Back to Journals » Veterinary Medicine: Research and Reports » Volume 15
Canine Atopic Dermatitis: Prevalence, Impact, and Management Strategies
Authors Drechsler Y , Dong C, Clark DE, Kaur G
Received 30 September 2023
Accepted for publication 26 January 2024
Published 13 February 2024 Volume 2024:15 Pages 15—29
DOI https://doi.org/10.2147/VMRR.S412570
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Yvonne Drechsler,1 Charli Dong,2 David E Clark,1 Gagandeep Kaur1
1College of Veterinary Medicine, Western University of Health Sciences, Pomona, CA, USA; 2Animal Dermatology Clinic, Pasadena, CA, USA
Correspondence: Yvonne Drechsler, College of Veterinary Medicine, Western University of Health Sciences, 309 E. Second Street, Pomona, CA, 91711, USA, Tel +1 909 706 3535, Email [email protected]
Abstract: Atopic dermatitis (AD) is a common inflammatory and pruritic allergic skin disease in humans and dogs worldwide. The pathogenesis of AD is multifactorial, immunologically complex, and may involve genetic factors, epidermal barrier dysfunction, microbiome changes, immune dysregulation, and allergic sensitization. Across species, prevalence of AD is on the rise. At present, there is no cure for canine AD (CAD). The treatment for CAD is multifaceted and aimed at controlling the pruritus, associated inflammation, and infections, repairing the skin barrier function, and dietary management. This review presents data on prevalence, impact, and complex immunological interactions in AD with a focus on subsequent management of the disease in the canine population. A multimodal approach for management of CAD to address varying clinical signs and responses to therapies is discussed.
Keywords: immunopathology, topical therapies, systemic therapies, pruritus, immune therapies, alarmins
Prevalence
Canine atopic dermatitis (CAD) is a multifactorial, pruritic disease, with genetic and environmental factors playing an important role in development and pathophysiology.1,2 Skin barrier dysfunction and aberrant immune response are the characteristics of CAD.2,3 The prevalence of CAD has been estimated at 3–15%.4 However, as per the American College of Veterinary Dermatology (ACVD) task force on atopic dermatitis (AD), these percentages are not based on reliable epidemiological data, and the ACVD task force concluded that the actual incidence and prevalence of CAD in the canine population is not well known.5
Comparative studies of patients presented to veterinary facilities for skin diseases can be helpful to assess prevalence. In a study of dogs presented for skin problems at 52 veterinary practices in the US, prevalence of atopic or allergic dermatitis, and atopy, was 4.7% in 31,484 dogs examined.6 In a study from the Small Animal Clinic at University of Montreal, 18.8% of the dogs presented for dermatological disorders of which 12.7% were atopic.7 A recent retrospective study from a teaching hospital in Brazil reports an upward trend in diagnosis of CAD with 25.65% AD cases of all the dogs examined. Although the numbers from these studies provide insight into the cases presented, it is difficult to derive prevalence from these studies as populations at the teaching hospitals do not represent the general canine population.
Although many studies provide some insight on the prevalence of CAD, these data are influenced by geographical regions, survey methods, selection of populations, types of veterinary practices, and criteria used for diagnosis of CAD and other dermatoses.5 The prevalence and risk of developing CAD is also influenced by environmental factors. Dogs living indoors have a higher frequency of developing CAD.8,9 In a study of Golden and Labrador retrievers, environmental factors such as living in rural areas, or with other animals, and being walked in forest areas were associated with a decreased risk of CAD development, whereas, early adoption, and living in a shed in puppyhood were associated with a higher risk of CAD development.10 Another study in Labrador and Golden retrievers reports factors such as dogs born in rural locations, living with other dogs, and those walked on woodlands, fields, and beaches reduced the risk of developing CAD.11
