predictors and appropriate therapeutic options for DAC with liver metastasis remain unknown. The aim of this study was to investigate the correlation between clinicopathological characteristics and survival in DAC patients with liver metastasis using data retrieved from the SEER database, and to explore appropriate treatment options.
Materials and Methods
Data Source
The SEER database has been set up by the National Cancer Institute to collate the clinical information of cancer patients in the United States.15 The SEER*Stat software (version 8.4.0.1) was used to extract the data of DAC patients, which was then screened using site codes and histological codes defined by the third edition of the International Classification of Diseases for Oncology (ICD-O-3). The inclusion criteria were as follows: 1) pathological diagnosis of DAC, 2) presence of liver metastasis, 3) single primary tumor, and 4) diagnosed between 2011–2020. Patients with incomplete follow-up information and survival time less than one month were excluded. The suitable authorization was obtained to access and use the data from SEER database, and all protocols were followed to protect patient privacy. The SEER database is a publicly available database. The data published by the SEER database does not require informed patient consent because cancer is a reportable disease in the United States. This study complies with the Declaration of Helsinki and its subsequent amendments.
Study Variables
The demographic and clinicopathological data obtained from the Surveillance, Epidemiology, and End Results (SEER) database encompassed variables such as gender, age, ethnicity, marital status, tumor grade, tumor size, presence of organ metastasis (specifically in the bone, brain, or lung), utilization of radiotherapy, chemotherapy, and surgery. The patients were classified on the basis of the age at diagnosis (<60 and ≥ 60 years), ethnicity (Caucasian, African American and others) and marital status (married and unmarried including divorced, widowed, separated and single based on codes in SEER database). In addition, the G1 (well differentiated) and G2 (moderately differentiated) tumors were pooled as low grade, and G3 (poorly differentiated) and G4 (undifferentiated or anaplastic) as high grade according to the SEER tumor grading system. The X-tile software (version 3.6.1) was used to determine the optimal cutoff for tumor size, and the patients were divided accordingly (<4 and ≥4 cm). Additionally, the patients were categorized into two groups, namely the metastasis and non-metastasis groups, according to the presence or absence of metastatic growth in other organs such as bone, brain, or lung. Similarly, the participants were categorized into distinct cohorts based on their exposure to radiotherapy, chemotherapy, and surgery. The local therapeutic interventions employed in this investigation, encompassing surgery and radiotherapy, were specifically directed towards the primary site of DAC with liver metastasis.
Overall Survival (OS) and Cancer-Specific Survival (CSS)
OS was defined as the time from cancer diagnosis to death caused by any cause. CSS was defined as the time from diagnosis to death due to the cancer.
Statistical Analyses
Statistical analyses were performed using IBM SPSS statistical software (version 22.0). Univariate Cox regression model was established to evaluate the correlation of sex, age, ethnicity, marital status, year of diagnosis, grade, size, organ metastasis (bone/brain/lung), radiotherapy, chemotherapy, and surgery with OS and CSS. The independent predictors of OS and CSS were identified with the multivariate Cox regression model. Kaplan-Meier curves were plotted using R software to determine the independent risk factors associated with survival, and the results were expressed as hazard ratios (HR) with 95% confidence intervals (CI). All statistical tests were two-sided, and P<0.05 was considered statistically significant at.
Results
Clinicopathological Characteristics of Patients
A total of 482 DAC patients with liver metastasis were selected, of which 274 (56.8%) were male and 208 (43.2%) were female. In addition, 72.6% of the patients were white, and 19.1% were African-American. Most patients were older than 60 years (73.9%). Furthermore, 288 patients (59.8%) were married and 173 (35.9%) were unmarried. Tumor grade was classified as low grade (170, 35.3%), high grade (118, 24.5%) and unknown (194, 40.2%). There were 156 patients with defined tumor size, of which 91 (18.9%) and 65 (13.5%) had tumors measuring < 4 cm and ≥ 4 cm in diameter respectively. 108 patients also complicated with bone, brain or lung metastasis. In addition, 54 (11.2%) patients received radiotherapy, and chemotherapy was performed for 328 (68.0%). A total of 44 patients underwent surgery. The 1-year OS and CSS rates for all patients were 25.4% and 28.3%, and the 5-year OS and CSS rates were 2.4% and 2.9% respectively. The clinicopathological characteristics of DAC patients with liver metastasis are summarized in Table 1.
