Back to Journals » International Journal of Women's Health » Volume 16
Causal Relationship Between Endometriosis and Pelvic Inflammatory Diseases: Mendelian Randomization Study
Authors Liu K, Liu X, Cao T, Cui X, Sun P, Zhang L, Wu X
Received 13 September 2023
Accepted for publication 15 April 2024
Published 24 April 2024 Volume 2024:16 Pages 727—735
DOI https://doi.org/10.2147/IJWH.S440110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Kang Liu, Xiaochun Liu, Tao Cao, Xianmei Cui, Pengyu Sun, Liang Zhang, Xiaoqin Wu
Department of Obstetrics and Gynecology, Shanxi Bethune Hospital & Shanxi Academy of Medical Sciences, Shanxi Bethune Hospital Affiliated to Shanxi Medical University, Taiyuan, 030032, People’s Republic of China
Correspondence: Xiaoqin Wu, Department of Obstetrics and Gynecology, Shanxi Bethune Hospital & Shanxi Academy of Medical Sciences, Shanxi Bethune Hospital affiliated to Shanxi Medical University, Taiyuan, 030032, People’s Republic of China, Email [email protected]
Objective: This study explores the causal relationship between endometriosis and pelvic inflammatory diseases (PID).
Methods: The study utilized genome-wide association study (GWAS) datasets for endometriosis (“finn-b-N14_ENDOMETRIOSIS“) and PID (”finn-b-N14_OTHFEMPELINF”). Subsequently, two-sample Mendelian randomization (MR) analyses were conducted using inverse variance weighting (IVW), Egger regression (MR-Egger), and weighted median (WM) methods. Heterogeneity was evaluated using Cochran’s Q test, and in case of detected outliers, they were removed for re-evaluation of MR causality.
Results: From the endometriosis GWAS dataset, 33 single nucleotide polymorphisms (SNPs) were selected as instrumental variables. All three methods, IVW (OR = 1.39, P < 1× 10− 8), MR-Egger (OR = 1.41, P = 0.003), and WM (OR = 1.37, P = 1.16× 10− 5) confirmed a causal relationship between endometriosis and PID. The association between endometriosis and pelvic inflammation remained unaffected by the exclusion of individual SNPs. Lastly, Cochran’s Q test and funnel plots showed no evidence of SNP asymmetry.
Conclusion: The results of the MR analysis support a potential causal relationship between endometriosis and an increased risk of PID.
Keywords: endometriosis, pelvic inflammatory diseases, genome-wide association study, Mendelian randomization
Introduction
Pelvic inflammatory disease (PID) is an infection affecting the endometrium, fallopian tubes, ovaries, and pelvic peritoneum.1,2 The patient’s clinical symptoms mainly include pelvic pain, abnormal vaginal discharge, fever and chills, and painful urination.3,4 Due to its nonspecific symptoms, misdiagnosis or delayed diagnosis are usually happened which can lead to inflammatory sequelae including infertility, ectopic pregnancy, and chronic pelvic pain.1,2 According to 2013–2014 data, there were 110,000 cases of PID among women aged 18–44.5,6 Considering the estimated treatment cost of $1995 per patient in previous years, this undeniably poses a significant healthcare concern.1 Sexually transmitted infections and bacterial vaginosis have been considered as predominantly causes of PID. However, research shows that as society evolves, a variety of factors may be associated with this disease. In the 1950s, PID was associated with Mycobacterium tuberculosis and Neisseria gonorrhoeae, while in the 1980s, Chlamydia trachomatis became linked to PID.7 Recent clinical trial results for PID in American women showed that only 25% of cases were due to sexually transmitted infections.8,9 Advances in clinical screening technology have led to the recognition of certain factors, such as postpartum or postabortion infection, intrauterine devices, endometrial surgery, and endometriosis, as predisposing factors for PID.
