Back to Journals » Research and Reports in Urology » Volume 16
Catheter-Free Urodynamics Testing: Current Insights and Clinical Potential
Authors Vogt B
Received 22 September 2023
Accepted for publication 19 December 2023
Published 3 January 2024 Volume 2024:16 Pages 1—17
DOI https://doi.org/10.2147/RRU.S387757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Benoît Vogt
Department of Urology, Polyclinique de Blois, La Chaussée Saint-Victor, France
Correspondence: Benoît Vogt, Department of Urology, Polyclinique de Blois, 1 Rue Robert Debré, La Chaussée Saint-Victor, 41260, France, Tel +33 254906511, Fax +33 254906566, Email [email protected]
Abstract: Lower urinary tract dysfunction not only interferes with the health-related quality of life of patients but may also lead to acute kidney injury and infections. To assess the bladder, urodynamic studies (UDS) have been implemented but the use of catheters leads to discomfort for the patient. Catheter-free long-term UDS would be useful and a potential solution could be ambulatory wireless devices that communicate via telemetry. Such sensors can detect pressure or volume. Numerous types of potential catheter-free sensors have been proposed for bladder monitoring. Despite substantial innovation in the manufacturing of implantable biomedical electronic systems, such sensors have remained at the laboratory stage due to a number of critical challenges. These challenges primarily concern hermeticity and biocompatibility, sensitivity and artifacts, drift, telemetry, and energy management. Having overcome these challenges, catheter-free ambulatory urodynamic monitoring could combine a synchronized intravesical pressure sensor with a volume analyzer but only the steps of cystometry and volume measurement are currently sufficiently reproducible to simulate UDS results. The measurement of volume by infrared optical sensors, in the form of abdominal patches, appears to be promising and studies are underway to market a telemetric ambulatory urodynamic monitoring system that includes an intravesical pressure sensor. There has been considerable progress in wearable and conformable electronics on many fronts, and continued collaboration between engineers and urologists could quickly overcome current challenges. In addition, to the diagnosis of UDS, such sensors could be useful in the development of a long-term closed-loop neuromodulation system. In this review, we explore the various types of catheter-free bladder sensors, inherent challenges and solutions to overcome these challenges, and the clinical potential of such long-term implantable sensors.
Keywords: urodynamic studies, ambulatory monitoring, wearable devices, telemetry, pressure sensors, bladder volume
Introduction
Population studies in numerous countries have reported the prevalence of urinary incontinence to range from approximately 25 to 45%. It is strongly related to age and more than 40% of woman aged ≥ 70 years are affected.1,2 Lower urinary tract dysfunction (LUTD) not only interferes with the health-related quality of life of patients but may also lead to acute kidney injury and infections.3 The management of urinary incontinence, including bladder monitoring, is a vital component of urological care. For this purpose, urodynamic studies (UDS) have been implemented to measure relevant parameters of the lower urinary tract and assess their function and dysfunction during filling and emptying of the bladder. Its main objective is to reproduce patient symptoms to identify the underlying cause and provide an assessment of the lower urinary tract.4,5 However, a lack of privacy and the use of catheters during the procedure leads to discomfort for the patient. Furthermore, it is not possible to monitor the bladder under “normal life” conditions, as the patient has to remain in an uncomfortable position, without moving, during a short duration of assessment.4,6,7 Thus, when UDS are inconclusive, long-term UDS are necessary. Ambulatory urodynamic monitoring (AUDM) has been developed to allow continuous monitoring in a domestic setting.8–10 However, AUDM also involves the use of catheters and catheter displacement may occur during movement.10
To address the non-physiological nature of UDS and AUDM, a potential solution could be telemetric ambulatory urodynamic monitoring (TAUDM), which uses a wireless device that communicates via telemetry.11–14 Since the days of the first pacemakers, implantable electronic systems have undergone a major transformation. The advent of micro-, and nano-scale technologies have brought about tremendous miniaturization of all components of the sensors and exciting developments in electronics on many fronts.12,13 However, the development of wearable systems for LUTD monitoring is less mature13 and new approaches for bladder monitoring require addressing several old and new challenges.15 Despite current challenges, the potential benefit that wearable bladder-monitoring devices could have on the lives and health of patients, including even integration into a closed-loop neuromodulation device, could be considerable.13
In this review, we describe the main points of UDS and the challenges of fully converting UDS to catheter-free wireless ambulatory urodynamic studies. Then, we explore the various types of wearable catheter-free bladder sensors and propose an association of sensors to approach, as closely as possible, TAUDM. We also discuss the challenges faced in the development of wearable devices and provide a brief overview of the solutions proposed to overcome them. Finally, we discuss a number of possible future perspectives, highlighting the clinical potential of such sensors in long-term monitoring.
Databases such as PubMed, PubMed Central (PMC), and IEEE Xplore were used to search for material for this study. Keyword combinations such as “urodynamic studies”, “bladder pressure”, “bladder volume”, and “wearable devices” were used to identify relevant articles. The initial search results were refined by exploring the most recent and relevant cited articles. A total of 148 papers were analyzed for the review. The considered articles presented information on catheter-free technologies applied to bladder monitoring, and recent advances in electronic technologies.
Standard Urodynamic Studies
Urodynamic studies are a central element of the urologist’s toolkit, but their overuse is a subject of continued debate. Nonetheless, UDS are useful in situations of a doubtful diagnosis.5,16 The main indications for performing UDS are classically the identification of LUTD, the predicted consequences of LUTD on the upper urinary tract, the predicted outcomes of management or an intervention, and the evaluation of treatment failure. UDS aim to evaluate the nature and cause of a patient’s symptoms and attempts to replicate them.4
UDS currently allow physicians to evaluate the filling and emptying of the bladder to identify and treat a variety of LUTDs, such as overactive bladder, stress urinary incontinence, bladder outlet obstruction, and neurogenic bladder.5
UDS usually include a review of the patient’s medical history and medications, a physical examination, and noninvasive and invasive multi-channel urodynamic tests, with uroflowmetry, filling cystometry, pressure-flow studies, urethral function tests, and electromyography.4,5 The required instrumentation is cumbersome (Figure 1A).
Uroflowmetry is a non-invasive assessment and is completed by an assessment of the post-void residual urinary volume. The act of micturition is affected by psychological factors. A lack of privacy leads to many individuals being unable to void during UDS, which greatly limits the information obtained from such studies.5 In a questionnaire-based study completed by 314 patients immediately after undergoing UDS, Suskind et al observed that anxiety was the most commonly reported component of emotional discomfort.17
Cystometry is an invasive dynamic measurement of detrusor pressure during the continuous filling of the bladder with warmed sterile physiological saline. Using a local anesthetic lubricating gel, the procedure consists of introducing a catheter directly into the bladder through the urethra, allowing the study of pressure variations under filling and voiding conditions. A second catheter is introduced into the rectum or vagina to measure abdominal pressure. The catheters remain in place during the procedure but introduce discomfort. Suskind et al observed that placement of the urethral catheter was the most commonly reported component of physical discomfort (approximately 43% of patients).17
A pressure-flow study during the voiding phase is an invasive assessment and allows the measurement of the pressure generated by the detrusor muscle and the resulting flow. Once again, a post-void residual urinary volume is estimated.
During catheter removal, the urethral pressure profile is estimated by measurement of urethral length and the competence of the sphincter.
