Back to Journals » Lung Cancer: Targets and Therapy » Volume 11
Clinical Outcomes of Proton Beam Therapy for Ground-Glass Opacity-Type Lung Cancer
Authors Nagata I, Ogino T, Arimura T , Yoshiura T
Received 4 July 2020
Accepted for publication 22 September 2020
Published 9 October 2020 Volume 2020:11 Pages 105—111
DOI https://doi.org/10.2147/LCTT.S270283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Ichiro Nagata,1,2 Takashi Ogino,1 Takeshi Arimura,1 Takashi Yoshiura2
1Medipolis Proton Therapy and Research Center, Ibusuki, Kagoshima, Japan; 2Department of Radiology, Graduate School of Medical and Dental Sciences, Kagoshima University, Kagoshima, Japan
Correspondence: Ichiro Nagata
Medipolis Proton Therapy and Research Center, 4423 Higashikata, Ibusuki, Kagoshima 891-0304, Japan
Tel +81 993 23 5188
Fax +81 993 24 3450
Email [email protected]
Purpose: Surgery is the standard treatment for early-stage non-small cell lung cancer (NSCLC), including ground-glass opacity (GGO)-type lung cancer. However, some patients are inoperable or refuse to undergo surgery. To explore whether proton beam therapy (PBT) can be an alternative to surgical resection in these patients, this study aimed to examine the retrospective treatment outcomes of patients with GGO-type lung cancer who underwent PBT.
Patients and Methods: Patients with stage I NSCLC and GGOs who underwent PBT at the Medipolis Proton Therapy and Research Center (Kagoshima, Japan) between April 2011 and September 2015 were included. Patients were treated with a total dose of 66 GyE delivered in 10 fractions. Survival curves were calculated using the Kaplan–Meier method, and treatment-related adverse events (AEs) were assessed.
Results: A total of 48 patients (median age: 70.9 ± 9.2 years; men: 54.2%) were analyzed, among whom 53 tumors were observed. The 3-year overall survival rate after PBT was 91.7% (95% confidence interval [CI], 79.3– 96.8%), the 3-year disease-free survival rate was 85.4% (95% CI: 71.8– 92.8%), and the 3-year local control rate among 53 tumors was 92.5% (95% CI: 81.1– 97.1%). During the 3-year follow-up period, 4 patients died, and 3 survived despite recurrence or metastasis. Common AEs were radiation pneumonitis (89.6%), rib fracture (27.1%), and cough (27.1%). None of the patients developed grade ≥ 3 treatment-related AEs.
Conclusion: The results of this study suggest that PBT may be a promising alternative for patients with GGO-type lung cancer when surgical resection is not feasible, with excellent survival outcomes and tolerable treatment-related AEs.
Keywords: proton therapy, carcinoma, non-small-cell lung, ground-glass opacity, adenocarcinoma, Japan
Introduction
Lung cancer is the leading cause of cancer-related deaths in Japan. In 2017, 74,120 people died of lung cancer, which accounted for 19.9% of all cancer deaths in Japan.1 Surgical resection is the standard treatment for stage I non-small cell lung cancer (NSCLC) including ground-glass opacity (GGO)-type lung cancer.2 However, some patients are medically inoperable due to presence of comorbidities or advanced age, and even operable patients may refuse to undergo surgery. For these patients, stereotactic body radiation therapy (SBRT), a technique used to deliver a high dose of radiation to the target, is a good alternative. However, the use of X-rays still involves the risk of radiation-induced complications, such as radiation pneumonitis, because X-rays require irradiation from multiple directions, thereby increasing the risk of radiation injury to normal tissues.