Endometriosis is a disease in which tissue similar to the lining of the uterus (endometrium) grows on the ovaries, fallopian tubes, and other pelvic structures. It affects 6–10% of reproductive-aged women, causing symptoms such as dysmenorrhea, dyspareunia, chronic pelvic pain, irregular uterine bleeding, and/or infertility, significantly impacting their quality of life.10,11 Moreover, endometriosis is linked to various diseases including ovarian cancer, breast cancer, autoimmune disorders, cardiovascular diseases, and asthma.12,13 PID and endometriosis can share common symptoms and pathogenesis factors. An increasing number of researchers are turning their attention to the connection between PID and endometriosis. Lei Wan et al analyzed public data from the National Health Insurance Research Database (NHIRD) and demonstrated that patients with PID had a three-fold increase in the risk of developing endometriosis.14 On the other hand, studies confirmed that having endometriosis is a high-risk factor for PID and PID recurrence.10,15 However, traditional observational studies have some limitations, and the associations between endometriosis and PID reported in previous studies may still be explained by reverse causality and residual confounding. Thus, whether there is a causal relationship between endometriosis and PID remains uncertain.
To study the cause-and-effect link between endometriosis and PID, we can employ Mendelian randomization (MR). MR is a method that uses genetic variants as instrumental variables to estimate the causal impact of risk factors on health outcomes.16 In comparison to traditional multivariate observational analysis, Mendelian Randomization (MR) is less susceptible to the influence of confounding variables and measurement errors, and it avoids biases arising from reverse causation. Therefore, MR has become a reliable method for obtaining robust estimates of the causal effects of various risk factors on health outcomes, often yielding results similar to those of randomized controlled trials.
In the present study, we employ a two-sample MR approach to evaluate the influence of endometriosis on PID risk, revealing potential associations between the two conditions. Our results support a potential causal relationship between endometriosis and an increased risk of PID.
Methods
Study Design
The study utilized the Integrative Epidemiology Unit Open Genome-Wide Association Studies database (IEU openGWAS, https://gwas.mrcieu.ac.uk/), which aggregates data from various studies involving genetic variations related to endometriosis (GWAS ID: finn-b-N14_ENDOMETRIOSIS, a European database has 16,377,306 SNPs from 77,257 samples.)17 and pelvic inflammatory disease (GWAS ID: finn-b-N14_OTHFEMPELINF, a European database has 16,379,585 SNPs from 114,771 samples.). We selected genetic variants associated with endometriosis and pelvic inflammatory disease, ensuring a minimum minor allele frequency (MAF) greater than 0.01 in the European population (Figure 1). The study adhered to the three core assumptions of MR research: (1) the selected genetic variants SNPs should be closely related to the exposure, (2) the selected genetic variants SNPs should be independent of confounding factors, and (3) the selected genetic variants SNPs should affect the outcome only through the exposure, but not directly related. This study was based on publicly available pooled data, and the protocol and data collection were approved by the ethics committee of each original association study, following the Strengthening the Reporting of Observational Studies in Epidemiology-Mendelian Randomization (STROBE-MR) guidelines.18
Pelvic Inflammatory Disease GWAS Summary Statistics
To investigate the causal relationship between endometriosis and pelvic inflammatory disease risk, we systematically retrieved summary data from the pelvic inflammatory disease GWAS. The IEU OpenGWAS database’s pelvic inflammatory disease GWAS data (finn-b-N14_OTHFEMPELINF) consisted of summary statistics from a study involving 2913 cases and 111,858 female controls, encompassing 16,379,585 SNP loci.
Genetic Instrument Variable Selection
To study the causal relationship between endometriosis and pelvic inflammatory disease risk, we employed instrumental variables to assess the relationship between the two conditions. We identified the endometriosis GWAS to identify genetic variants associated with endometriosis. Initially, we selected SNPs associated with endometriosis as candidate instrumental variables for MR analysis (P < 5e-06). Subsequently, to ensure exposure-related instrumental variables were independent, we used linkage disequilibrium (LD) clumping to exclude SNPs in LD (r2 = 0.01, clumping window = 10Mb). Finally, to avoid bias from weak instrumental variables, we calculated the strength of instrumental variables using the F-statistic, with an F-statistic greater than 10 indicating a low likelihood of weak instrumental variables. Instrumental variable selection and quality control were conducted using the R package TwoSampleMR (v 0.5.6).