In general, the onset for clinical signs of CAD is between 4 months to 3 years of age.8,9 However, the age of onset can vary between different breeds,12 and thus cause a change in the prevalence reported per breed, especially if a breed is represented highly in a specific geographic area. Sex-related predispositions for CAD have not been reported, but studies have shown higher numbers in either sex. In a retrospective study from Brazil, where 25.65% of dogs presented to the teaching hospital were diagnosed with CAD, 62.4% were females.13 However, studies have shown that male dogs, especially neutered males, are more likely to develop CAD than intact female dogs.11 In a study from Australia, a higher risk in males from Pug and Bichon Frisch breeds was identified.14 However, it should be noticed that most studies do not notice any sex predilection for development of CAD. In another study, a negative correlation between feeding a non-commercial homemade diet of the nursing dog and the development of CAD in her litter has also been reported.15
The prevalence of CAD also depends on the representation of specific breeds in geographical areas where studies are conducted. Multiple studies report that Golden Retrievers, Labrador Retrievers, West Highland Terriers, German Shepherds, and French Bulldogs are at an increased risk of developing CAD.3 In a study from Australia, 11 dog breeds with significantly increased odds risk for CAD were identified worldwide, such as Boxer, Labrador Retriever, Pug, Bulldog, and West Highland Terrier, and classified as predisposed.14 In a retrospective study from Brazil, CAD was most prevalent in mixed breed dogs, followed by Shih Tzu and Poodles.13 Several factors influence the breed representations in CAD, including regional popularity of the breeds, genetic susceptibility, and geographical area.13
Despite all the studies, it should be noted that true prevalence and incidence of CAD is hard to determine due to the complex development, presentation, and management of the disease. Many CAD cases are managed by veterinarians without a specific diagnosis, such as mild presentations of CAD that are managed symptomatically or specific clinical presentations such as chronic otitis, which may not be identified as CAD by clinicians,5 affecting incidence reporting. Furthermore, the influence of a variety of environmental risk factors in disease development affects the prevalence reported.1 Considering all the factors that can affect diagnosis and presentation of CAD, there is a need for detailed epidemiological studies on the prevalence and incidence of CAD.
Impact
The prevalence numbers discussed above indicate that CAD is a significant malady of the pet population. Veterinarians rely on clinical signs and behavior of the animal when assessing the effect of a disease on the wellbeing of pets. Diseases of pets can affect quality of life of the pets as well as that of their owners.16,17 Since 2010, several questionnaires have been developed for skin diseases in small animals, and some of these also assess owner quality of life. Some questionnaires have been validated, and assessing quality of life in therapy studies is becoming more common.16 In the study by Linek and Favrot,17 almost half of the owners of dogs with CAD reported the disease affected their own quality of life, and a majority of owners felt the disease affected their pet’s quality of life. Those effects included changes or interruptions in mood, family life, leisure activities, and sleep. Some owners were also concerned with expenses and the burden of treatment. The pet’s quality of life was affected by changes in the dog’s activities of playing, walking, and sleeping.17
There are studies that indicate pruritus and atopic dermatitis can affect canine behavior in negative ways. One study found increases in behaviors associated with fear, anxiety, aggression, and decreased trainability.18 Another study did not find increases in fear and anxiety behaviors, while an increase in numerous unwanted or problem behaviors was identified, along with a decrease in trainability.11 The authors postulated that behavioral changes could be related to stress associated with pruritus. Behavioral problems are potential reasons for euthanasia of companion animals. According to a study by Pegram et al, behavioral problems were one of the most common reasons for euthanasia of dogs in the United Kingdom.19 However, the study by Linek et al did not show increased thoughts of euthanasia by the owners.17
CAD requires ongoing management that entails a long-term financial commitment from the pet owner, which may be a burden and could affect access to care in some instances. However, based on prevalence of the disease, the treatment and management of CAD is potentially a significant source of veterinary industry income. Information on the financial impact of CAD for patients, pet owners, and the veterinary industry, though, is limited. The inclusion of the use of quality-of-life data is helping to improve our understanding and treatment of CAD. There is a need for focused studies on the financial, pet behavioral, and human emotional impacts of CAD.