Electromyography records the electrical potentials generated by the pelvic floor muscle activity using electrodes.4
Aside from the discomfort introduced by the catheters, UDS may be unable to reproduce the symptoms or, on the contrary, trigger them by nonphysiological retrograde bladder filling. For example, UDS showed normal results, without detrusor-overactivity, in more than 42% of women with wet or dry overactive bladder.18 Moreover, UDS are unable to study bladder function under “normal-life” conditions, as the patient has to remain in an uncomfortable position, without moving and during an insufficient duration of assessment (20 to 40 minutes).6,7,9
Thus, when UDS are inconclusive, AUDM may be helpful in diagnosing the cause of the symptoms and guiding more appropriate management of patients.8 AUDM is performed similarly to conventional cystometry but certain specific elements differ: it is based on natural filling of the bladder (the patients are usually asked to drink extra fluids) and testing lasts for approximately 2 to 4 h. Patients are fully dressed after the initiation of the test and are able to leave the urodynamics examination room, which may reduce embarrassment (Figure 1B).19 AUDM has been shown to be more sensitive than UDS for the diagnosis of overactive bladder7,10 but the clinical role of AUDM is still debated. AUDM is recommended as a second-line diagnostic tool when UDS have failed to provide a diagnosis.19 However, AUDM also involves the use of catheters, and catheter displacement may occur during movement.5,10
Given the invasiveness of the catheters, the integration of wearable wireless and catheter-free sensors has been proposed. According to Stuart et al, the devices for TAUDM must provide a robust platform that features body-like mechanical properties, long-range power transfer capabilities, and miniaturized electronics that provide reliable functioning of the system and a small footprint.11 The power source and encapsulation components are the major contributors to the overall weight and size of the device, whereas the size of electronic circuitry components has decreased dramatically with advancements in micro-electromechanical systems and nanotechnology.12 Wearable systems have become increasingly important over the last few decades and assistive devices for patients affected by cardiac diseases have already reached the market. However, the development of wearable systems for LUTD monitoring is less mature.13 and implantable sensors for bladder monitoring have remained at the laboratory stage due to ongoing critical challenges.
To date, the most promising device for TAUDM to reach the market appears to be the UroMonitor. Bright Uro has developed the UroMonitor to conduct ambulatory urodynamic monitoring that is wireless and catheter-free. The device can remain in the bladder for up to seven days in the home setting and record bladder pressure.20 When produced with a voiding diary, uroflowmetry, and the ability to record other physiological data, the UroMonitor will provide the most comprehensive UDS available to clinicians. A Phase II Small Business Innovation Research Proposal project has started in 2023 and will continue to 2025, with the primary objectives of developing the UroMonitor and evaluating the safety and feasibility of this device in a Clinical Feasibility Study and then bringing this device to market.21 Thus, Bright Uro has created the Glean Urodynamics System, a catheter-free urodynamics testing system that consists of an insertion tool, a Bluetooth®-enabled pressure sensor in a flexible silicone tube, a software application for use by clinicians and patients, and a uroflowmeter to sense volume and flow.14
Potential Wireless and Catheter-Free Devices for Bladder Monitoring
A biosensor is defined as a bio-analytical device comprised of three essential components: 1) a biomarker connected to 2) a transducer component and 3) a signal processing system.22 The urinary bladder has two important functions, urine storage and emptying.
Biomarkers should be chosen to reproduce the UDS steps as closely as possible. Therefore, bladder pressure and contraction and the post-void urinary volume could be candidate biomarkers and provide physicians with valuable information in controlling bladder function during UDS.23
The transducer depends on the transduction methods, such as acoustic, optical, capacitive, resistive, piezoelectric, or resonant. For example, for piezoresistive pressure sensors, changes in applied pressure on a sensitive diaphragm (polysilicon, graphene) is transferred into voltage due to the piezoresistive effect. As the metal strain gauge has been replaced by piezoresistors, the size of the pressure sensors has been gradually reduced from the centimeter to millimeter scale. The miniaturization of sensors provides multiple benefits, such as low power consumption, light weight, a small volume, accurate measurement in a space-limited region, low cost, and little influence on the objects being detected.24
The generated signal is then stored in the device for later playback or transmitted in real time to outside the body to a receiver that records the results.
Pressure Monitoring Devices
Extraluminal Vesical Devices
Pressure sensors consist of a miniaturized pressure sensing unit in contact with urine connected to an implanted data-recording or transmitting unit and properly encapsulated to protect the active components from the harsh working environment, which tends to lead to fluid infiltration and corrosion.13
In 2009, Tan et al designed a fully implantable wireless pressure sensor system for intraluminal pressure monitoring covering the range of expected bladder pressure. The device was surgically implanted alongside the bladder and a catheter placed through the detrusor muscle and into the bladder lumen. A computer received the wireless data, which were collected at 1 Hz. This device has been successfully maintained for three days in a porcine model.25
A similar design was proposed by Basu et al but the device was implanted with a cystoscope by raising a flap of the mucosa and tested on five calves. Simultaneous pressure recordings from the sensor and an intravesical catheter showed good agreement for pressure values for bladder volumes below 100 mL.13,26 Cystoscopic implantation is minimally invasive but all the intra-detrusor devices tested to date have eroded or migrated into the bladder and no devices remained at the implant location for > 4 weeks.5,26 Such invasive methods are excessive in the diagnostic setting of UDS.
Intraluminal Vesical Devices
In 2009, the prototype of Jourand et al was designed to store data in its built-in internal flash memory and required subsequent downloading after extraction from the bladder.27 It was less than 40 mm in length and 5 mm in diameter, allowing insertion through a 20 French cystoscope, and it contained battery power for over 24 h and an on/off switch for extended shelf life.6
In 2013, Wille et al developed a system for long-term UDS without a catheter. The intravesical pressure was measured but the data storage capacity used efficiently, with an algorithm that selected relevant changes in pressure.28 Then, they designed an intravesical capsule for which expulsion during voiding was avoided through a novel C-shaped design of the device.9
Previous work initially concentrated on a battery-powered device with on-board data storage that had a short lifespan and required decapsulation of the “pill” for the stored data to be accessed. In 2016, Bakula et al proposed an improvement of the bladder pill, measuring as little as 5×30 mm and using resonant inductive coupling for wireless power and communication via an omnidirectional receiving coil. Powering of the device and communication were successfully achieved at a distance of up to 12 cm. Data were collected at 10 Hz and integrated into an external belt suitable for in-vivo testing in anaesthetized mini-pigs.29 In 2019, Soebadi et al demonstrated the feasibility and performance of this device in vitro and in vivo. Bench testing showed agreement with pressure measurements with catheter-based methods in awake pigs.30
In 2016, Lee et al presented a sensitivity-enhanced wireless passive resonant pressure sensor for monitoring bladder pressure that can be minimally invasively implanted in the bladder and that avoids battery lifetime problems.31
In 2020, Li et al developed a mini-implantable wireless sensor for monitoring bladder pressure by placing the entire module into the bladder cavity of a rabbit. After the device detected the digital pressure value from the pressure sensor, the data were wirelessly transmitted at a carrier frequency of 433 MHz via a miniature radio frequency transmitter in real time to the host unit. Based on this frequency of radio transmission, the applicable transmission distance was determined to be approximately 2 m, which is adequate for placing a module into the bladder cavity for many biomedical applications. The bladder pressures were simultaneously detected by the sensor and conventional cystometry, showing similar signals during the voiding phase. There was no corrosion or damage to the implantable module after implantation for seven days in rabbits.32
In 2021, Majerus et al designed a minimally invasive bladder pressure sensor. It consisted of a curled silicone tube measuring 5 mm in diameter with internal electronics and a battery. The sensor was intended to be inserted through the urethra, similarly to a catheter, and to curl into a loop when in the bladder (Figure 1C). In the study, the authors demonstrated the feasibility of automatically applying closed-loop or conditional sacral neuromodulation using a wireless bladder pressure sensor (Figure 1C), a real-time control algorithm, and a neurostimulation system over five days in four female sheep.14
Volume Monitoring Devices
After a retrospective evaluation of 976 UDS in patients showing no pathological findings, the normal bladder volume of a healthy adult had a limit of comfortable tolerance of approximately 500 mL and the post-void residual urinary volume was negligible.33 Volume estimations are usually performed as indirect measurements. Based on the measured parameters to be correlated with bladder volume, wearable sensors can be grouped into three classes, sensors that detect variations in electrical properties, bladder wall deformation, and changes in bladder dimension.13
Variation of Bladder Electrical Properties
In 2018, McAdams et al suggested an unanchored implantable volume and pressure sensor within the bladder. It consisted of a low-power pressure transducer and microcontroller, a customizable number of electrodes (Figure 2A), and improved power management, with a rechargeable coin-cell battery, an energy-harvesting microcontroller with inductive recharge input, and data inductive transmission (Figure 2B). The volume estimate was determined by analysis of the direct current conducted by the fluid (conductance). The detected current depended on both urine volume (number of ions) and urine concentration (density of ions). Under worst-case load conditions, the active power draw allowed an estimated ~40 hours in the active mode prior to recharge. This non-hermetic encapsulation approach suggested good tolerability in the feline bladder.34 This device appears to be the only floating device that measures both bladder pressure and volume. However, further studies are necessary for larger bladder volumes.