Mendelian Randomization Estimation
The study employed the inverse variance weighting (IVW) method to assess the effect size of each instrumental variable, estimating the causal impact of exposure on outcomes using the Wald ratio. The MR Egger regression intercept was utilized to assess pleiotropy, and if p > 0.05, it was determined that there was no evidence of pleiotropy. MR-Egger and weighted median (WM) results further confirmed the causal relationship between endometriosis and pelvic inflammatory disease. The primary distinction between MR-Egger and IVW is that MR-Egger considers the presence of an intercept and fits using the inverse of the outcome variance as weights, while IVW does not consider the presence of an intercept. Furthermore, Cochran’s Q test was used to assess heterogeneity, and if outliers were detected, they were removed, and MR causality was reassessed. Statistical analysis and data visualization were performed using R software version 4.1.3 (https://www.r-project.org/).
Results
Characteristics of Instrumental Variable SNPs
The study initially selected 35 SNPs highly associated with endometriosis (P < 5e-06, r2 = 0.01, clumping window = 10Mb). Subsequently, SNPs with inconsistent alleles and those with irreparable palindromic SNPs (rs9482771) were discarded, leaving 33 remaining SNPs. These 33 SNPs were rs112726611, rs77971566, rs749749, rs10757277, rs9706167, rs45497400, rs34297508, rs12213593, rs17150482, rs9383585, rs364950, rs106871, rs115448220, rs6908034, rs2249850, rs11031005, rs113470730, rs9600230, rs57439315, rs34324306, rs55667387, rs140993356, rs28517654, rs35961234, rs58415480, rs17694933, rs34505094, rs8086179, rs1566646, rs6546324, rs10917151, rs2092753, rs9485296. To assess the strength of each instrumental variable, we calculated the F-statistic for each instrumental variable. In our study, the F-statistic was substantially greater than 10, indicating these SNPs are strong instrumental variables (Table 1).
 |
Table 1 Information on Single Nucleotide Polymorphism (SNP) Loci Associated with Endometriosis |
Causal Relationship Between Endometriosis and Pelvic Inflammatory Disease
Based on summary data from the pelvic inflammatory disease GWAS (2913 cases and 111,858 female controls), MR analysis was conducted to evaluate the causal impact of endometriosis on pelvic inflammatory disease. MR estimates from various methods all indicated a causal relationship between endometriosis and pelvic inflammatory disease (Figure 1, Table 2). The specific results were as follows: IVW, OR = 1.39, P < 1×10‒8; MR-Egger, OR = 1.41, P = 0.003; WM, OR = 1.37,P = 1.16×10‒5; Weighted mode, OR = 1.64, P = 0.0003. Furthermore, the intercept represents the average pleiotropic effect of genetic variation, and IVW, MR-Egger, WM, and Weighted mode all indicated minimal presence of confounding factors within the data (Figure 2).
 |
Table 2 Evaluation of the Impact of Endometriosis on the Causal Relationship with Pelvic Inflammatory Disease Using Inverse Variance Weighting (IVW), MR-Egger, and Weighted Median (WM) Methods |
Heterogeneity and Sensitivity Analysis
Heterogeneity refers to the variability of causal estimates obtained from each SNP. Low heterogeneity suggests increased reliability of MR estimates. Cochran’s Q test indicated low heterogeneity for IVW and MR-Egger, further confirming the increased reliability of the causal relationship between endometriosis and pelvic inflammatory disease (Table 3). After performing individual SNP exclusion tests, the association between endometriosis and pelvic inflammatory disease remained unaffected by any single SNP (Figure 3A). Subsequently, the funnel plot revealed no evidence of asymmetric SNP distribution (Figure 3B).
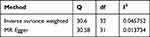 |
Table 3 Cochran’s Q Assessment of Heterogeneity for Inverse Variance Weighting (IVW) and MR-Egger Results in the Relationship Between Endometriosis and Pelvic Inflammatory Disease |
Discussion
In this study, we employed extensive GWAS data from publicly available databases and conducted an unbiased two-sample MR analysis to investigate the causal associations between endometriosis and PID. Following rigorous quality control measures, we obtained the results to confirm that there is a causal relationship between endometriosis and PID. Our results provide a new theoretical basis for clinical diagnosis and prevention of PID.