Unlike photon beams such as X-rays, proton beams have a very rapid energy loss immediately before they stop within the body, resulting in a sharply localized peak of dose (Bragg peak).3 As the depth of the Bragg peak is determined based on the energy of protons, varying the amount of energy enables the delivery of a desired dose over the target tumor volume, with minimal damage to normal tissues. With this characteristic, proton beam therapy (PBT) has the potential to improve the clinical outcomes of lung cancer patients with decreased toxicity, compared with photon radiation therapy.4 In recent years, studies have demonstrated the efficacy and safety of PBT for early-stage NSCLC, some of which have supported PBT as a promising alternative for medically inoperable patients.5–8
The characteristics of GGOs on computed tomography (CT) scans can be defined as “hazy increased opacity of the lung, with preservation of the bronchial and vascular margins”9 and observed in various pathologic entities from benign to malignant, including inflammation, organizing pneumonia, focal fibrosis, and lung adenocarcinoma.10,11 Because most benign conditions usually resolve within 3 months10,12 and even growing GGOs typically show an indolent course,13 the current treatment strategies basically consider that GGOs <5 mm do not require treatment.13 However, a portion of GGOs increase, although usually slowly, in size or density,13 and persistent GGOs are highly suggestive of neoplastic conditions, including atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), and minimally invasive adenocarcinoma (MIA).10 As these lesions can develop into invasive adenocarcinoma, early treatment of these adenocarcinomas (GGO-type lung cancer) is important to achieve a better prognosis.12
Although the data on PBT for early-stage NSCLC are accumulating, no studies have specifically examined the efficacy and safety of PBT in patients with GGO-type lung cancer among early-stage NSCLCs. Additionally, a study published by Japan Clinical Oncology Group 0201 demonstrated that the lower a consolidation/tumor ratio, the better in prognosis.14 Therefore, in this study, we aimed to retrospectively explore the treatment outcomes of patients with GGO-type lung cancer who underwent PBT at our center to generate new knowledge in order to determine whether PBT may be a good alternative for these patients when surgical resection is not feasible.
Patients and Methods
Patients
This retrospective observational study included all patients with GGO-type lung cancer who underwent PBT at the Medipolis Proton Therapy and Research Center (Kagoshima, Japan) between April 2011 and September 2015. Patients who had 1) histologically or clinically diagnosed stage I (T1a‒c or T2a N0 M0) NSCLC through biopsy or high-resolution CT scans, and 2) GGOs identified on high-resolution CT scans were eligible for the study. All patients were diagnosed with GGO-type lung cancer at the time of referral to the facility. Additionally, the treatment decisions of these patients were confirmed at the cancer board conference held within the facility.
Information about this study, such as purposes, methods, and contact details, was posted at the study site, providing patients with an opportunity to opt out of the study. Thus, the study was exempted from taking individual agreement from each patient. This study was approved by the ethics committee of the Medipolis Proton Therapy and Research Center and was conducted in accordance with the Declaration of Helsinki (R2018-1).
Proton Beam Therapy
The treatment plan was developed using a CT-based three-dimensional treatment planning system (XiO-M; Elekta, Stockholm, Sweden). Patients were immobilized in the supine position with a thermoplastic cast, and 2-mm-thick CT images were obtained during the exhalation phase using a respiratory gating system. The gross tumor volume (GTV) was identified from the images in the pulmonary window setting. The clinical target volume (CTV) was defined as the GTV plus a 5-mm margin in all directions. The planning target volume (PTV) was defined as the CTV plus a set-up margin of 5 mm and an internal margin, which was determined depending on the respiratory movements. The relative biological effectiveness (RBE) of the proton beam was determined to be 1.1, according to a previous study that compared the RBE of proton beam and photon (GyE =Proton Gy×1.1).15 In all patients, a total dose of 66 GyE in 10 fractions, which is a Japanese standard for proton beam therapy for Stage I lung cancer,16 was delivered to the PTV. The patients were treated equal doses every time over 10 days (5 days a week). Patients were treated with proton beams produced by a PBT system employing the beam-wobbling method with a ridge filter to spread the Bragg peak (Mitsubishi Electric Corporation, Tokyo, Japan). Patients were treated with 2-portal irradiation. The dose–volume constraint was set as lung V20 (lung volume receiving more than 20 Gy) ≤30%. Although this rule follows the Japanese standard, V20 is usually much lower in the actual treatments in both SBRT and proton beam therapy. Moreover, a study that compares dose volume of proton beam therapy and SBRT demonstrated that proton has less dose distributions for all OAR including V20 for lung.17 Treatment was administered during the end-expiration phase using a respiratory gating system with two or four portals of proton beams arranged in a way that would minimize dose exposure to the normal tissue. The wobbler method was used to produce the spread out Bragg Peak with 150 MeV proton beams.
After PBT, patients underwent follow-up examinations every 3 months for the first year and every 6 months thereafter. Regular follow-up examinations included chest CT and blood tests to identify the tumor markers.