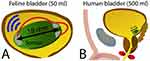 |
Figure 2 Volume monitoring devices: variations in electrical properties of the bladder. (A) The sensor consists of a small panel measuring 12×18 mm with three electrodes and was surgically implanted in a feline bladder and remained unanchored. The volume estimate was determined by the analysis of direct current conducted by the fluid between the electrodes (red arrows). This device could measure both bladder pressure and volume and allowed wireless transmission to a receiver. Data from McAdams et al34 (B) Because the feline bladder is small (50 mL), this approach requires further studies using a larger bladder volume, such as that of humans, for which the volume is 10 times greater. |
Bladder Wall Deformation
Several strategies have been proposed to measure bladder wall deformation based on an estimation of the distance between pairs of sensors anchored to the external bladder walls.13 Such bladder sensors are typically available in magnetic, capacitive, potentiometric, and resistive types.
The placement of the sensors detailed in this section require at least laparoscopic surgery. Such invasive methods are excessive in the diagnostic setting of UDS but may be relevant for long-term closed-loop systems. However, the aim is to provide the reader with a current overall vision of the field using recent examples of wearable sensors and to explore new research avenues.
In 2009, Wang et al designed a coin-shaped permanent magnet stitched onto the anterior bladder wall. A magnetic field sensor was fixed onto the external lower abdominal wall in male dogs. After progressive filling of the bladder, sensor readings positively correlated with bladder volume up to 200 mL.35
In 2013, Cao et al proposed a wireless capacitive sensor to monitor bladder volume in a small animal model. It consisted of a metal interdigitated finger-like structure. The sensor was tested in rats in a miniaturized configuration to be wrapped around the bladder. However, further studies of such technology would be needed for larger bladder volumes.36
Resistive sensors in the shape of true potentiometers were reported in 2015 by Chen et al, with the sliding rails placed on the dorsal side of the pig bladder wall in vitro (Figure 3A).37 On the contrary, other authors have attempted to maximally approach the flexibility of the bladder wall by using materials designed to be both conformable and biocompatible. A soft, conformable, biocompatible strain sensor based on ultra-thin stretchable electronics was reported in 2019 by Hannah et al.35 In 2017, Kim et al reported an implantable bladder volume sensor with a multi-level resistor ladder that estimated the bladder volume through discrete resistance values. Discretization allowed more accurate volume monitoring.38 The sensor was composed of a biocompatible polypyrrole/agarose hydrogel composite with a Young’s modulus of the composite comparable to that of the bladder wall, which made it possible to limit the effect of mechanical loading of the sensor on bladder movement.39 The resistance response to stretching was linear for a change in strain of 20 to 40% (Figure 3B).40
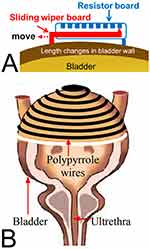 |
Figure 3 Volume monitoring devices: bladder wall deformation. (A) Using a resistive strain sensor, the bladder volume was calculated from an estimation of the distance between pairs of sensors anchored to the external bladder walls. Adapted from Chen SC, Hsieh TH, Fan WJ et al. Design and evaluation of potentiometric principles for bladder volume monitoring: a preliminary study. Sensors (Basel). 2015;15(6):12802–12815.37 (B) Use of an electronically conducting polymer that produced a reproducible change in electrical resistance on stretching. Volume estimations were based on the resistance analysis to stretching. Rajagopalan S, Sawan M, Ghafar-Zadeh E, Savadogo O, Chodavarapu VP. A Polypyrrole-based Strain Sensor Dedicated to Measure Bladder Volume in Patients with Urinary Dysfunction. Sensors (Basel). 2008;8(8):5081–5095.40 |
Changes in Bladder Dimensions
Several strategies have been proposed to measure changes in bladder dimension based on an estimation of the distance between opposite walls of the bladder. Such bladder sensors are typically available in acoustic, optical, and impedancemetric types. Only a few noninvasive wearable devices able to monitor bladder volume have reached the market.
Acoustic Assessment of Bladder Dimension
Transcutaneous ultrasound is the most highly explored method for bladder volume monitoring. However, the commercial CE marked US systems (BladderScan BVI 9400 and the Prime – Verathon Medical, Bothell, WA, USA) may not be suitable for domestic use because the instrumentation, even if portable, is cumbersome and expensive.13,41 Indeed, transcutaneous ultrasound requires a large number of transducers to establish an interpretable image in a two-dimensional sector (Figure 4A).
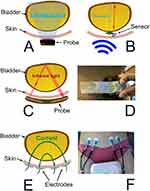 |
Figure 4 Volume monitoring devices: changes in bladder dimensions. (A) Geometries for ultrasound volume analysis. B-mode imaging with an array of transducers scanning a two-dimensional sector. (B) Theoretical model offering A-mode scans using an implantable single transducer. Ultrasound reflections from the front and rear walls of the bladder made it possible to estimate the volume with the antero-posterior diameter of the bladder (red arrow). An inductive link was used for both power transfer and data telemetry with the implanted unit. Data from Mandal S et al42 (C) Near infrared spectroscopy technique using light sources of approximately 975 nm to detect variations in the water content and oxygenation status and hemodynamics of the bladder. By placing the emission and detection probes on opposite sides of the bladder, an increase in bladder volume results in a decrease in the detected-light intensity and makes it possible to estimate the volume. (D) Positioning of the wearable device over the bladder using the bony landmark of the symphysis pubis. Reprinted from Stothers L, Macnab A, Mutabazi S, Mukisa R, Molavi B, Shadgan B. Near-Infrared Spectroscopic Screening for Bladder Disease in Africa: Training Rural Clinic Staff to Collect Data of Diagnostic Quality. Journal of Spectroscopy. 2016;2016:1241862.43 (E) Bioimpedance. Using electrodes, the passage of a small alternating current across tissues is opposed by body tissue impedance. Volume estimations are based on the measured parameters. (F) Tetrapolar bioimpedance measurements carried out on a volunteer. Adapted from Gaubert V, Gidik H, Koncar V. Proposal of a Lab Bench for the Unobtrusive Monitoring of the Bladder Fullness with Bioimpedance Measurements. Sensors (Basel). 2020;20(14):3980.44 |
In 2022, Mandal et al described an implantable wireless sensor anchored to the anterior bladder wall for making ultrasound distance measurements using an A-mode approach (a single transducer). An inductive link was used for both power transfer and data telemetry with the implanted unit. In vitro tests of the device on bladder phantoms filled with water and immersed in saline solution showed a maximum operating depth of 3.2 cm from a 10 cm diameter external coil. Bladder volume measurements showed estimation errors of approximately 15% for volumes in the 50 to 300 mL range, but only a single-axis wall-to-wall distance measurement was used to estimate bladder volume (Figure 4B).42
In 2017, van Leuteren et al developed a wearable and wireless ultrasound device named the URIKA bladder monitor. This device estimates the anterior–posterior bladder dimension and when it exceeds a critical threshold, the patient is notified of a full bladder.45 A subsequent pilot study on 18 participants using the DFree device attached to the lower abdomen was conducted by Hofstetter et al The authors suggested that the device may be beneficial as a support for patients with bladder dysfunction.46,47
In 2018, Van Leuteren et al presented another wearable ultrasound device called the SENS-U. It was based on a combination of four ultrasound transducers that are positioned on the lower abdomen using a skin-friendly adhesive. The sensor continuously estimated bladder volume. The device was able to detect a full bladder with a success rate of 90%.47,48