Immunopathology
Initially, AD was understood simply as an IgE mediated allergic response or hypersensitivity I reaction, disrupting skin barrier function and activating Th2 responses. Different hypotheses were put forward, such as Outside-In with epidermal barrier dysfunction as primary cause leading to immune activation, or Inside-Out, where epidermal barrier dysfunction is secondary.20 However, it has become evident that there are more nuanced and complex underpinnings of the disease, involving a variety of immune cell subsets and responses, types of barrier function disruption and neuroimmune feedback loops that contribute to pathophysiology.21–24
In healthy skin, a balance of skin barrier integrity, commensal skin microbiota, and cellular skin populations contribute to skin function and response to pathogens. Disruption of any of these factors leads to dysregulation of this balance with negative effects. Several strong candidate genes for development of AD in humans have been identified, such as mutations in filaggrin, an important epidermal protein,25 or immune genes found in a cluster on human chromosome 5, such as IL4, and IL13 with over 30 loci implicated.26 In dogs, recent genome-wide association studies (GWAS) on canine AD have also shown a strong connection with the filaggrin gene locus, and genes involved in immune responses and skin barrier function, with over 15 loci connected to CAD development.27 As in humans, it is not clear how many mutations in these genes contribute to CAD. Certain dog breeds, such as German Shepherd dogs and Golden and Labrador retrievers, are overrepresented in prevalence of CAD.28,29 However, while genetics play certainly a role, multiple factors including the environment and nutrition add to a complex trait such as AD, as evidenced by the fact that mutations are not found in all cases of AD, and even carriers of these mutations do not all develop AD.1,30
Epidermal barrier disruption promotes inflammation, either due to filaggrin mutation as a primary cause, or secondary, subsequent to inflammatory/immune signaling. Other causes of skin dysbiosis occur with the itch-scratch cycle caused by irritants or allergens. These disruptions favor colonization with opportunistic microbiota, negatively affecting the skin by creating a feedback loop between immune response and epidermal inflammation. Staphylococcus spp. are commonly found in atopic lesions, in both humans (S. aureus)31,32 and dogs (S. pseudintermedius),33,34 in conjunction with overall decreased microbial diversity.34 Other microbiota found associated with AD skin are Malassezia,35,36 or Corynebacterium.34 All of these can aggravate already inflamed skin, and in several studies the use of antimicrobials and medicated shampoos has been found to be beneficial in combination with other treatments for AD,37,38 however, there is concern about increasing resistance of bacteria with the continued use of antibiotics.39 As with epidermal barrier dysfunction, changes in microbiota community diversity and prevalence might be primary or secondary to AD development.
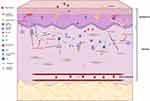
In non-lesional skin and early acute stage of AD, tissue damage leads to the release of a class of proteins by keratinocytes, such as IL25, IL33, or thymic stromal lymphopoietin (TSLP), which are also called alarmins,40–42 and chemokines CCL17 and CCL2243 (Figure 1). Alarmins are strong activators of Innate Lymphocyte Cell type 2 (ILC2) which release IL5 and IL13, both cytokines involved in TH2 signaling.42,44 ILC2 has been found in acute AD lesions of humans and mice,42,45 but have not been investigated in the skin of dogs. A study on peripheral blood in atopic dogs did not show elevated levels of these cells, which is in agreement with studies on ILCs in human patients.46 The alarmin TSLP has been shown to activate dermal dendritic cells to express OX40 and consequently activate naïve CD4 T cells, further amplifying a Th2 response.47–49 Expression of TSLP was significantly increased in lesional and nonlesional skin of dogs with CAD.50 Another aspect of alarmin release from keratinocytes, particularly TSLP and IL33, is the subsequent initiation of itch sensations. Scratching leads to more epidermal damage, followed by more release of alarmins, release of histamines from a variety of cells including mast cells, and Th2 mediated activation of IL31, which acts on sensory neurons to induce an itch sensation.51,52 The itch-scratch cycle reinforces itself and can eventually lead to thickening of the epidermis and chronic inflammation.
Chemokines CCL17 and CCL22 also induce Th2 responses, and combined, these inflammatory events lead to a self-amplifying release of IL4, IL5, IL13, and IL31, increased infiltration of CD4 and CD8 T cells, activation of B cells and IgE class switching, activation of mast cells, and recruitment of eosinophils.44,53,54 The key intracellular pathway involved in inflammatory and particularly Th2 signaling is Janus activated kinase/signal transducer and activator of transcription (JAK/STAT) mediated,55 where binding of cytokines to their cellular receptors activates JAK, leading to phosphorylation of STAT inducing gene transcription of inflammatory signaling molecules. Due to its importance in Th2 signaling, this pathway has become a major focus for drug development to combat pruritus and inflammation in AD.