Endometriosis and PID are two distinct gynecological conditions, sometimes they may have interconnect and impact on reproductive health.19 More and more researchers are paying attention to the relationship between endometriosis and PID. Current reports suggest that endometriosis may affect PID through multiple potential pathways. Firstly, the infiltration of macrophages and abnormal activation of vascularization in endometriosis lead to adhesions and structural changes in pelvic organs.20–22 Subsequently, these altered structures make it easier for bacteria to thrive, thus increasing the risk of PID. Secondly, the presence of stagnant blood in endometriotic lesions may raise the pH of surrounding organs, impacting the local microbial environment, lead to bacterial infection, and subsequently induce PID.23,24 Furthermore, the cyclic renewal of the lesion site during the menstrual cycle creates a favorable nutritional environment for bacterial proliferation, promoting the spread of infection.25,26 Thirdly, the microbiota normally regulates inflammation, immunity, and metabolism, forming a protective barrier against pathogen invasion and eliminating pathogenic microbes.27–29 Changes in the microbiota around endometriotic lesions—specifically, decreased abundance of lactobacilli in the lower reproductive tract and diverse anaerobic bacteria in the upper reproductive tract, peritoneum, and endometriotic lesions, including Gardnerella, Streptococcus, Enterococcus, and Escherichia coli—facilitate the propagation of pathogens associated with bacterial vaginosis, leading to the development of PID.30–32 Fourthly, dysregulation of the local immune system in patients with endometriosis, such as increased number and activation of peritoneal macrophages and dysregulation of T lymphocytes, can further lead to changes in local and systemic inflammatory mediators.33–36 Abnormally levels of inflammatory factors can lead to persistent inflammation, impeding timely clearance of bacteria and leading to PID.2,37 Additionally, endometriosis exhibits abnormal DNA methylation, histone modifications, and non-coding RNA expression, inducing further changes in immune cell function, quantity, and phenotype, compromising the immune defense mechanisms and exacerbating PID progression.38–40
Consistent with previous observational studies. Our study employed MR analysis to assess the causal relationship between endometriosis and PID. Given that MR studies can be influenced by genetic variation and associations with multiple variables, we used the weighted median (WM) method to address pleiotropy, conducted MR-Egger for assessing directional pleiotropy, and conducted sensitivity analysis to examine the robustness of our MR study. The results from IVW, MR-Egger, and WM methods consistently confirmed the causal relationship between endometriosis and PID.
This study has its own limitations: (1) The GWAS data are derived exclusively from European populations. Therefore, the findings may not be extrapolated to other ethnic groups and regions. More comprehensive studies across different ethnic groups are needed; (2) Although utilizing the largest available large-scale GWAS data, follow-up research efforts should focus on further expanding the sample size to produce more precise assessments.
Conclusion
In conclusion, our study findings demonstrate that endometriosis is a risk factor for PID, suggesting a potential causal relationship between endometriosis and increased PID risk. This sheds new light on the underlying mechanisms of the potential association between endometriosis and PID, providing a novel direction for further exploration.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This study was supported by the Science and Technology Innovation Program of Shanxi Higher Education Institutions (grant Nos. 2022L161) and the Natural Science Foundation of Shanxi Province (grant Nos. 20210302124591).
Disclosure
The authors declared that they have no conflicts of interest in this work.