In 2021, Fournelle et al developed a new wearable low-cost ultrasound device for long-term and automated bladder monitoring without user interaction (MoUsE). It includes 32 transducers. A wireless interface and a battery powered version are in development.49
In 2021, Jo et al proposed a wearable bladder scanner system that can continuously measure bladder volume in daily life for patients who need UDS. The sensor was placed 2 cm above the pubic bone, in line with the navel, and was secured with wires or belts. For an injection volume ranging from 50 mL to 450 mL, the proposed system had an average error of 24 mL and 29 mL, respectively. This flexible system could contribute to the development of electronic voiding diaries as part of the home healthcare system.50
Optical Assessment of Bladder Dimensions
Near infrared spectroscopy (NIRS) is a non-invasive optical technique that uses light sources of approximately 975 nm, which is the absorption peak of water, to detect the oxygenation status and hemodynamics of various organs (Figure 4C). It has been hypothesized that bladder storage and emptying leads to real-time changes in oxygenation and hemodynamics in the bladder wall as the bladder fills and empties.23
Since 2013, several authors have reported a significant difference in light absorption between the full and empty state.23,43,47,51 The device is composed of a wearable optode sensor intended to be attached to the skin of the lower abdomen (Figure 4D). The sensor can also be a patch that can remain constantly attached to the body. The optode sensor can be controlled wirelessly using a mobile phone. The validated feasibility of the sensor showed almost the same results as an ultrasound scanner, which is routinely used in the clinical field.51 Moreover, this device was able to detect voiding (just before the start of urinary flow) and not only the state of a full bladder. The fact that a difference in light absorption occurs when the bladder volume remains constant suggests that the NIRS data represent a physiologically relevant measurement. In addition, the device was very well accepted by children and these wireless devices make ambulatory monitoring possible.52
Assessment of Bladder Dimensions by Bioimpedance
Another method explored in recent years concerns the analysis of bioimpedance, which is the measurement of the resistance of the soft tissues in the presence of an alternating current. Using conventional or dry electrodes, the passage of a small alternating current across tissues is opposed by impedance of the body tissues (Figure 4E).13,47
In 2015, Palla et al investigated whether the BodyGateWay (STMicroelectronics), an electronic patch for the remote monitoring of cardiac and respiratory function, could be successfully used for the development of a wearable real-time bladder volume monitoring system. Four sensor electrodes were placed above the bladder. After the reduction of artifacts due to the volunteer’s movements, the results obtained showed the validity and effectiveness of the proposed solution.53
In 2017, Shin et al proposed a comfortable waist-belt-type device that uses the body impedance analysis technique with a motion artifact reduction algorithm and applications to connect the device to a smartphone.54
In 2020, Gaubert et al designed a realistic pelvic phantom, incorporating ex vivo tissues, such as pig bladder and skin. The manikin was suitable for wearing textile boxer underwear and the impedance meter recorded variations in impedance at the surface of the phantom’s skin using four electrodes in real time. The authors concluded that in the future, underwear incorporating textile electrodes would be able to monitor the fullness of the bladder of its wearer (Figure 4F).44
In 2018, Leonhäuser et al conducted a comparison study of the electrical impedance tomography technique using ultrasound technology for the measurement of bladder volume. Electrical impedance tomography measurements were taken using a commercial device (Goe MF II) that uses 16 electrodes placed at the level of the lower abdomen. The estimation of maximum bladder capacity by electrical impedance tomography was similar to that calculated by ultrasound or measured after voiding, but the estimation of residual urine volume showed significantly worse accuracy for electrical impedance tomography.55
Potential Wireless and Catheter-Free Devices for Telemetric Ambulatory Urodynamic Monitoring
Uroflowmetry, urethral pressure profiles, and pressure-flow studies, with the observation of the pressure during uroflowmetry for the voided volume, must be assessed during the urodynamics evaluation. Electromyography is not suitable for TAUDM because the recorded signal is of very small amplitude and the electrodes need to be implanted.56 Cystometry and volume measurement are the only UDS steps that can be reproduced for TAUDM. Thus, the bladder pressure and post-void urinary volume are efficient biomarkers for the assessment of bladder function during UDS. The placement of the sensors, requiring at least laparoscopic surgery, is excessive in the diagnostic setting of UDS. The potential number of sensors for TAUDM is thus limited to intravesical and transcutaneous sensors.
Pressure Integration
The assessment of pressure absolutely requires the presence of a pressure sensor in contact with the bladder cavity. The uroMonitor and sensor of McAdams et al represent an important step forward in the development of TAUDM.14,34 On the other hand, the mobility of the intravesical sensor, not attached to the wall of the bladder, could induce urinary symptoms, in the same way that the mobile bladder loop of the double-pigtail stent induces stent-related symptoms, by bladder friction.57 These additional symptoms demonstrated in a randomized study58 must be integrated into the interpretation of the UDS.
Volume Integration
The commercial ultrasound systems currently available may not be suitable for domestic use because the instrumentation, even if portable, is cumbersome and expensive. Lighter ultrasonic sensors or solutions based on NIRS or bioimpedance sensors with a limited number of electrodes integrated into a belt or boxer shorts could provide volume assessment.44,45,50,52,54 However, continuous recording with adequate sampling is necessary to couple volume to bladder pressure. NIRS sensors could provide additional information, given that changes in the signal can be detected while the volume remains unchanged.52
Integration of Pressure-Volume Coupling
During cystometry, the pressure trace should be marked with annotations of the patient’s subjective sensations. These markers should signify the patient’s “first sensation of filling”, “first desire to void”, and “strong desire to void.”4 Simultaneous recordings of pressure and volume, associated with the recording of events (efforts, coughing), are therefore key elements for interpretation.5 Coupling between the bladder sensor and the volume detector is therefore necessary. Currently, only the sensor from McAdams et al can integrate this double function.34
After listing the numerous innovations in the field of bladder monitoring, remote ambulatory urodynamic monitoring could combine a pressure sensor, such as the UroMonitor or the device by McAdams et al, synchronized with a NIRS sensor system in the form of patches to assess volume. To further approach UDS, Abelson et al proposed that urine leakage could also be detected using sensors placed in underwear and uroflowmetry equipment in the patient’s home could help to determine voiding frequency and flow data.5 The Glean Urodynamics system created by Bright Uro could be a good compromise to approach UDS in the domestic setting.
Current Challenges and Emerging Solutions
Despite substantial innovations in the manufacturing of implantable biomedical electronic systems, implantable sensors for bladder monitoring have remained at the laboratory stage due to a number of challenges. The objective here is to provide readers with an overview of the challenges and a range of solutions that new technologies offer and those that, to date, remain underused or even unknown. With these new technologies, continued collaboration between engineers and urologists could resolve issues such as sensitivity and motion artefacts, power supply and telemetry, drift, hermeticity and biocompatibility, data sampling rate, and compatibility issues with imaging modalities, such as magnetic resonance imaging.