Outcomes and Analyses
Data on patient characteristics, tumor characteristics, PBT, and follow-up evaluations (eg, survival, tumor control, and adverse events [AEs]) were retrospectively collected. The efficacy outcomes were 3-year overall survival (OS), disease-free survival (DFS), and local control (LC) rates. The OS, DFS, and LC were calculated from the date of PBT using the Kaplan–Meier method. For safety, treatment-related AEs reported at any time during the follow-up were assessed and graded according to the Common Terminology Criteria for Adverse Events v4.0. All analyses were performed using SAS Release 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient and Tumor Characteristics
Of the 49 patients included, 1 who was lost to follow-up was excluded from the analysis. Thus, 48 patients were analyzed. The median ± standard deviation (SD) age of these 48 patients was 70.9 ± 9.2 years, and 54.2% were men (Table 1). The median follow-up period was 49 months. Of these, 11 (22.9%) patients had a past history of lung cancer, among whom 9 had surgery, 1 had chemoradiation therapy, and 1 had chemoradiation therapy and radiofrequency ablation. Patients for whom PBT was chosen over surgery for clinical reasons (ie, complication, high age, and clinical preference) accounted for 20.8% of the study population, while those for whom PBT was chosen as their preferred treatment or as they refused to undergo surgery accounted for 79.2% of the population.
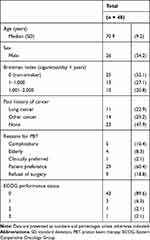 |
Table 1 Patient Characteristics |
Among the 48 patients, 53 tumors were observed. The characteristics of these tumors are summarized in Table 2. Of these, 44 (83.0%) were stage IA1‒3, while 9 (17.0%) were stage IB. According to the radiological diagnosis, the most frequent subtypes of GGO adenocarcinomas were lepidic-predominant adenocarcinoma (LPA, 52.8%), followed by AIS (28.3%), MIA (17.0%), and AAH (1.9%). Only 16 of 53 tumors were diagnosed through biopsy, whose histological types were adenocarcinomas (10/16), alveolar cell carcinoma (1/16), and unknown despite biopsy (5/16).
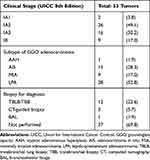 |
Table 2 Tumor Characteristics |
Survival and Local Control
The 3-year OS rate after PBT among 48 patients was 91.7% (95% confidence interval [CI], 79.3–96.8%) (Figure 1). The 3-year DFS rate among 48 patients was 85.4% (95% CI: 71.8–92.8%). The 3-year LC rate among 53 tumors was 92.5% (95% CI: 81.1–97.1%).
During the 3-year follow-up, 4 patients died (patient #1, 2, 5, and 6), and 3 survived despite recurrence or metastasis (patient #3, 4, and 7). Data of these 7 patients with death or recurrence during the 3-year follow-up are summarized in Table 3.
 |
Table 3 Summary of Patients Who Died or Experienced Recurrence Following PBT (n = 7) |
Adverse Events
Overall, none of the patients developed grade ≥3 treatment-related AEs (Table 4). The most common AE was radiation pneumonitis: 37 (77.1%) developed grade 1, while 6 (12.5%) developed grade 2 radiation pneumonitis. Of the 48 patients, 13 (27.1%) had rib fractures, all of which were grade 1. Cough was reported in 13 (27.1%) patients, among which 11 (22.9%) had grade 1 and 2 (4.2%) had grade 2 cough. Other reported AEs were grade 1 pleural effusion (n = 1), grade 1 pleurisy (n = 1), and grade 1 chest pain (n = 8). None of the patients reported dermatitis.
 |
Table 4 Adverse Events Reported During the Follow-Up Period (n = 48) |
Discussion
In this study, to generate new knowledge on the potential of PBT as an alternative to surgical resection, particularly in patients with GGO-type lung cancer among early-stage NSCLC, we retrospectively examined the treatment outcomes of 48 patients with GGO-type lung cancer who received PBT at our center. Although some AEs such as radiation pneumonitis, rib fracture, and cough occurred at a relatively high rate, these patients had excellent survival with no grade ≥3 treatment-related AEs, and a high local control rate was achieved.
The 3-year OS rate after PBT in patients with GGO-type lung cancer was 91.7%. Although direct comparison is not appropriate, our results were similar to or better than those reported in previous retrospective studies of PBT for stage I NSCLC in Japan (3-year OS = 61–90%),5–7 and even higher than the 3-year OS after SBRT for early-stage NSCLC (55.8–76.5%),18–20 The standard treatment option for GGO-type lung cancer is still surgical resection. However, our results suggest that the prognosis after PBT may not be far inferior to that after lobectomy or segmentectomy for stage I lung adenocarcinomas (3-year OS = 94.1–96.9%21,22), which would support PBT as a positive alternative, especially when surgical resection is not feasible.
