Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Clinical outcomes and risk factors in patients with cervical metastatic spinal cord compression after posterior decompressive and spinal stabilization surgery
Authors Lei M, Yu J, Yan S, An X, Liu Y
Received 19 August 2018
Accepted for publication 15 November 2018
Published 11 January 2019 Volume 2019:15 Pages 119—127
DOI https://doi.org/10.2147/TCRM.S184497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Mingxing Lei,1,* Jun Yu,2,* Shiju Yan,1 Xiao An,1 Yaosheng Liu3
1Department of Orthopedic Surgery, Hainan Hospital of the PLA General Hospital, Sanya, People’s Republic of China; 2Department of Anesthesiology, The Fourth Medical Center of PLA General Hospital, Beijing, People’s Republic of China; 3Department of Orthopedic Surgery, The 307th Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Purpose: The aim of this study was to investigate the clinical results of surgery for cervical spine metastasis and identify clinical risk factors affecting postoperative survival and neurological outcome.
Patients and methods: A retrospective analysis of medical records was performed on 19 patients who had undergone decompressive surgery and spine stabilization due to metastatic spinal cord compression in the cervical spine. All patients had severe pain before surgery. Worst pain, average pain, and pain interference were evaluated using the visual analog scale (range, 0–10) for each patient at baseline and following surgery. Neurological recovery was assessed using the Japanese Orthopaedic Association Score (JOAS). In addition, associations between ten characteristics and postoperative survival and neurological outcomes were analyzed in the study.
Results: The mean worst pain score in a 24-hour period was 8.6 before the operation. At 1 day, 1, 3, 6, and 12 months after the operation, the mean worst pain scores decreased to 5.6, 4.5, 3.8, 2.6, and 2.4 (all P<0.001 vs baseline), respectively. Similar decreases in average pain and pain interference were also observed. The median JOAS in a 24-hour period was 11.0 before the operation. At 1 day, 1, 3, 6, and 12 months after the operation, the median JOAS increased to 12.0 (P=0.469), 13.0 (P=0.010), 14.0 (P<0.001), 15.0 (P<0.001), and 14.0 (P<0.001), respectively. According to the multivariate analysis, postoperative survival was significantly associated with the type of primary tumor (P=0.033), preoperative ambulatory status (P=0.004), extra-spinal bone metastasis (P=0.021), 125I seed brachytherapy (P=0.014), and complication status (P=0.009). Better neurological outcome was found to be correlated with higher JOAS (P=0.013). Surgery-related complications occurred in 26.3% of patients.
Conclusion: Posterior decompression and spine stabilization for painful cervical spine metastasis resulting from spinal cord compression were found to be effective for neurological recovery and pain control with a tolerable rate of complications.
Keywords: cervical spine metastasis, surgery, survival prognosis, neurological outcome, visual analog scale, Japanese Orthopaedic Association Score
Introduction
Cervical spine metastasis is less frequent than metastasis to other parts of the spine.1 Unfortunately, metastatic disease in the cervical spine can result in severe pain, respiratory failure, and neurological quadriplegia due to metastatic spinal cord compression (MSCC).2 MSCC is regarded as an oncologic emergency that occurs in up to 10% of adult patients with cancer during their disease courses and can become symptomatic, which involves intractable pain, neurological deficits, or even bladder and bowel dysfunction, adversely affecting the patient’s quality of life.2,3 Treatments for MSCC in the cervical spine include radiotherapy,4 vertebroplasty,5 surgery,6 corticosteroids, and symptom control, alone or in combination, which are effective for improving or maintaining neurological function, relieving pain, stabilizing the spine, and positively improving the patient’s quality of life.7
In 2005, a phase III trial strongly suggested that direct decompressive surgery following postoperative radiotherapy was superior to treatment with radiotherapy alone for MSCC in terms of ambulatory status, regaining the ability to walk, ambulatory duration, and survival in selected patients. Notably, patients with MSCC in the cervical spine were included in this study.8 Therefore, posterior approaches, starting with decompressive laminectomy followed by spine stabilization, have traditionally been the most common surgical procedures for MSCC.