Motion Artefacts and Sensitivity
Sensor sensitivity is essential to detecting bladder pressure. However, good pressure sensitivity of the sensor may decrease after encapsulation with a silicone rubber coating.31 Furthermore, too much sensitivity affects data analysis, such as the presence of abdominal pressure artefacts on vesical pressure measurements. To interpret the pressure measured during TAUDM, it is necessary to couple it with vaginal or rectal pressure, but the aim of TAUDM is to eradicate catheters. Thus, algorithms based on signal properties of bladder contraction and extravesical motion can classify relevant bladder events to substitute for abdominal pressure measurements and produce data comparable to that of UDS.59,60
Power Supply and Telemetry
Currently, two main methods are used to transmit power to components within the implanted device: battery-based approaches and radio frequency-based approaches that require efficient coupling.61
There is a strong trend towards producing devices of ever diminishing size and weight to make them compatible with normal human activity. Batteries, whether single-use or rechargeable, significantly contribute to the overall weight and size of the device and have a limited lifetime.12,32 For example, the maximum active mode of the rechargeable battery by McAdams et al was estimated to be ~40 hours prior to recharge.34 A hybrid system with a rechargeable battery based on radio frequency could extend battery life.3 For devices intended for TAUDM or applications for which a high data sampling rate is required, a battery-based system may be more suitable One approach to extend battery life is to create a “sleeping mode.” However, these methods do not provide truly continuous monitoring of bladder pressure.61
The second approach is to use an inductively powered bladder sensor receiving power from an external power transfer system, although there is a loss of power during transfer between the external system and the bladder sensor.3 Biological tissues significantly attenuate wireless signals, especially at commonly used frequencies such as 2.4 GHz, and there is a need to limit the absorption of damaging energy into the tissues. Such attenuation can be minimized by using frequencies below 4 MHz.62
Communication distance is a challenging issue because inductive coupling requires alignment and proper positioning of both the external and internal coils for efficient power transfer. The proper positioning of the device is not specific to inductive coupling, as it was also reported that proper SENS-U positioning and childhood obesity were factors that should be taken into account when using the device in daily clinical practice.47,48 Most importantly, externally powered devices have functional distances of 2 to 20 cm between the power source and the device, which severely limits their use in the general population, given that obesity rates are increasing.5,29,42,63
In addition, the relatively large size and heavy construction of the external coil limits its continual wearability during real-life activity. Improper coupling between the implanted and external coils can result in poor power or data transmission. By contrast, the pressure sensor of Lee et al used a miniature radio frequency transmitter (433 MHz) and the applicable transmission distance was close to 2 m.32 Thus, active telemetry with a battery appears to be more suitable for TAUDM.61
Recent research has focused on using energy harvesters for self-powered sensor systems. Triboelectric nano-generators (TENG) generate static electricity from friction and electrostatic induction between two types of surface materials. Under an external force, the two friction layers continuously contact and separate from each other.64,65 For example, for the treatment of obesity, a battery-free implantable vagal nerve stimulation device was built based on a flexible TENG that was attached to the stomach wall of rats and could generate biphasic electric pulses when the stomach wall moved. The TENG electrodes were directly connected to the vagal nerve and stimulated the vagal afferent fibers to reduce food intake and achieve weight control.66 To prevent cardiovascular diseases, Ouyang et al proposed a flexible self-powered pulse sensor adapted to various human arteries and based on an active triboelectric sensor, with excellent output performance. Using a Bluetooth chip, communications could wirelessly transmit a pulse waveform to a smartphone and monitor the pulse data in real-time.67
Drift
To achieve reliable long-term measurements, implanted sensors should possess a stable, consistent response over their lifetime.61 A change in the response of a sensor that is inconsistent with the rate of change of the quantity to be measured is known as drift.3 Signal drift encountered in pressure sensors can be divided into offset drift, in which the baseline measurement slowly drifts to obscure the desired pressure measurement, and sensitivity drift, in which the sensitivity of the device decreases over time.61 The in-vivo environment is dynamic. For example, the sensors of McAdams et al showed a slow drift over time that could have been due to moisture uptake by the encapsulation gel.34 It is important that implanted sensors have drift compensation circuits. For example, the addition of temperature data or offset-cancellation circuitry could assist the calibration of the sensor response in the in-vivo environment.61,68
Sensor drift of sensitivity can be caused by the accumulation of biological material. With the aim of limiting biofouling material, Kim et al described a universal packaging technique for reducing sensor drift in which the sensor was encased in a silicone-filled medical-grade polyurethane balloon. In-vitro soak tests of 100 days in pigs using commercial micromachined piezoresistive pressure sensors demonstrated stable operation.69
Hermeticity and Biocompatibility
The durability of an implantable bladder sensor is an important factor of the design. Hermetic packaging can prevent failure of the implanted active circuitry because human urine creates a hostile and corrosive environment for bladder sensors. Moreover, many miniaturized sensors use bio-incompatible materials, such as silicon. Thus, a biocompatible watertight encapsulant is essential for these implantable devices.3,61 For certain membrane-based pressure sensors, the hermetic packaging can interfere with proper pressure transduction to the pressure sensitive membrane.31 To maintain acceptable sensitivity, Majerus et al used an encapsulation method that resulted in a watertight device with a low-attenuation pressure “window.” A silicone encapsulant was applied to the entire device, except for the membrane, which was left uncoated.70 Encapsulation can increase the overall weight and size of the device. Yao et al developed a micro−nano composite coating for implantable pressure sensors to protect them from corrosion by body fluids while not increasing their size or impairing their function. The micro−nano composite coating was composed of a nano-scale silane layer coupled with a microscale parylene layer, in which the nano-scale layer improves adhesion. The sensitivity was almost unchanged after immersion in a simulated body fluid for 434 days.71 For new wearable sensors, the materials used in their design are mostly chosen based on their technical properties. However, in the case of electronic skin patches, choosing the adhesive or the material to use for a wearable device on the skin should be taken into consideration.72
Data Sampling Rate
A high sampling rate is desired because it provides more information about the quantity measured. However, a high sampling rate translates to more power consumption and a shorter battery life.3 A pressure sampling rate < 1 Hz is probably unsuitable for TAUDM, particularly given the duration of relevant events, such as coughing or sneezing. To detect the onset of bladder contractions, frequencies > 20 Hz have been suggested.5,26,73
Magnetic Resonance Imaging (MRI)
Another problem that implantable bladder sensors encounter is their poor compatibility with current imaging devices, such as MRI. The use of ferrites for enhanced inductive coupling in implantable bladder sensors to effectively improve wireless communication could interfere with MRI.3
Future Insights
These challenges have persisted since the beginning of the development of implantable sensors. Despite these challenges, it is possible that sensors for TAUDM could be commercialized in the near future and, as several authors indicate, the repercussions on the lifestyle and health of patients would be significant. Indeed, due to advances in electronics and diverse innovations, TAUDM could be used more widely for the evaluation of treatment or for therapeutic management. Thus, bionic solutions to assist bladder function could experience accelerated development.3,5,13,74
Exciting Advances in Electronic Performance
There is a recent high demand for novel approaches in healthcare and the diagnosis of diseases at early stages. Pressure sensing of diverse tissues and organs, such as bladder pressure, will be much more important for diverse biomedical applications in the future. However, helping to diagnose a disease only requires the temporary presence of the sensor. Thus, the use of biodegradable sensors in the biomedical field will grow, with the advent of recent developments in micro-/nanotechnologies. Biodegradable pressure sensors or biodegradable-TENG have already been developed for biomedical applications.75,76 Biodegradable piezoelectric force sensors can be used as implantable medical devices for monitoring pressure inside the body. Cheng et al reported a mechanical-annealing strategy for engineering all-organic biodegradable piezoelectric force sensors using natural amino acid crystals as piezoelectric materials.77
Semiconducting materials are essential in most electronic devices, such as transistors, diodes, radio frequency inductors, capacitors, and power-harvesting devices.74 There are significant real-life translations of these exciting technologies.61,78 For example, nano-transistor sensors have been designed by modifying channels and attaching the prostate-specific antigen monoclonal antibody to them, allowing a very low detection limit of 10 fg.mL−1.78,79 However, traditional semiconducting materials, such as silicon, are considered to be non-degradable. Interestingly, when its structure is scaled down to the nano scale, it can be fully biodegraded in aqueous solutions. These advances open perspectives for complete and biodegradable organic electronics.75
Flexible electronic textiles could replace certain traditional bulky, rigid, and uncomfortable wearable electronics.80 By manufacturing nanofibers with tunable properties, electrospinning has emerged as a versatile platform that can be potentially used to design sensors, energy harvesters, batteries, and antennae on flexible and breathable textile substrates.81,82 These new technologies could be adapted to design patches for NIRS or bioimpedance that can be worn for prolonged periods.