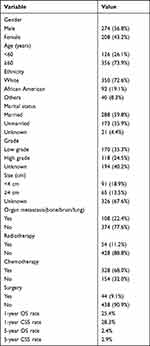 |
Table 1 Baseline Demographic and Clinicopathologic Characteristics of 482 Duodenal Adenocarcinoma with Liver Metastasis |
 |
Table 2 Univariate Cox Analysis of Variables in Duodenal Adenocarcinoma Liver Metastasis |
 |
Table 3 Multivariate Cox Analysis of Variables in Duodenal Adenocarcinoma Liver Metastasis |
Univariable and Multivariable Cox Regression Analysis
The univariate analysis showed that older age, organ metastasis (bone, brain, or lung), lack of chemotherapy, and no surgery were predictors of lower OS and CSS. Marital status was associated with patient OS, not CSS. Patients who were married had better OS. However, gender, ethnicity, tumor grade, tumor size, and radiotherapy were not significantly associated with the OS or CSS (Table 2). Furthermore, multivariate analysis identified lack of chemotherapy and no surgical resection of the primary tumors as independent predictors of reduced OS and CSS (Table 3).
Kaplan-Meier Survival Analysis
As shown in Figure 1, without organ metastasis (bone/brain/lung) resulted in a significant survival benefit for the patients (p < 0.05). However, tumor grade and tumor size had no significant effect on the survival of patients (p > 0.05). As shown in Figure 2, chemotherapy and surgery resulted in a significant survival benefit for the patients (p < 0.05). However, radiotherapy had no significant effect on the survival of patients (p > 0.05).
Discussion
Advanced duodenal carcinoma presents a poor prognosis, with only a few reports available regarding its clinical features, prognostic risk factors and appropriate treatment. We conducted the largest study to date in patients with DAC with liver metastases to explore clinicopathological features, prognostic factors, and treatment options. In our study, the majority of the patients in our cohort were elderly (≥ 60 years), and the number of males was slightly more than that of females. Our results validated that gender, age, and ethnicity were not independent predictor associated with the OS and CSS. Marital status was associated with patient OS, not CSS.
The prognosis of DAC is generally dismal, with a 5-year OS of only 32.3%; in addition, none of the patients with advanced cancer survived for five years.7 In our study, the 1-year OS and CSS rates for DAC patients with liver metastasis were 25.4% and 28.3%, and the 5-year OS and CSS rates were 2.4% and 2.9%. We did not observe any significant association between tumor size and prognosis. In contrast, previous studies have found tumor size to be an independent predictor of survival in DAC patients.10,16,17 Nevertheless, the primary tumor size may not have an impact on the prognosis of patients with metastasis. Tumor grade is one of the significant factors affecting the prognosis of DAC, and high tumor grade portends worse prognosis,10,18 which was also confirmed in our study. In addition, 22.4% of the DAC patients with liver metastasis had metastases to other organs (bone, brain, or lung), but distant metastasis was not identified as a significant prognostic factor.
Traditional medical treatments for DAC patients include surgery, radiotherapy, and chemotherapy. However, few data factually support their survival benefits in DAC patients with liver metastasis. This is partly due to the fact that some advanced patients reject the above treatment options for palliative care.19 Surgical resection is currently the only treatment for DAC with curative potential. Ryder et al showed that the 5-year OS rate was 43% in DAC patients with tumor resection and only 13% in patients without tumor resection.16 The efficacy of adjuvant chemoradiotherapy for DAC remains controversial. Ecker et al showed that adjuvant radiotherapy did not confer any survival benefit on DAC patients, while Lim et al demonstrated that post-operative radiotherapy in DAC patients improved the OS and CSS.20,21 A study based on the National Cancer Database reported that adjuvant chemotherapy prolonged the OS in DAC patients with locally advanced disease.22 However, a population-based, case-matched analysis showed that adjuvant chemotherapy in stage II and III DAC patients had no significant survival benefit.23 Furthermore, a study based on data from the National Clinical Oncology Database revealed that combining radiotherapy with adjuvant chemotherapy did not significantly improve survival.20 For advanced DAC, the efficacy of radiotherapy remains equivocal. Surgery and palliative chemotherapy improve survival in patients with advanced DAC.24–26 However, little data is available regarding the effects of these treatments on the survival of DAC patients with liver metastasis. We found that chemotherapy and surgery, but not radiotherapy, was associated with favorable prognosis. Thus, multimodal therapy for DAC patients with liver metastasis is strongly recommended.