Surgery is technically challenging in the cervical spine due to the complicated anatomy of this region, and metastatic disease in the cervical spine is considered a poorer prognosis than in the thoracic and lumbar spine. Few publications have addressed the surgical results and clinical outcomes of cervical spine metastasis, let alone MSCC in cervical spine treated with posterior decompression and spine stabilization. In this study, pain outcome and neurological recovery were used to evaluate surgical results, and factors for survival and neurological outcomes were also analyzed.
Patients and methods
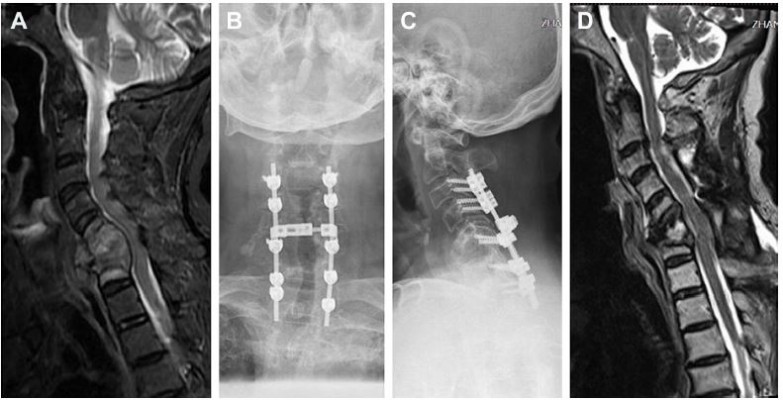
Nineteen patients with MSCC in the cervical spine who had been operated with decompression and spine stabilization were retrospectively analyzed in our department between May 2013 and May 2017. The indication for surgery was neurological deficit (sensory and/or motor function impairment, sphincter dysfunction) due to MSCC which had been confirmed by spinal magnetic resonance imaging, and a life expectancy of at least 3 months. Surgery was not performed on those whose expected survivals were <3 months and in those whose health was too poor to undergo surgery. That was consistent with the Spine Oncology Study Group.9 All patients had severe pain before surgery. The diagnosis of cancer or bone metastasis was confirmed histologically. All the patients were operated with posterior decompression and spine stabilization in our department (Figure 1). Corticosteroids were routinely used before surgery. Local radiotherapy, systemic chemotherapy, and targeted therapy were performed after the wound healed, about 3–4 weeks after the surgery. Patients with missing data were not included. The Medical Research Ethics Board of the Affiliated Hospital of Academy of Military Medical Sciences approved this retrospective study and waived patient consent for review of medical images and records, as all data were anonymized. This study was conducted in accordance with the Declaration of Helsinki.
Visual analog scale (VAS), ranging from 0 to 10, was used to evaluate the worst pain, average pain, and pain interference for each patient at baseline and following surgery within 24 hours and at 1, 3, 6, and 12 months. Neurological recovery was assessed based on Japanese Orthopaedic Association Score (JOAS; Table 1) within 24 hours before the operation and 24 hours, 1, 3, 6, and 12 months after the operation. In addition, 10 characteristics were analyzed for postoperative neurological and survival outcome. These characteristics included age (≤57 years vs >57 years, median age: 57 years), sex (female vs male), type of primary cancer (slow growth vs rapid growth), ambulatory status (can vs cannot), extra-spinal bone metastasis (no vs yes), visceral metastasis (no vs yes), time for developing motor deficit (≤2 weeks vs >2 weeks), 125I seed brachytherapy (no vs yes), complication (no vs yes), and JOAS (severe: score <10 vs mild: score ≥10). All the above-mentioned characteristics were commonly analyzed in previous studies.
  | Table 1 Neurological function as determined using the JOAS system |
Analysis of the VAS and JOAS in a 24-hour period was performed via repeated measures of the correlated variance model across each time point, supplemented by Wilcoxon rank-sum test. Univariate and multivariate analyses of the survival outcome were performed with the Kaplan–Meier method and the log-rank test and the multiple Cox regression model, respectively. Both univariate and multivariate analyses of the neurological prognosis were performed with the ordered-logit model. A P-value of ≤0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.2 software (SAS Institute Inc, Cary, NC, USA).