Kim et al introduced epidermal electronics by laminating devices composed of sensors for temperature and strain and supporting electronics, such as transistors, ring oscillators, diodes, and radio frequency inductors in serpentine patterns, onto the skin.74 Thus, various strategies have been investigated, with the development of intrinsically stretchable organic electronics to enable a seamless and unobtrusive interface with the skin.15,72 For example, to limit the development of pressure ulcers in patients with limited mobility, Farooqui et al presented a continuous wireless monitoring system with inkjet printing on a standard bandage that can send early warnings.83 Finally, other innovations make it possible, for example, to develop a contact lens equipped with a light-emitting diode that lights up depending on the patient’s glycemic state using induction.84
The recent advances in electronics are not only limited to the detection of biomarkers but also allow mechanical actions. Hassani et al presented a bi-stable actuator to empty the bladder by incorporating shape memory alloy components. This polymer wrapped around a rat bladder allowed itself to be gently distended by bladder filling. After electrical stimulation, bladder emptying was obtained by the polymer returning to its initial shape.85
Conceivable Bionic Solutions
For patients with LUTD and poor outcomes from traditional treatment approaches, neuromodulation systems could offer an alternative.13 The current system of neuromodulation uses an open-loop system that only delivers continuous stimulation without considering changes in the state of the patient. The most promising biomarkers are currently the measurement of bladder pressure or the detection of bladder events based on an analysis of afferent nerve activity from the bladder to the brain.56,86 This last method is invasive and excessive in the diagnostic setting of UDS but may be relevant for long-term closed-loop systems.
Continuous sacral neuromodulation is used to treat overactive bladder. A study demonstrated the feasibility of automatically applying closed-loop sacral neuromodulation using a bladder pressure sensor.14 However, the long-term tolerance of a mobile intravesical sensor or a system implanted in direct communication with urine is yet to be evaluated. Closed-loop neuromodulation could also include a system to monitor afferent nerve activity and respond by stimulating the sacral nerve with a fully automated system for which the patient does not need to provide a trigger.
A simplified view of neurogenic lower urinary tract dysfunction after spinal cord injury may be described as improper sensory awareness for the need to void or improper stimulus delivery to cause the bladder to void.87 In this case, using newly available technology, closed-loop neuromodulation could allow voluntary urination. Measuring the volume by a resistive or optical sensor implanted around the bladder would signal a full bladder via an implanted vibrator. A wireless radio frequency remote control would allow emptying of the bladder by at the desired moment by sacral anterior root or shape memory alloy component stimulation.85,87,88
Conclusion
Urodynamic studies aim to evaluate the nature and cause of a patient’s symptoms and attempts to replicate them. The fact that these tests are conducted within the doctor’s urodynamics examination room, with short measurement times, can lead to unreliable results. Domestic bladder monitoring by telemetric ambulatory urodynamic monitoring devices would be highly suitable, but remain an open challenge. Indeed, the design of wearable systems for bladder monitoring is still in its early stages but the performance of the myriad of new electronics systems is truly exciting. We hope that the convergence of cutting-edge science and medicine will enable the development of wearable systems for telemetric ambulatory urodynamic monitoring and closed-loop neurostimulation to provide patients with a better quality of life.
Abbreviations
UDS, urodynamic studies; AUDM, ambulatory urodynamic monitoring; TAUDM, telemetric ambulatory urodynamic monitoring; LUTD, lower urinary tract dysfunction; NIRS, near infrared spectroscopy; TENG, triboelectric nano-generators; MRI, magnetic resonance imaging.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The author certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript are the following: Benoît Vogt received royalties from Rocamed for the treatment of ureteral stones but there are no financial competing interests in the manuscript. The author reports no other conflicts of interest in this work.
References
1. Milsom I, Gyhagen M. The prevalence of urinary incontinence. Climacteric. 2019;22(3):217–222. doi:10.1080/13697137.2018.1543263
2. Schreiber Pedersen L, Lose G, Høybye MT, Elsner S, Waldmann A, Rudnicki M. Prevalence of urinary incontinence among women and analysis of potential risk factors in Germany and Denmark. Acta Obstet Gynecol Scand. 2017;96(8):939–948. doi:10.1111/aogs.13149
3. Dakurah MN, Koo C, Choi W, Joung YH. Implantable Bladder Sensors: a Methodological Review. Int Neurourol J. 2015;19(3):133–141. doi:10.5213/inj.2015.19.3.133
4. Yao M, Simoes A. Urodynamic Testing and Interpretation. Treasure Island (FL): StatPearls Publishing; 2023.
5. Abelson B, Majerus S, Sun D, Gill BC, Versi E, Damaser MS. Ambulatory urodynamic monitoring: state of the art and future directions. Nat Rev Urol. 2019;16(5):291–301. doi:10.1038/s41585-019-0175-5
6. Jourand P, Puers R. The BladderPill: an in-body system logging bladder pressure. Sens Actuators A. 2010;162(2):160–166. doi:10.1016/j.sna.2010.01.035
7. Radley SC, Rosario DJ, Chapple CR, Farkas AG. Conventional and ambulatory urodynamic findings in women with symptoms suggestive of bladder overactivity. J Urol. 2001;166(6):2253–2258.
8. Cantu H, Sharaf A, Bevan W, Hassine A, Hashim H. Ambulatory urodynamics in clinical practice: a single centre experience. Neurourol Urodyn. 2019;38(8):2077–2082. doi:10.1002/nau.24153
9. Wille S, Schumacher P, Paas J, et al. Catheterless long-term ambulatory urodynamic measurement using a novel three-device system. PLoS One. 2014;9(5):e96280. doi:10.1371/journal.pone.0096280
10. Pannek J, Pieper P. Clinical usefulness of ambulatory urodynamics in the diagnosis and treatment of lower urinary tract dysfunction. Scand J Urol Nephrol. 2008;42(5):428–432. doi:10.1080/00365590802299056
11. Stuart T, Hanna J, Gutruf P. Wearable devices for continuous monitoring of biosignals: challenges and opportunities. APL Bioeng. 2022;6(2):021502. doi:10.1063/5.0086935
12. Bazaka K, Jacob MV. Implantable Devices: issues and Challenges. Electronics. 2012;2(4):1–34. doi:10.3390/electronics2010001
13. Semproni F, Iacovacci V, Menciassi A. Bladder Monitoring Systems: state of the Art and Future Perspectives. IEEE Access. 2022;10:125626–125651. doi:10.1109/ACCESS.2022.3221816
14. Majerus SJA, Offutt SJ, Brink TS, et al. Feasibility of Real-Time Conditional Sacral Neuromodulation Using Wireless Bladder Pressure Sensor. IEEE Trans Neural Syst Rehabil Eng. 2021;29:2067–2075. doi:10.1109/TNSRE.2021.3117518
15. Greco F, Bandodkar AJ, Menciassi A. Emerging technologies in wearable sensors. APL Bioeng. 2023;7(2):020401. doi:10.1063/5.0153940
16. Clement KD, Lapitan MC, Omar MI, Glazener CM. Urodynamic studies for management of urinary incontinence in children and adults: a short version Cochrane systematic review and meta-analysis. Neurourol Urodyn. 2015;34(5):407–412. doi:10.1002/nau.22584
17. Suskind AM, Clemens JQ, Kaufman SR, et al. Patient perceptions of physical and emotional discomfort related to urodynamic testing: a questionnaire-based study in men and women with and without neurologic conditions. Urology. 2015;85(3):547–551. doi:10.1016/j.urology.2014.11.001
18. Hashim H, Abrams P. Is the bladder a reliable witness for predicting detrusor overactivity? J Urol. 2006;175(1):191–194. doi:10.1016/S0022-5347(05)00067-4
19. Digesu GA, Gargasole C, Hendricken C, et al. ICS teaching module: ambulatory urodynamic monitoring. Neurourol Urodyn. 2017;36(2):364–367. doi:10.1002/nau.22933
20. Frainey BT, Majerus SJA, Derisavifard S, et al. pd66-05 safety, feasibility, and accuracy of the uromonitor: a catheter-free wireless ambulatory cystometry device. J Urol. 2021;206(Supplement 3). doi:10.1097/JU.0000000000002110.05
21. Bright Uro. Feasibility Assessment of Glean Urodynamics System. Available from: https://clinicaltrials.gov/study/NCT05694793.