Aside from Th2, several studies have looked at Th1, Th9, Th17 and Th22 activation both in humans and dogs.53,56–58 The involvement of additional cell types and cytokines is reflective of an increasingly complex and widening adaptive immune response, transitioning to chronic disease. Th22 is associated with expression of IL22, which is present in the onset of acute lesions, progressively increasing in chronic disease.56 On the other hand, Th1 and Th17 tend to be more variable in chronic stages of AD, but are also associated with bacterial colonization, as both of these pathways are typically considered classic inflammatory pathogen responses.59 Different endotypes of atopic dermatitis in humans have been shown to reflect differences in the activation of these Th responses.44,60 As an added complication, in humans difference in Th1 vs Th22 or Th17 involvement has been associated with ethnicity, with European vs Asian subtypes, but recent transcriptomic studies reveal a rather heterogeneous pathogenesis involving Th17, Th22, and Th1 types.44,59–61 Dog breed-specific Th subtypes have so far not been identified.
Recent advances in single-cell transcriptomics are increasingly delineating these complex responses on a cellular level both in humans and dogs.22,62,63 Not only do these studies show the cellular subpopulations and detailed activated genes and pathways involved but also identify different cells previously not well investigated in the context of AD. For example, fibroblasts were identified in these single-cell sequencing studies as having a role in immunomodulation, opening up new avenues of investigation for therapeutics. In the canine study, gamma delta T cells were additionally identified as highly increased in the atopic skin of dogs, and several inflammatory markers previously not detected discovered.63
Considering the variability of responses, but common themes such as Th2 responses, neuroinflammation and itch, the treatment of CAD has focused on the pathways involved in these areas as detailed in the following management practices.
Management of CAD
CAD requires a multimodal approach for management. Each dog has varying degrees of clinical signs and responses to therapies; thus, it is best to tailor the management plan for each individual patient. It is also important to emphasize that secondary infection and other factors need to be addressed for these management strategies to be effective. Clinicians should always treat for secondary bacteria and yeast pyoderma or pododermatitis prior to reaching for symptomatic care, especially when there is a sudden increase in the pruritus score or if a patient’s pruritus is localized. Of equal importance, it is pertinent to investigate other causes of pruritus with diet trials and parasite control. If there is a limited response to these management therapies, clinicians should reconsider their diagnosis. Lastly, to ensure successful long-term management, client education is most essential. Chronicity and incurable aspects of CAD in addition to expectations ought to be addressed. Frustration and ongoing expenses are common concerns, and owners should be counseled accordingly.
Immunotherapy
Allergen-specific immunotherapy (ASIT) has been at the forefront of managing AD in both humans and veterinary patients since 1911.64–66 The first report of successfully treating an allergic dog with ASIT was published in 1941.67 It is the most effective and proactive way to manage CAD. ASIT is generally safe, helps reduce clinical signs, and decreases the overall need for medication.68 ASIT refers to a mix of allergens given in progressively increasing doses and typically consists of an induction phase followed by maintenance. Traditionally, these doses are given as subcutaneous injections. Frequencies and amounts should be customized to each individual depending on the individual’s clinical responses and adverse events. The average observable clinical response occurs between six and eight months but because response rates are variable, subcutaneous ASIT should be continued for at least 1 year to properly evaluate efficacy.64,68,69 For some patients, especially seasonal cases, 2–3 years of follow-up are needed to make a suitable assessment. According to a retrospective study of 664 client owned dogs, more than 50% of clinical signs improved in 59.9% of atopic dogs with ASIT. Beneficial effects are notably higher if dogs are examined regularly and consistently, and if no systemic long-term corticosteroids are given in the first 9 months of therapy.70 Nevertheless, owner compliance is the main factor as the cause for reduced efficacy of ASIT. In another retrospective study of 145 CAD patients managed with ASIT, the duration of treatment was strongly linked with efficacy.71 Dogs treated for less than 1 year only showed an efficacy rate of 22% compared to dogs treated for more than 1 year (65%). In addition, concomitant medications in dogs treated for more than 1 year were reduced by 87%.71
Some clinicians, including the authors of this paper, have found RUSH (“sped-up”) immunotherapy to be an effective protocol because RUSH immunotherapy can decrease the time necessary to achieve maintenance doses as well as reducing questionable side effects monitored by owners. In RUSH protocols, doses are administered at 15–60 minute intervals over 1–3 days, until maintenance is achieved.72
Sublingual immunotherapy (SLIT) is a form of immunotherapy where allergens are given orally instead of by traditional subcutaneous injection. For humans, it has been an efficacious treatment and received United States regulatory approval in 2014.73 In people, the major use is for allergic rhinoconjunctivitis or asthma but has also been used for AD.74,75 Protocols usually include hooking the plastic dispensing nozzle over the lower arcade of teeth or the commissure, with the goal that the solution be dispensed sublingually.74 Protocols generally consist of an induction period ranging from lower to higher concentrations but can vary. Most protocols consist of one to two pumps twice daily. Patients should avoid eating or drinking for 10 minutes before and after administration for best absorption.