Results
Patient characteristics
Patients’ demographic data are summarized in Table 2. The median age of the patients (9 females and 10 males) was 51 years (range: 29–69 years). Cases were classified into two groups according to primary tumor’s growth rate. The slow growth group included breast cancer (four cases), thyroid cancer (three cases), and renal cancer (one case) while the rapid growth group included lung cancer (eight cases), esophagus cancer, colorectal cancer, and unknown original cancer site (one case each). The involved levels were C1 in one case (5%), C2 in three cases (16%), C3 in three cases (16%), C4 in four cases (21%), C5 in five cases (26%), C6 in seven cases (37%), and C7 in seven cases (37%), which showed low incidence in the upper cervical spine. The median length of follow-up was 10 months (range: 3–18 months).
Surgical results
The mean worst pain score in a 24-hour period was 8.6 before the operation. At 1 day, 1, 3, 6, and 12 months after the operation, the mean worst pain scores decreased to 5.6, 4.5, 3.8, 2.6, and 2.4, respectively (all P<0.001 vs baseline; Table 3, Figure 2). Similar decreases in average pain and pain interference were observed. In detail, the worst pain relief (a 2-score drop in pain was defined as clinically significant relief) was observed in 78.9% of the patients, average pain relief was achieved in 89.5% of the patients, and pain interference relief occurred in 84.2% of the patients after surgery. The median JOAS in a 24-hour period was 11.0 before the operation. At 1 day, 1, 3, 6, and 12 months after the operation, the median JOAS increased to 12.0 (P=0.469), 13.0 (P=0.010), 14.0 (P<0.001), 15.0 (P<0.001), and 14.0 (P<0.001), respectively.
  | Table 3 Brief inventory and JOAS at baseline and following surgery |
Clinical outcomes
In the entire cohort of 19 patients, the overall median survival time was 11.5 months; 6-month and 12-month survival rates were 73.7% and 46.3%, respectively. According to the univariate analysis, factors that were found to be related to longer survival were slow growth cancer, ambulatory ability, 125I seed brachytherapy, no surgery-related complication, and higher JOAS (Table 4). Median survival time was 13.7 months (95% CI, 3.0–45.1 months) for patients with slow growth cancer and 6.4 months (95% CI, 1.9–11.5 months) for those with rapid growth cancer, and the difference was significant (P<0.001). Besides, patients who were ambulatory had longer median survival time than those who were not (13.7 months; 95% CI, 6.2–31.4 months vs 5.4 months; 95% CI, 0.5–8.2 months) (P=0.024). And patients who had undergone 125I seed brachytherapy also had longer median survival (20.8 months; 95% CI, 6.4–35.0 months vs 6.2 months; 95% CI, 1.9–13.6 months) (P=0.034). Furthermore, patients with postoperative complications (4.1 months; 95% CI, 0.5–11.4 months) were found to have shorter median survival than those without any postoperative complication (13.7 months; 95% CI, 6.4–31.4 months) (P<0.001). Finally, compared with patients with lower JOAS (4.4 months; 95% CI, 1.9–11.4 months), patients with higher JOAS were observed to have longer median survival (13.7 months; 95% CI, 6.4–31.4 months) (P=0.010). According to the multivariate analysis of survival outcome, the type of primary cancer (P=0.033), ambulatory status (P=0.004), extra-spinal bone metastasis (P=0.021), 125I seed brachytherapy (P=0.014), and surgery-related complication (P=0.009) maintained significance. JOAS lost significance, while extra-spinal bone metastasis became significant. As for the neurological outcome, ambulatory status (P=0.028), surgery-related complication (P=0.019), and JOAS (P=0.013) had significant impact based on the univariate analysis, while only JOAS (P=0.013) showed significance based on the multivariate analysis. Notably, patients with ambulatory ability (P=0.028), no surgery-related complication (P=0.019), and higher JOAS (P=0.013) had better neurological outcome. Besides, patients with no visceral metastasis and shorter time for developing motor deficit also had a better neurological outcome, but both did not reach significance.
Complications
Surgery-related complications occurred in five patients (26.3%). Operation site infection was observed in two cases, which was successfully treated by continuous irrigation. One patient showed epidural hematoma and required surgical removal. Cerebrospinal fluid leakage was found in one case and required percutaneous lumbar drainage. Pneumonia occurred in one case and was controlled by antibiotics.