22. Rodrigues D, Barbosa AI, Rebelo R, Kwon IK, Reis RL, Correlo VM. Skin-Integrated Wearable Systems and Implantable Biosensors: a Comprehensive Review. Biosensors. 2020;10(7):79. doi:10.3390/bios10070079
23. Koven A, Herschorn S. NIRS: past, Present, and Future in Functional Urology. Curr Bladder Dysfunct Rep. 2022;17(4):241–249. doi:10.1007/s11884-022-00665-4
24. Song P, Ma Z, Ma J, et al. Recent Progress of Miniature MEMS Pressure Sensors. Micromachines. 2020;11(1):56. doi:10.3390/mi11010056
25. Tan R, McClure T, Lin CK, et al. Development of a fully implantable wireless pressure monitoring system. Biomed Microdevices. 2009;11:259–264. doi:10.1007/s10544-008-9232-1
26. Basu AS, Majerus S, Ferry E, Makovey I, Zhu H, Damaser MS. Is submucosal bladder pressure monitoring feasible? Proc Inst Mech Eng. Nanomaterials. 2019;233(1):100–113. doi:10.1177/0954411918754925
27. Jourand P, Puers R, Autonomous A. Capacitive Sensor Based and Battery Powered Internal Bladder Pressure Monitoring System. Procedia Chem. 2009;1(1):1263–1266. doi:10.1016/j.proche.2009.07.315
28. Wille S, Tenholte D, Engelmann U. A system for long-term urodynamic studies without catheters. Eur Urol. 2013;63(5):966–968. doi:10.1016/j.eururo.2013.01.029
29. Bakula M, Soebadi A, De Ridder D, Puers R. The Bladder Pill: developments Toward Bladder Pressure Measurement in Wake Mini-pigs. Procedia Eng. 2016;168:193–196. doi:10.1016/j.proeng.2016.11.215
30. Soebadi MA, Bakula M, Hakim L, Puers R, De Ridder D. Wireless intravesical device for real-time bladder pressure measurement: study of consecutive voiding in awake minipigs. PLoS One. 2019;14(12):e0225821. doi:10.1371/journal.pone.0225821
31. Lee HY, Choi B, Kim S, Kim SJ, Bae WJ, Kim SW. Sensitivity-Enhanced LC Pressure Sensor for Wireless Bladder Pressure Monitoring. IEEE Sensors Journal. 2016;16(12):4715–4724. doi:10.1109/JSEN.2016.2533262
32. Li YT, Yang LY, Hsu WT, Peng CW. Designing and Implementing an Implantable Wireless Micromanometer System for Real-Time Bladder Pressure Monitoring: a Preliminary Study. Sensors. 2020;20(16):4610. doi:10.3390/s20164610
33. Alloussi SH, Lang C, Eichel R, Ziegler M, Stenzl A, Alloussi S. Urodynamical benchmarks: a retrospective analyses of 976 combined urodynamics with no pathological findings to evaluate standard values. Eur Urol Suppl. 2010;9(2):227. doi:10.1016/S1569-9056(10)60679-3
34. McAdams I, Kenyon H, Bourbeau D, Damaser MS, Zorman C. Low-cost, Implantable Wireless Sensor Platform for Neuromodulation Research. IEEE Biomed Circuits Syst Conf. 2018;2018. doi:10.1109/BIOCAS.2018.8584729
35. Wang J, Hou C, Zheng X, Zhang W, Chen A, Xu Z. Design and evaluation of a new bladder volume monitor. Arch Phys Med Rehabil. 2009;90(11):1944–1947. doi:10.1016/j.apmr.2009.06.013
36. Cao H, Tata U, Landge V, Li AL, Peng YB, Chiao JC A wireless bladder volume monitoring system using a flexible capacitance-based sensor.
37. Chen SC, Hsieh TH, Fan WJ, et al. Design and evaluation of potentiometric principles for bladder volume monitoring: a preliminary study. Sensors. 2015;15(6):12802–12815. doi:10.3390/s150612802
38. Kim MK, Lee S, Yoon I, et al. Polypyrrole/Agarose Hydrogel-Based Bladder Volume Sensor with a Resistor Ladder Structure. Sensors. 2018;18(7):2288. doi:10.3390/s18072288
39. Kim MK, Kim H, Jung YS, et al. Implantable bladder volume sensor based on resistor ladder network composed of conductive hydrogel composite. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:1732–1735. doi:10.1109/EMBC.2017.8037177
40. Rajagopalan S, Sawan M, Ghafar-Zadeh E, Savadogo O, Chodavarapu VP. A Polypyrrole-based Strain Sensor Dedicated to Measure Bladder Volume in Patients with Urinary Dysfunction. Sensors. 2008;8(8):5081–5095. doi:10.3390/s8085081
41. Verathon. BladderScan Prime Plus. Available from: https://www.verathon.com/bladderscan-prime-plus/.
42. Mandal S, Dehghanzadeh P, Zamani H, Shaik S. A Wireless Implantable Microsystem for Real-Time Bladder Volume Monitoring. TechRxiv. 2022. doi:10.36227/techrxiv.20063783.v1
43. Stothers L, Macnab A, Mutabazi S, Mukisa R, Molavi B, Shadgan B. Near-Infrared Spectroscopic Screening for Bladder Disease in Africa: training Rural Clinic Staff to Collect Data of Diagnostic Quality. J Spectrosc. 2016;2016:1241862. doi:10.1155/2016/1241862
44. Gaubert V, Gidik H, Koncar V. Proposal of a Lab Bench for the Unobtrusive Monitoring of the Bladder Fullness with Bioimpedance Measurements. Sensors. 2020;20(14):3980. doi:10.3390/s20143980
45. van Leuteren PG, de Vries BA, de Joode-Smink JCJ, ten Haken B, de Jong TPVM, Dik P. URIKA, continuous ultrasound monitoring for the detection of a full bladder in children with dysfunctional voiding: a feasibility study. Biomed Phys Eng Express. 2017;3(1):017005. doi:10.1088/2057-1976/aa589f
46. Hofstetter S, Zilezinski M, Wolf A, et al. Dfree ultrasonic sensor in supporting quality of life and patient satisfaction with bladder dysfunction. Int J Urol Nurs. 2022;17:62–69. doi:10.1111/ijun.12334
47. Hafid A, Difallah S, Alves C, et al. State of the Art of Non-Invasive Technologies for Bladder Monitoring: a Scoping Review. Sensors. 2023;23(5):2758. doi:10.3390/s23052758
48. van Leuteren PG, Klijn AJ, de Jong TPVM, Dik P. SENS-U: validation of a wearable ultrasonic bladder monitor in children during urodynamic studies. J Pediatr Urol. 2018;14(6):569.e1–569.e6. doi:10.1016/j.jpurol.2018.07.018
49. Fournelle M, Grün T, Speicher D, et al. Portable Ultrasound Research System for Use in Automated Bladder Monitoring with Machine-Learning-Based Segmentation. Sensors. 2021;21(19):6481. doi:10.3390/s21196481
50. Jo HG, Park BH, Joung DY, et al. Forward-Looking Ultrasound Wearable Scanner System for Estimation of Urinary Bladder Volume. Sensors. 2021;21(16):5445. doi:10.3390/s21165445
51. Kang BI, Kim A, Kim S. Advancing Patient Care: innovative Use of Near-Infrared Spectroscopy for Monitoring Urine Volume in Neurogenic Bladder. Int Neurourol J. 2023;27(Suppl 1):S27–33. doi:10.5213/inj.2346100.050
52. Macnab AJ, Shadgan B, Stothers L, Afshar K. Ambulant monitoring of bladder oxygenation and hemodynamics using wireless near-infrared spectroscopy. Can Urol Assoc J. 2013;7(1–2):E98–E104. doi:10.5489/cuaj.271
53. Palla A, Rossi S, Fanucci L. Bioimpedance based monitoring system for people with neurogenic dysfunction of the urinary bladder. Stud Health Technol Inform. 2015;217:892–896.