References
1. Hillier SL, Bernstein KT, Aral S. A review of the challenges and complexities in the diagnosis, etiology, epidemiology, and pathogenesis of pelvic inflammatory disease. J Infect Dis. 2021;224(12 Suppl 2):S23–S28. doi:10.1093/infdis/jiab116
2. Darville T. Pelvic inflammatory disease due to Neisseria gonorrhoeae and chlamydia trachomatis: immune evasion mechanisms and pathogenic disease pathways. J Infect Dis. 2021;224(12 Suppl 2):S39–S46. doi:10.1093/infdis/jiab031
3. Eggert J, Sundquist K, van Vuuren C, Fianu-Jonasson A. The clinical diagnosis of pelvic inflammatory disease--reuse of electronic medical record data from 189 patients visiting a Swedish University Hospital emergency department. BMC Womens Health. 2006;6(1):16. doi:10.1186/1472-6874-6-16
4. Risser WL, Risser JM, Risser AL. Current perspectives in the USA on the diagnosis and treatment of pelvic inflammatory disease in adolescents. Adolesc Health Med Ther. 2017;8:87–94. doi:10.2147/AHMT.S115535
5. Shroff S. Infectious vaginitis, cervicitis, and pelvic inflammatory disease. Med Clin North Am. 2023;107(2):299–315. doi:10.1016/j.mcna.2022.10.009
6. Frock-Welnak DN, Tam J. Identification and treatment of acute pelvic inflammatory disease and associated sequelae. Obstet Gynecol Clin North Am. 2022;49(3):551–579. doi:10.1016/j.ogc.2022.02.019
7. Mitchell CM, Anyalechi GE, Cohen CR, Haggerty CL, Manhart LE, Hillier SL. Etiology and diagnosis of pelvic inflammatory disease: looking beyond gonorrhea and chlamydia. J Infect Dis. 2021;224(12 Suppl 2):S29–S35. doi:10.1093/infdis/jiab067
8. Price MJ, Ades AE, Welton NJ, Simms I, Macleod J, Horner PJ. Proportion of pelvic inflammatory disease cases caused by chlamydia trachomatis: consistent picture from different methods. J Infect Dis. 2016;214(4):617–624. doi:10.1093/infdis/jiw178
9. Lillis R, Kuritzky L, Huynh Z, et al. Outpatient sexually transmitted infection testing and treatment patterns in the United States: a real-world database study. BMC Infect Dis. 2023;23(1):469. doi:10.1186/s12879-023-08434-2
10. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–852. doi:10.1016/S0140-6736(21)00389-5
11. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15(11):666–682. doi:10.1038/s41574-019-0245-z
12. Saunders PTK, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021;184(11):2807–2824. doi:10.1016/j.cell.2021.04.041
13. Salliss ME, Farland LV, Mahnert ND, Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. 2021;28(1):92–131. doi:10.1093/humupd/dmab035
14. Tai FW, Chang CY, Chiang JH, Lin WC, Wan L. Association of pelvic inflammatory disease with risk of endometriosis: a nationwide cohort study involving 141,460 individuals. J Clin Med. 2018;7(11):379. doi:10.3390/jcm7110379
15. Zografou Themeli M, Nirgianakis K, Neumann S, Imboden S, Mueller MD. Endometriosis is a risk factor for recurrent pelvic inflammatory disease after tubo-ovarian abscess surgery. Arch Gynecol Obstet. 2023;307(1):139–148. doi:10.1007/s00404-022-06743-6
16. Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–1621. doi:10.1001/jama.2021.18236
17. Garitazelaia A, Rueda-Martinez A, Arauzo R, et al. A systematic two-sample Mendelian randomization analysis identifies shared genetic origin of endometriosis and associated phenotypes. Life. 2021;11(1):24. doi:10.3390/life11010024
18. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. doi:10.7554/eLife.34408
19. Huang JY, Yang SF, Wu PJ, Wang CH, Tang CH, Wang PH. Different influences of endometriosis and pelvic inflammatory disease on the occurrence of ovarian cancer. Int J Environ Res Public Health. 2021;18(16). doi:10.3390/ijerph18168754
20. Mabrouk M, Di Donato N, Montanari G, Savelli L, Ferrini G, Seracchioli R. Do women with deep infiltrating endometriosis have more tubal alterations? Objective evaluation of 473 patients. J Reprod Med. 2013;58(9–10):417–424.