In addition to the survival rate, the 3-year LC rate after PBT was also high (92.5%). Although direct comparison is not appropriate due to the differences in study designs and methods, the LC rate observed in this study was high, similar to those achieved by SBRT for stage I lung cancer (80–97%).23 However, one study previously reported that the 3-year LC after SBRT for biopsy-proven or radiographically diagnosed bronchioloalveolar carcinoma (ie, MIA and AIS) was 100%, although the sample size was small (n=18).24 The efficacy of PBT versus SBRT specifically for GGO-type lung cancer may be worthy of further study.
Of the 48 patients included in this study, 3 patients survived despite recurrence or metastasis, and 4 patients died in the 3-year follow-up period. The number of patients in this study with worse prognosis was relatively small, and other techniques also share these risks. For example, one study previously reported that 5 of 97 (5.1%) patients with GGOs experienced recurrence after wedge resection (4 had local recurrence and 1 had distant metastasis).25 Another study reported that 9 of 18 patients with biopsy-proven or radiographically diagnosed bronchioalveolar carcinoma developed metastases after SBRT, although none had local failure.24 Considering these, the recurrence or metastasis rates may not be high for PBT; nevertheless, it is important to acknowledge the existence of these risks.
Although a large proportion of patients (89.6%) developed radiation pneumonitis, most of them (37/43) were grade 1 and none were grade ≥3. A meta-analysis previously reported that early-stage NSCLC patients who received particle beam therapy, including PBT, had a lower incidence of grade ≥3 radiation pneumonitis than those with photon SBRT (0.9% vs 3.4%, p< 0.001).26 As the reduced dose to the normal lung is one advantage of PBT over photon SBRT,27 PBT may be associated with a lower risk of severe radiation pneumonitis, which is worth investigating in further research. Another noticeable AE was rib fracture (27.1%), which was consistent with previous reports on PBT for stage I NSCLC (26.3–35.7%).5–7 Although the proportion was relatively high, all cases were grade 1, indicating that they were asymptomatic or required no treatment. Cough was also relatively common (27.1%) among our study population, which may be the clinical manifestation of radiation pneumonitis. Although dermatitis has been frequently reported in previous studies,5–7 no dermatitis was reported in our study population. Overall, as no grade ≥3 AEs were reported in this study, PBT may be considered a relatively tolerable treatment.
For the first time, this study suggested that PBT may be a good alternative for patients with GGO-type lung cancer. However, because this study had no control group, conclusions could not be drawn on whether PBT was indeed comparable or superior to existing techniques such as photon SBRT, which needs to be confirmed in future research. One limitation inherent in its retrospective design was the lack of available data, such as the operability of patients. Because PBT was chosen due to patient preference rather than clinical reasons for the majority of patients, most of our study population may have been operable, which may have contributed to the better survival outcomes. Second, because of the relatively short follow-up period (median: 49 months), the survival analysis was limited to 3 years. Long-term efficacy and safety should be examined in a future study. Third, because 37 out of 53 tumors were diagnosed clinically through high-resolution CT scan, malignancy of these tumors was uncertain compared with those diagnosed histologically. This uncertainty could influence the outcome. Furthermore, while radiation pneumonia and rib fractures were detected using imaging modalities, other AEs basically depended on patients’ self-reports, which may have resulted in underestimation of these AEs. In addition, data of AEs were unavailable for some patients, which implies a potential for underreporting of some important AEs. Thus, our results need to be interpreted with caution.
Conclusion
The results of this study suggest that PBT may be a promising alternative for patients with GGO-type lung cancer when surgical resection is not feasible, with excellent survival outcomes and tolerable treatment-related AEs. Future prospective, confirmatory studies are warranted to elucidate the advantages of PBT over other treatment options for the treatment of these patients.
Disclosure
The authors declare that they have neither conflict of interest nor financial support.
References
1. Cancer Registry and Statistics. Cancer information service, national cancer center, Japan, cancer mortality (1958‒2017). n.d. Available from: https://ganjoho.jp/en/professional/statistics/table_download.html.
2. The Japan Lung Cancer Society. Practice guidelines for lung cancer 2018, n.d. Available from: https://www.haigan.gr.jp/guideline/2018/1/2/180102010100.html.