Intra-lymphatic immunotherapy (ILIT) is a relatively new form of ASIT.72,76 In dogs, protocols include administration with ultrasound guidance into a lymph node, usually the popliteal, as it is the easiest lymph node to access. Induction phases can vary but one published protocol consists of one injection every 4 weeks for four treatments, followed by the maintenance phase of subcutaneous ASIT injections thereafter.76 In a prospective comparison study comparing three different protocols (subcutaneous ASIT, ILIT and SLIT), ILIT outperformed both groups in returning to normal and improvement. In another study, no significant differences between RUSH immunotherapy and ILIT induction protocol were noted.72 ILIT has been found to be safe and identical in efficacy to traditional subcutaneous ASIT.75
Currently, there is no standardization for immunotherapy protocols no matter the methodology. Protocols can be used as a starting point, but the clinician should make adjustments based on each individual patient’s response. Presently, it is essential to highlight that CAD is a diagnosis via exclusion and that neither ASIT nor IgE serologies can be used to diagnose CAD. Yet these diagnostics are helpful in identifying hypersensitivity to environmental allergies in CAD patients after a diagnosis has been made.
Drugs
Antihistamines make a minor impact on the overall management of CAD, but they continue to be one of the most frequently used medications to manage CAD.77 Type I oral antihistamines include hydroxyzine, diphenhydramine, chlorpheniramine, clemastine, and dimetindene. Their principal mechanism of action was considered to interfere with histamine-mediated pruritus by blocking the histamine H1 receptor.78,79 Histamine is an inflammatory mediator released from several cells, such as mast cells and basophils, leading to further degranulation of mast cells and inflammatory cell migration and is thought to be involved in the excitation of unmyelinated C-fibers via increase in calcium in neurons, leading to itch.80,81 First-generation antihistamines block histaminic and muscarinic receptors and are able to pass the blood–brain barrier.82 Second-generation antihistamines predominantly block histaminic receptors and are less likely to pass blood–brain barrier.82 First-generation antihistamine studies tend to have conflicting results, but it can be agreed that results are generally trivial or ineffective. A randomized control trial reported that two first generation oral antihistamines, consisting of a hydroxyzine/chlorpheniramine combination (Histacalmine®, Virbac, Carros, France) and dimetindene (Fenistil®, Novartis, Basel Switzerland) barely improved dermatological lesions and pruritus in dogs with CAD.83 In contrast, the development of skin lesions in an experimental model of acute CAD in house dust mite-sensitized dog was not prevented by the administration of hydroxyzine.78 In another study, it was demonstrated that cetirizine, a second-generation antihistamine, had no effect on pruritus after 14 days of administration in chronic CAD patients.79 Loratadine, cetirizine, and fexofenadine are commonly prescribed second-generation antihistamines.84 For optimal benefit, it is recommended that type I antihistamines be given either prior to a flare, or given to dogs with mild forms of CAD.68 Certain antihistamines (hydroxyzine, cetirizine, and cyproheptadine) may also block serotonin receptors.69 Some antihistamines modify behavior, notably, tricyclic antidepressants that include amitriptyline and doxepin. Adverse effects are often mild, largely being pruritus, but dry mouth, panting, sedation, drowsiness, hypersalivation, ataxia, trembling, hyperesthesia, and excitation have all been reported.69 Second-generation antihistamines should theoretically provide fewer side effects because they do not pass the blood–brain barrier, but this is not guaranteed.84 Despite all these negative data, in a study performed by Dell and collaborators, owners believe antihistamines to be an effective part of multimodal therapy for CAD.77 Benefits are only seen in a minority of dogs and benefits usually occur in the first 7–14 days of treatment.80 Antihistamines are better suited as an additive or used synergistically with other medications.80 Their lack of efficacy is probably linked to the fact that histamine is not the only molecule causing pruritis, as detailed in the immunopathology section.