54. Shin SC, Moon J, Kye S, Lee K, Lee YS, Kang HG. Continuous bladder volume monitoring system for wearable applications. Annu Int Conf IEEE Eng Med Biol Soc Jeju, Korea. 2017;2017:4435–4438. doi:10.1109/EMBC.2017.8037840
55. Leonhäuser D, Castelar C, Schlebusch T, et al. Evaluation of electrical impedance tomography for determination of urinary bladder volume: comparison with standard ultrasound methods in healthy volunteers. Biomed Eng Online. 2018;17(1):95. doi:10.1186/s12938-018-0526-0
56. Park E, Lee JW, Kang M, Cho K, Cho BH, Lee KS. Detecting Bladder Biomarkers for Closed-Loop Neuromodulation: a Technological Review. Int Neurourol J. 2018;22(4):228–236. doi:10.5213/inj.1836246.123
57. Vogt B, Desgrippes A, Desfemmes FN. Changing the double-pigtail stent by a new suture stent to improve patient’s quality of life: a prospective study. World J Urol. 2015;33:1061–1068. doi:10.1007/s00345-014-1394-2
58. Bosio A, Alessandria E, Agosti SC, et al. Pigtail suture stents significantly reduce stent-related symptoms compared to conventional double J Stents: a prospective randomized trial. Eur Urol Open Sci. 2021;29:1–9. doi:10.1016/j.euros.2021.03.011
59. Karam R, Bourbeau D, Majerus S, et al. Real-Time Classification of Bladder Events for Effective Diagnosis and Treatment of Urinary Incontinence. IEEE Trans Biomed Eng. 2016;63(4):721–729. doi:10.1109/TBME.2015.2469604
60. Karam R, Bhunia S, Majerus S, Brose SW, Damaser MS, Bourbeau D. Real-time, autonomous bladder event classification and closed-loop control from single-channel pressure data. Annu Int Conf IEEE Eng Med Biol Soc. 2016;2016:5789–5792. doi:10.1109/EMBC.2016.7592043
61. Yu L, Kim BJ, Meng E. Chronically implanted pressure sensors: challenges and state of the field. Sensors. 2014;14(11):20620–20644. doi:10.3390/s141120620
62. Nelson BD, Karipott SS, Wang Y, Ong KG. Wireless Technologies for Implantable Devices. Sensors. 2020;20(16):4604. doi:10.3390/s20164604
63. Suster MA, Young DJ Wireless recharging of battery over large distance for implantable bladder pressure chronic monitoring.
64. Park YG, Lee S, Park JU. Recent Progress in Wireless Sensors for Wearable Electronics. Sensors. 2019;19(20):4353. doi:10.3390/s19204353
65. Wang C, Shi Q, Lee C. Advanced Implantable Biomedical Devices Enabled by Triboelectric Nanogenerators. Nanomaterials. 2022;12(8):1366. doi:10.3390/nano12081366
66. Yao G, Kang L, Li J, et al. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat Commun. 2018;9(1):5349. doi:10.1038/s41467-018-07764-z
67. Ouyang H, Tian J, Sun G, et al. Self-Powered Pulse Sensor for Antidiastole of Cardiovascular Disease. Adv Mater. 2017;29(40). doi:10.1002/adma.201703456
68. Majerus SJ, Garverick SL, Suster MA, Fletter PC, Damaser MS. Wireless, Ultra-Low-Power Implantable Sensor for Chronic Bladder Pressure Monitoring. ACM J Emerg Technol Comput Syst. 2012;8(2):11. doi:10.1145/2180878.2180883
69. Kim A, Powell CR, Ziaie B. An Universal packaging technique for low-drift implantable pressure sensors. Biomed Microdevices. 2016;18(2):32. doi:10.1007/s10544-016-0058-y
70. Majerus SJ, Fletter PC, Damaser MS, Garverick SL. Low-power wireless micromanometer system for acute and chronic bladder-pressure monitoring. IEEE Trans Biomed Eng. 2011;58(3):763–767. doi:10.1109/TBME.2010.2085002
71. Yao J, Qiang W, Wei H, et al. Ultrathin and Robust Micro-Nano Composite Coating for Implantable Pressure Sensor Encapsulation. ACS Omega. 2020;5(36):23129–23139. doi:10.1021/acsomega.0c02897
72. Levin A, Gong S, Cheng W. Wearable Smart Bandage-Based Bio-Sensors. Biosensors. 2023;13(4):462. doi:10.3390/bios13040462
73. Majerus SJ, Fletter PC, Ferry EK, Zhu H, Gustafson KJ, Damaser MS. Suburothelial Bladder Contraction Detection with Implanted Pressure Sensor. PLoS One. 2017;12(1):e0168375. doi:10.1371/journal.pone.0168375
74. Kim DH, Lu N, Ma R, et al. Epidermal electronics. Science. 2011;333(6044):838–843. doi:10.1126/science.1206157
75. Shin YK, Shin Y, Lee JW, Seo MH. Micro-/Nano-Structured Biodegradable Pressure Sensors for Biomedical Applications. Biosensors. 2022;12(11):952. doi:10.3390/bios12110952
76. Zheng Q, Zou Y, Zhang Y, et al. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci Adv. 2016;2(3):e1501478. doi:10.1126/sciadv.1501478
77. Cheng Y, Xu J, Li L, et al. Boosting the Piezoelectric Sensitivity of Amino Acid Crystals by Mechanical Annealing for the Engineering of Fully Degradable Force Sensors. Adv Sci. 2023;10(11):e2207269. doi:10.1002/advs.202207269
78. Amen MT, Pham TTT, Cheah E, Tran DP, Thierry B. Metal-Oxide FET Biosensor for Point-of-Care Testing: overview and Perspective. Molecules. 2022;27(22):7952. doi:10.3390/molecules27227952
79. Hossain MM, Shabbir B, Wu Y, et al. Ultrasensitive WSe2 field-effect transistor-based biosensor for label-free detection of cancer. 2D Mater. 2021;8(4):045005. doi:10.1088/2053-1583/ac1253
80. Shak Sadi M, Kumpikaitė E. Advances in the Robustness of Wearable Electronic Textiles: strategies, Stability, Washability and Perspective. Nanomaterials. 2022;12(12):2039. doi:10.3390/nano12122039
81. Du K, Lin R, Yin L, Ho JS, Wang J, Lim CT. Electronic textiles for energy, sensing, and communication. iScience. 2022;25(5):104174. doi:10.1016/j.isci.2022.104174
82. Das R, Zeng W, Asci C, Del-Rio-Ruiz R, Sonkusale S. Recent progress in electrospun nanomaterials for wearables. APL Bioeng. 2022;6(2):021505. doi:10.1063/5.0088136
83. Farooqui MF, Shamim A. Low Cost Inkjet Printed Smart Bandage for Wireless Monitoring of Chronic Wounds. Sci Rep. 2016;6:28949. doi:10.1038/srep28949
84. Park J, Kim J, Kim SY, et al. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci Adv. 2018;4(1):eaap9841. doi:10.1126/sciadv.aap9841
85. Arab Hassani F, Mogan RP, Gammad GGL, et al. Toward Self-Control Systems for Neurogenic Underactive Bladder: a Triboelectric Nanogenerator Sensor Integrated with a Bistable Micro-Actuator. ACS Nano. 2018;12(4):3487–3501. doi:10.1021/acsnano.8b00303
86. Khurram A, Ross SE, Sperry ZJ, et al. Chronic monitoring of lower urinary tract activity via a sacral dorsal root ganglia interface. J Neural Eng. 2017;14(3):036027. doi:10.1088/1741-2552/aa6801
87. Dodd W, Motwani K, Small C, et al. Spinal cord injury and neurogenic lower urinary tract dysfunction: what do we know and where are we going? J Mens Health. 2022;18(1):24. doi:10.31083/j.jomh1801024
88. Arab Hassani F, Jin H, Yokota T, Someya T, Thakor NV. Soft sensors for a sensing-actuation system with high bladder voiding efficiency. Sci Adv. 2020;6(18):eaba0412. doi:10.1126/sciadv.aba0412
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.