Discussion
MSCC is an oncological emergency, which initially and reversibly causes edema, venous congestion, and demyelination, and prolonged compression can lead to vascular injury, cord necrosis, and permanent damage. Importantly, patients who have no neurological function for >48 hours are unlikely to improve.10 Current data suggest that radiation therapy, corticosteroids, and surgery, alone or in combination, can relieve pain and preserve neurologic function.7 The commonly used surgical procedures for patients with MSCC include excisional surgery, palliative decompression, and minimally invasive surgery. Excisional surgery can remove the whole metastasis and may realize long-term survival without cancer burden, but its complication rates are very high. Generally speaking, palliative decompression is the standard surgical procedure for MSCC. Palliative decompression, posterior decompression, and spine stabilization, had lesser complication rates, lower bleeding risks, and adequate decompressions as compared with excisional surgery. Thus, rapid decompression and immediate spine stabilization for MSCC has become the standard treatment for MSCC due to its increased efficacy over conventional radiotherapy in preserving neurological function and improving survival prognosis.8,11
Reduction in pain and the preservation of motor function in patients with MSCC may significantly improve the patients’ quality of life. Surgery for MSCC in the cervical spine was found to be effective in terms of pain control and neurological recovery. In the present study, the worst pain, average pain, and pain interference showed improvement when preoperative and postoperative pain scores were compared at each time point. In detail, the worst pain relief (a 2-score drop in pain was defined as clinically significant relief) was observed in 78.9% of the patients, average pain relief was achieved in 89.5%, and pain interference relief occurred in 84.2% after surgery. Regarding neurological outcome, postoperative JOAS was increased at each time point as compared with preoperative JOAS. 68.4% of the patients could walk 4 weeks after surgery, 15.8% of the nonambulatory patients before operation regained the ability to walk, and 52.6% of the patients maintained their ambulatory status. Similar results were obtained in another prospective multicenter study. Fehlings et al12 concluded that surgical intervention provided immediate and sustained improvement in pain, neurologic, functional, and patients’ quality of life after analyzing 142 MSCC patients. There was an improvement in ambulatory status, lower extremity, and total motor scores at 6 months after surgery. Oswestry Disability Index and pain interference also improved at 6 weeks and 3, 6, and 12 months, postoperatively. Moreover, at 3 months after surgery, the American Spinal Injury Association impairment scale grade improved. Regarding surgical procedure, surgical levels were similar with the cancer-involved levels. Generally, the fusion range was the upper and lower two vertebrae of the surgical levels.
Several prognostic factors have been identified to assess survival prognosis after surgery for spinal metastasis. Tokuhashi et al13,14 presented a scoring system including six parameters, ie, general condition, number of extra-spinal bone metastases, number of spinal metastases, the incidence of metastases to a major internal organ, type of primary malignancy, and finally grade of neurological deficit. Sioutos et al15 found that preoperative neurological status, the anatomic site of the primary carcinoma, and the number of vertebral bodies involved were significantly associated with survival. Robson10 reported that prognostic indicators that suggest surgery would more likely be beneficial are histological findings such as multiple myeloma, lymphoma, or breast, prostate, or renal cancers, good motor function at presentation, good performance status, limited comorbidity, single-level spinal disease, absence of visceral metastasis, and long interval from primary diagnosis. Bauer and Wedin16 showed that absence of visceral metastases, solitary skeletal metastases, and type of primary cancer (not lung, breast, and renal cancer, lymphoma or myeloma) were significantly associated with longer survival. Heidecke et al17 concluded that histology of the primary tumor, the extent of metastasis, and baseline general condition were the most important prognostic factors for survival in patients with cervical metastasis after surgery.