21. Liu W, Zhang Z, Li D. Primary ovarian abscess in virginal young woman with huge endometriosis cyst: a case report. Medicine. 2022;101(21):e29463. doi:10.1097/MD.0000000000029463
22. Matsuda N, Jwa SC, Tamura S, et al. Factors associated with an unfavorable clinical course in hospitalized patients with pelvic inflammatory disease: a retrospective cohort study of 117 patients from a Japanese academic institution. BMC Womens Health. 2022;22(1):348. doi:10.1186/s12905-022-01925-5
23. Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res Microbiol. 2017;168(9–0):782–792. doi:10.1016/j.resmic.2017.04.001
24. Awulachew E, Diriba K, Awoke N. Bacterial isolates from CSF samples and their antimicrobial resistance patterns among children under five suspected to have meningitis in Dilla University Referral Hospital. Infect Drug Resist. 2020;13:4193–4202. doi:10.2147/IDR.S264692
25. Profet M. Menstruation as a defense against pathogens transported by sperm. Q Rev Biol. 1993;68(3):335–386. doi:10.1086/418170
26. Mak P, Wojcik K, Wicherek L, Suder P, Dubin A. Antibacterial hemoglobin peptides in human menstrual blood. Peptides. 2004;25(11):1839–1847. doi:10.1016/j.peptides.2004.06.015
27. Chen C, Song X, Wei W, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8(1):875. doi:10.1038/s41467-017-00901-0
28. Christen U. Pathogen infection and autoimmune disease. Clin Exp Immunol. 2019;195(1):10–14. doi:10.1111/cei.13239
29. Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18(2):105–120. doi:10.1038/nri.2017.111
30. Jiang I, Yong PJ, Allaire C, Bedaiwy MA. Intricate connections between the microbiota and endometriosis. Int J Mol Sci. 2021;22(11):5644. doi:10.3390/ijms22115644
31. Noh EJ, Kim DJ, Lee JY, et al. Ureaplasma urealyticum infection contributes to the development of pelvic endometriosis through toll-like receptor 2. Front Immunol. 2019;10:2373. doi:10.3389/fimmu.2019.02373
32. Khan KN, Kitajima M, Yamaguchi N, et al. Role of prostaglandin E2 in bacterial growth in women with endometriosis. Hum Reprod. 2012;27(12):3417–3424. doi:10.1093/humrep/des331
33. Hill JA, Faris HM, Schiff I, Anderson DJ. Characterization of leukocyte subpopulations in the peritoneal fluid of women with endometriosis. Fertil Steril. 1988;50(2):216–222. doi:10.1016/S0015-0282(16)60062-6
34. Berbic M, Fraser IS. Immunology of normal and abnormal menstruation. Womens Health. 2013;9(4):387–395. doi:10.2217/whe.13.32
35. Olkowska-Truchanowicz J, Bocian K, Maksym RB, et al. CD4(+) CD25(+) FOXP3(+) regulatory T cells in peripheral blood and peritoneal fluid of patients with endometriosis. Hum Reprod. 2013;28(1):119–124. doi:10.1093/humrep/des346
36. Fainaru O, Adini A, Benny O, et al. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. FASEB J. 2008;22(2):522–529. doi:10.1096/fj.07-9034com
37. Miller JE, Lingegowda H, Symons LK, et al. IL-33 activates group 2 innate lymphoid cell expansion and modulates endometriosis. JCI Insight. 2021;6(23). doi:10.1172/jci.insight.149699
38. Khan KN, Yamamoto K, Fujishita A, et al. Differential levels of regulatory T cells and T-Helper-17 cells in women with early and advanced endometriosis. J Clin Endocrinol Metab. 2019;104(10):4715–4729. doi:10.1210/jc.2019-00350
39. Rowe JH, Ertelt JM, Way SS. Foxp3(+) regulatory T cells, immune stimulation and host defence against infection. Immunology. 2012;136(1):1–10. doi:10.1111/j.1365-2567.2011.03551.x
40. Gogacz M, Winkler I, Bojarska-Junak A, et al. T regulatory lymphocytes in patients with endometriosis. Mol Med Rep. 2014;10(2):1072–1076. doi:10.3892/mmr.2014.2294
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