Glucocorticoids have powerful immunologic and anti-inflammatory activities. They exhibit many inhibitory effects directly acting on target genes, subsequently affecting the production of inflammatory mediators as well as induction of anti-inflammatory cytokines and thereby modulating cell-mediated and humoral immunity.69 Glucocorticoids are effective and work quickly, but without proper management, undesirable adverse effects can occur. Typically, oral glucocorticoids are used for managing CAD. Oral prednisolone, prednisone, and methylprednisolone are given at 0.5–1 mg/kg per day.69 Adverse effects are generally proportional to steroid potency, dosage, and administration duration. Long-term control of CAD is best accomplished with alternate days of glucocorticoid therapies, at once every 48 hour or 72-hour dosing, to diminish side effects. Side effects include varying degrees of hypercortisolism-polydipsia, polyuria, polyphagia, weight gain, calcinosis cutis, dermal atrophy, pyoderma, panting, urinary tract infections, distended abdomen, depression, and diarrhea.69
Cyclosporine is an immunosuppressive drug that was originally developed to prevent organ transplant rejection.69 It is desired for its immunosuppressive benefits compared to its low cytotoxicity effects and does not interfere with the results of intradermal skin tests.69 Cyclosporine inhibits intracellular calcineurin, which is a serine/threonine protein phosphatase involved in activation of T cells. The enzyme dephosphorylates nuclear factor of the transcription factor called “nuclear factor of activated T cell cytoplasmic” (NFATc). Transcription factors function by translocation to the nucleus with subsequent binding to the promoter of a gene, inducing its transcription, which in case of NFATc includes interleukin-2 (IL-2), an important cytokine for T cell activation and proliferation. By blocking calcineurin, dephosphorylation and subsequent translocation of NFATc is blocked, decreasing IL-2 release and therefore T cell activation. Cyclosporine has been extensively used to treat CAD and many other diseases; its advantageous effects extend beyond IL-2 suppression as cyclosporine affects canine keratinocytes, potentially cutaneous dendritic cells, inhibits mast cell degranulation, and innate immunity.69,85 Different cyclosporine formulations are available: veterinary, human brand-named, and generic formulations. Microemulsified formulations are preferred, as the microemulsion concentration is absorbed quickly and effectively through the gastrointestinal tract of dogs. The problem with cyclosporine is variability in its bioavailability, between dogs and even in the same dog. Presence of food in the gastrointestinal tract can play a role in making bioavailability even more variable, especially when food is high in fat, so it was recommended to administer ultramicronized cyclosporine 2 hours before or after feeding.85,86 Conversely, a study demonstrated that food did not have any impact on the efficacy of ultramicronized cyclosporine clinically.87 At CAD specific dosing (5mg/kg PO per day), common side effects include vomiting, diarrhea, and anorexia. Less frequently observed side effects include hypertrichosis, gingival hyperplasia, papillomatosis, and psoriasiform lichenoid dermatosis.69,86 There is often delayed gratification as it may take 4 weeks of therapy to see optimal clinical responses. The authors recommend prescribing name-brand veterinary formulations of cyclosporine at the optimal dosing daily until remission is achieved before tapering to the lowest effective dose or using modified versions. In most cases, this includes daily administration for 1 month before tapering. Cyclosporine is metabolized in the liver and intestines, by the cytochrome P450 isoenzymes, specifically CYP3A4.85 Drugs inhibiting or inducing cytochrome P-450 may affect cyclosporine metabolism. CYP3A4 inducers, for example rifampin or carbamazepine, may decrease cyclosporine levels by increasing their clearance. On the other hand, P-450 inhibitors, most notably several antifungal drugs (ketoconazole, itraconazole, and fluconazole), decrease cyclosporine clearance and increase cyclosporine concentration.69 Some clinicians take advantage of this by combining cyclosporine with ketoconazole. When used in combination, cyclosporine can be reduced to 2.5 mg/kg PO per day, but note that this is a calculated hypothesis and true dosing would vary individually. While this is a method to decrease cyclosporine, the authors see a higher incidence of adverse effects and each patient's bioavailability is variable so the reduction serves only as a guide. Some clinicians use trough and peak cyclosporine levels to provide guidance.