This study has several limitations that should be considered. First, as a retrospective study, bias was inevitable Second, the data on treatment regimens was limited, and there was no information regarding the protocols and doses for chemotherapy. Third, since detailed data on tumor recurrence, metastasis and progression was lacking, we could not fully evaluate the survival outcomes. Despite these shortcomings, the SEER database provides clinicians with an invaluable tool for clinical cancer research, enabling large-scale studies of rare diseases.
Conclusions
We identified several prognostic factors for DAC with liver metastasis, including chemotherapy and surgery. This study provides insights into customized treatment regimens, although our findings will have to be validated in large-scale prospective, multicenter, randomized controlled studies.
Data Sharing Statement
The data analyzed during this study are available from the corresponding author on reasonable request.
Ethics Approval
All patient information was obtained from the SEER database, which is publicly accessible. Therefore, ethics committee review and informed consent requirements were exempted by the Ethics Committee of the Fuyang Hospital of Anhui Medical University.
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the construction of this database.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Anhui Province Higher Education Science Research Project (NO.2022AHO50715).
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Jiang S, Zhao R, Li Y, et al. Prognosis and nomogram for predicting postoperative survival of duodenal adenocarcinoma: a retrospective study in China and the SEER database. Sci Rep. 2018;8(1):7940. doi:10.1038/s41598-018-26145-6
2. Li D, Si X, Wan T, Zhou Y. Outcomes of surgical resection for primary duodenal adenocarcinoma: a systematic review. Asian J Surg. 2019;42(1):46–52.
3. Platoff RM, Kellish AS, Hakim A, et al. Simple versus radical resection for duodenal adenocarcinoma: a propensity score matched analysis of national cancer database. Am Surg. 2021;87(2):266–275. doi:10.1177/0003134820951432
4. Kato T, Ono Y, Oba A, et al. Comparison of the clinical efficacy of a new prognostic stratification for duodenal adenocarcinoma with that of TNM staging: the importance of T status with regard to the prognosis. Eur J Surg Oncol. 2023;49(1):122–128. doi:10.1016/j.ejso.2022.08.005
5. Topal U, Arıkan TB, Sozuer E, Akyıldız HY, Gok M. Surgical treatment and outcome for primary duodenal adenocarcinoma. Single Center Experience Ann Ital Chir. 2021;92:41–47.
6. López-Domínguez J, Busquets J, Secanella L, Peláez N, Serrano T, Fabregat J. [Duodenal adenocarcinoma: surgical results of 27 patients treated at a single center]. Adenocarcinoma duodenal: resultados del tratamiento quirúrgico de una serie unicéntrica de 27 pacientes. Cir Esp. 2019;97(9):523–530.
7. Kim MJ, Choi SB, Han HJ, et al. Clinicopathological analysis and survival outcome of duodenal adenocarcinoma. Kaohsiung J Med Sci. 2014;30(5):254–259.
8. Teufel A, Meindl-Beinker NM, Hösel P, et al. Characteristics and outcome of patients with small bowel adenocarcinoma (SBA). J Cancer Res Clin Oncol. 2023;149(8):4579–4590. doi:10.1007/s00432-022-04344-z
9. Zhang Z, Lei Y, Wang D, Yang L, Lou C. Case Report: a case of advanced duodenal adenocarcinoma in complete remission after chemotherapy combined with targeted therapy and radiotherapy. Front Oncol. 2022;12:968110. doi:10.3389/fonc.2022.968110
10. Zhu Z, Zhong F. Comparative analysis of outcomes and clinicopathological characteristics of duodenal adenocarcinoma: a SEER analysis. Cancer Invest. 2020;38(10):543–548. doi:10.1080/07357907.2020.1824260
11. Gibbs JF. Duodenal adenocarcinoma: is total lymph node sampling predictive of outcome?. Ann Surg Oncol. 2004;11(4):354–355. doi:10.1245/ASO.2004.02.914
12. Ecker BL, McMillan MT, Datta J, et al. Lymph node evaluation and survival after curative-intent resection of duodenal adenocarcinoma: a matched cohort study. Eur J Cancer. 2016;69:135–141.