3. Levin WP, Kooy H, Loeffler JS, DeLaney TF. Proton beam therapy. Br J Cancer. 2005;93(8):849. doi:10.1038/sj.bjc.6602754
4. Berman AT, James SS, Rengan R. Proton beam therapy for non-small cell lung cancer: current clinical evidence and future directions. Cancers (Basel). 2015;7(3):1178–1190. doi:10.3390/cancers7030831
5. Iwata H, Murakami M, Demizu Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer. 2010;116(10):2476–2485.
6. Makita C, Nakamura T, Takada A, et al. High-dose proton beam therapy for stage I non-small cell lung cancer: clinical outcomes and prognostic factors. Acta Oncol. 2015;54(3):307–314. doi:10.3109/0284186X.2014.948060
7. Hatayama Y, Nakamura T, Suzuki M, et al. Clinical outcomes and prognostic factors of high-dose proton beam therapy for peripheral stage I non-small-cell lung cancer. Clin Lung Cancer. 2016;17(5):427–432. doi:10.1016/j.cllc.2015.11.013
8. Chang JY, Zhang W, Komaki R, et al. Long-term outcome of phase I/II prospective study of dose-escalated proton therapy for early-stage non-small cell lung cancer. Radiother Oncol. 2017;122(2):274–280. doi:10.1016/j.radonc.2016.10.022
9. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi:10.1148/radiol.2462070712
10. Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ, Im JG. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. 2007;27(2):391–408. doi:10.1148/rg.272065061
11. Fan L, Liu SY, Li QC, Yu H, Xiao XS. Multidetector CT features of pulmonary focal ground-glass opacity: differences between benign and malignant. Br J Radiol. 2012;85(1015):897–904. doi:10.1259/bjr/33150223
12. Kodama K, Higashiyama M, Yokouchi H, et al. Natural history of pure ground-glass opacity after long-term follow-up of more than 2 years. Ann Thorac Surg. 2002;73(2):
13. Chang B, Hwang JH, Choi YH, et al. Natural history of pure ground-glass opacity lung nodules detected by low-dose CT scan. Chest. 2013;143(1):172–178. doi:10.1378/chest.11-2501
14. Asanuma H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: survival outcomes of Japan clinical oncology group 0201. J Thorac Cardiovasc Surg. 2013;146(1):24–30. doi:10.1016/j.jtcvs.2012.12.047
15. Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65(1):107–111. doi:10.1016/j.ijrobp.2005.10.031
16. JASTRO Japanese Society for Radiation Oncology. English translation of JASTRO treatment policy of proton beam therapy. Ver1.0. 2016.
17. Kadoya N, Obata Y, Kato T. Dose-volume comparison of proton radiotherapy and stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79(4):1225–1231. doi:10.1016/j.ijrobp.2010.05.016
18. Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer. 2004;101(7):1623–1631. doi:10.1002/cncr.20539
19. Nagata Y, Hiraoka M, Shibata T, et al. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93(5):989–996. doi:10.1016/j.ijrobp.2015.07.2278
20. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi:10.1001/jama.2010.261
21. Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg. 2013;146(2):358–364. doi:10.1016/j.jtcvs.2013.02.008
22. Okada M, Mimae T, Tsutani Y, et al. Segmentectomy versus lobectomy for clinical stage IA lung adenocarcinoma. Ann Cardiothorac Surg. 2014;3(2):153–159.
23. Nagata Y, Kimura T. Stereotactic body radiotherapy (SBRT) for stage I lung cancer. Jpn J Clin Oncol. 2018;48(5):405–409. doi:10.1093/jjco/hyy034
24. Badiyan SN, Bierhals AJ, Olsen JR, et al. Stereotactic body radiation therapy for the treatment of early-stage minimally invasive adenocarcinoma or adenocarcinoma in situ (formerly bronchioloalveolar carcinoma): a patterns of failure analysis. Radiat Oncol. 2013;8:4. doi:10.1186/1748-717X-8-4
25. Cho JH, Choi YS, Kim J, Kim HK, Zo JI, Shim YM. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg. 2015;99(1):218–222. doi:10.1016/j.athoracsur.2014.07.068
26. Chi A, Chen H, Wen S, Yan H, Liao Z. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: a systematic review and hypothesis-generating meta-analysis. Radiother Oncol. 2017;123(3):346–354. doi:10.1016/j.radonc.2017.05.007
27. Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol. 2010;97(3):425–430. doi:10.1016/j.radonc.2010.09.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