Except for the factors found in the study of Heidecke et al, the above prognostic factors were not specific to cervical spine metastasis after surgery. However, participants in the Heidecke et al study were operated for spine metastasis in general, not particularly for MSCC. In our series, the type of primary cancer, ambulatory status, extra-spinal bone metastasis, 125I seed brachytherapy, and surgery-related complication status were found to be significantly associated with postoperative survival in patients with MSCC in cervical spine after surgery. And, only JOAS had a significant impact on the postoperative neurological outcome. As for the postoperative overall survival and survival rates, the median overall survival was found to be 11.5 months and the 1-year survival rate was 46.3%, which conformed to other studies.17,18 Surgery-related complications occurred in 26.3% of the entire cohort of patients and were reported in other studies to occur in 10%–30% of patients.12,19,20
The limitation of this study includes the following aspects. First, the study was based on retrospective data, which unavoidably result in bias. Second, the statistical analyses included only 19 cases and over 10 years of data were used. The changes in diagnosis, treatment, clinical/medical knowledge, etc may remarkably influence the surgical results and postoperative outcomes. Finally, data on systemic treatment following treatment were not available in most patients, which also could bias survival outcome and surgical results. Therefore, a larger and prospective study is warranted.
In conclusion, posterior decompression and spine stabilization for painful cervical spine metastasis resulting from spinal cord compression was found to be effective for neurological recovery and pain control with a tolerable rate of complications. The type of primary cancer, ambulatory status, extra-spinal bone metastasis, 125I seed brachytherapy, and surgery-related complications were found to be significantly associated with postoperative survival, and JOAS has a significant impact on the postoperative neurological outcome. These factors can help select the individual treatment methodology for patients with MSCC in the cervical spine.
Acknowledgment
This study was funded by the Medical and Health Science and Technology Innovation Project of Sanya (no 2018YW04), and the PLA General Hospital Science and Technology Innovation Nursery Fund Project (no.17KMM31).
Disclosure
The authors report no conflicts of interest in this work.
References
Rajagopal T, Hutt N, Quraishi N. Analysis of levels of metastatic spinal cord compression (MSCC) and outcome of surgery in relation to the histological type of primary tumour. Eur Spine J. 2011;20:S475–S476. | ||
Cho W, Chang UK. Neurological and survival outcomes after surgical management of subaxial cervical spine metastases. Spine. 2012;37(16):E969–E977. | ||
Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15–24. | ||
Rades D, Abrahm JL. The role of radiotherapy for metastatic epidural spinal cord compression. Nat Rev Clin Oncol. 2010;7(10):590–598. | ||
Masala S, Anselmetti GC, Muto M, Mammucari M, Volpi T, Simonetti G. Percutaneous vertebroplasty relieves pain in metastatic cervical fractures. Clin Orthop Relat Res. 2011;469(3):715–722. | ||
Molina CA, Gokaslan ZL, Sciubba DM. Diagnosis and management of metastatic cervical spine tumors. Orthop Clin North Am. 2012;43(1):75–87. | ||
Hammack JE. Spinal cord disease in patients with cancer. Continuum. 2012;18(2):312–327. | ||
Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. | ||
Fehlings MG, David KS, Vialle L, Vialle E, Setzer M, Vrionis FD. Decision making in the surgical treatment of cervical spine metastases. Spine. 2009;34(22 Suppl):15:S108–S117. | ||
Robson P. Metastatic spinal cord compression: a rare but important complication of cancer. Clin Med. 2014;14(5):542–545. | ||
Chaichana KL, Woodworth GF, Sciubba DM, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62(3):683–692. | ||
Fehlings MG, Nater A, Tetreault L, et al. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol. 2016;34(3):268–276. | ||
Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15(11):1110–1113. | ||
Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30(19):2186–2191. | ||
Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76(8):1453–1459. | ||
Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand. 1995;66(2):143–146. | ||
Heidecke V, Rainov NG, Burkert W. Results and outcome of neurosurgical treatment for extradural metastases in the cervical spine. Acta Neurochir. 2003;145(10):873–881. | ||
Sung SO, Jeon BC, Hs O. Anterior cervical stabilization for cervical spine metastases. Kor J Spine. 2007;4:24–30. | ||
Oda I, Abumi K, Ito M, et al. Palliative spinal reconstruction using cervical pedicle screws for metastatic lesions of the spine: a retrospective analysis of 32 cases. Spine. 2006;31(13):1439–1444. | ||
Atanasiu JP, Badatcheff F, Pidhorz L. Metastatic lesions of the cervical spine. A retrospective analysis of 20 cases. Spine. 1993;18(10):1279–1284. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