13. Mann K, Gilbert T, Cicconi S, et al. Tumour stage and resection margin status are independent survival factors following partial pancreatoduodenectomy for duodenal adenocarcinoma.. Langenbecks Arch Surg. 2019;404(4):439–449. doi:10.1007/s00423-019-01779-w
14. Jiang QL, Huang XH, Chen YT, Zhang JW, Wang CF. Prognostic factors and clinical characteristics of patients with primary duodenal adenocarcinoma: a single-center experience from China. Biomed Res Int. 2016;2016:6491049. doi:10.1155/2016/6491049
15. Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) Program. Oncologist. 2007;12(1):20–37. doi:10.1634/theoncologist.12-1-20
16. Ryder NM, Ko CY, Hines OJ, Gloor B, Reber HA. Primary duodenal adenocarcinoma: a 40-year experience. Arch Surg. 2000;135(9):1070–1075. doi:10.1001/archsurg.135.9.1070
17. Hurtuk MG, Devata S, Brown KM, et al. Should all patients with duodenal adenocarcinoma be considered for aggressive surgical resection?. Am J Surg. 2007;193(3):319–325. doi:10.1016/j.amjsurg.2006.09.013
18. Zheng Z, Zhou X, Zhang J, et al. Nomograms predict survival of patients with small bowel adenocarcinoma: a SEER-based study. Int J Clin Oncol. 2021;26(2):387–398.
19. Duhan S, Keisham B, Duhan C, Singh S, Jain A. Duodenal adenocarcinoma with suspected brain metastasis. Cureus. 2023;15(4):e38199. doi:10.7759/cureus.38199
20. Ecker BL, McMillan MT, Datta J, et al. Adjuvant chemotherapy versus chemoradiotherapy in the management of patients with surgically resected duodenal adenocarcinoma: a propensity score-matched analysis of a nationwide clinical oncology database. Cancer. 2017;123(6):967–976. doi:10.1002/cncr.30439
21. Lim YJ, Kim K. Effect of postoperative radiotherapy on survival in duodenal adenocarcinoma: a propensity score-adjusted analysis of Surveillance, epidemiology, and end results database. Int J Clin Oncol. 2018;23(3):473–481. doi:10.1007/s10147-017-1226-7
22. Kaslow SR, Prendergast K, Vitiello GA, et al. Systemic therapy for duodenal adenocarcinoma: an analysis of the national cancer database (NCDB). Surgery. 2022;172(1):358–364. doi:10.1016/j.surg.2022.03.009
23. de Bakker JK, Meijer LL, Zonderhuis BM, van der Vliet HJ, Daams F, van Grieken NCT. Adjuvant chemotherapy for resected duodenal adenocarcinoma: a case-matched analysis in nation wide cohort. Acta Chir Belg. 2022;2022:1–7.
24. Sakae H, Kanzaki H, Nasu J, et al. The characteristics and outcomes of small bowel adenocarcinoma: a multicentre retrospective observational study. Br J Cancer. 2017;117(11):1607–1613. doi:10.1038/bjc.2017.338
25. Liu T, Wu Y, Jiang T. Efficacy of surgery and chemotherapy for stage IV small bowel adenocarcinoma: a population-based analysis using Surveillance, epidemiology, and end result program database. Cancer Med. 2020;9(18):6638–6645.
26. Hagihara S, Shimizu T, Inoue Y, et al. A complete response to capecitabine and oxaliplatin chemotherapy in primary duodenal carcinoma with liver and nodal metastases: a case report. Surg Case Rep. 2018;4(1):125. doi:10.1186/s40792-018-0532-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