Oclacitinib (Apoquel®, Zoetis, Parsippany-Troy Hill, NJ) is an immune-modulating drug that inhibits the JAK/STAT signaling pathway. As previously mentioned, this pathway plays a major role in Th2 mediated inflammatory responses. It has been approved for treating pruritus related to allergic dermatitis, including CAD.88 Oclacitnib has been popular among clinicians for its high degree of efficacy, rapid onset, and like cyclosporine, no interference with intradermal skin testing, and low degree of adverse effects. The labeled prescribed dosing is 0.4–0.6 mg/kg PO every 12 hours for up to the 14 days89 and then once daily for maintenance according to manufacturer’s instructions. However, the authors find that most cases do not require this induction dosing. Oclacitinib allows comparable reduction in pruritus and clinical signs, compared to prednisolone, and outperforms cyclosporine in pruritus resolution and speed of action.90–92 Side effects, mainly gastrointestinal signs, are rare. Skin infections are also noted as side effects,88 but dogs with CAD tend to develop recurrent skin infections without medications. At the label dose, changes in hematological and serum chemical parameters have been described, but are minimal, including slight leukopenia, mild hypercholesterolemia, and minor increases in alkaline phosphatase (ALP) activity levels.88 Apoquel® should not be administered in dogs less than 12 months of age, nor in dogs with serious infections, such as pneumonia. Apoquel may exacerbate neoplastic conditions or increase susceptibility to infection, notably demodicosis. There is now a chewable formulation available for dogs.93
Lokivetmab (Cytopoint®, Zoetis, Parsippany-Troy Hill, NJ) is indicated for the treatment of clinical ailments of CAD. It is a caninized anti-interleukin (IL)-31 monoclonal antibody that was designed to neutralize IL-31,94 which is a cytokine acting on sensory neurons in the skin causing neuroinflammation and itch.52 It is commercially available as a subcutaneous injection in 1 ml vials containing 10, 20, 30, or 40 mg of lokivetmab. The recommended minimum dose95 is 1 mg/kg bodyweight once a month. Contraindications include not using in cases of hypersensitivity and dogs that weigh less than 3 kg, according to manufacturer’s instructions. In a study of 274 CAD dogs, lokivetmab administered at about once monthly dosing of 1 mg/kg, provided pruritic relief within a day, lasting duration, and all with a good safety profile.96 Another study confirmed the same findings.97 A recent 2021 study found that a single subcutaneous injection of 2 mg/kg suppressed pruritus within 3 hour for forty-two days; however, this study was performed with purpose bred Beagles.98 In a study performed by Marsella and collaborators comparing prednisone, oclactinib, cyclosporine, and lokivetmab for treatment of CAD in which dogs were challenged twice weekly with allergens, lokivetmab could be applicable in preventing flares and improving Transepidermal Water Loss (TEWL).99
Pentoxifylline is a methylxanthine derivative that produces diverse physiological changes at the cellular level, such as white blood cell kinetics (responsiveness and activity), and platelet deformability and aggregation. It is used for a variety of inflammatory diseases. In a study performed by Singh and collaborators, pentoxifylline only provided low-quality evidence for the management of CAD.100 Pentoxifylline is available in a 400 mg coated tablet and dosing recommendations range between 20 and 30 mg/kg orally every 8–12 hours. Caution regarding the bioavailability of generic drugs ranging from 25% to 75%. Favorable response is not seen until 1–3 months of taking this drug. In general, serious side effects are not reported. Vomiting and diarrhea are seen infrequently. Dizziness and headaches have been reported in humans.101
Topical Active Ingredients
Antipruritics
Topical steroids are the mainstay of CAD topical treatments and are popular for their ability to quickly reduce inflammation and pruritus, ability to treat focal areas, and rarely produce undesired side effects in the short term. Topical glucocorticoids interfere with the inflammatory cascade and pruritogenic pathway by impeding the arachidonic acid pathway, some inflammatory cytokines, and growth factors and decreasing some adhesion molecules expression.102 Glucocorticoids come in varying degrees of potency and different formulations and should be considered when formulating a treatment plan. In North America, topical glucocorticoids are divided into seven classes based on their ability to cause blanching (vasoconstriction).102 Mild glucocorticoids (classes 6 and 7) are preferred when lesions are mildly inflamed, large areas to be treated, or thin skin at site of application (glabrous regions). These classes are preferred for long-term use. Ointments have an occlusive nature and improve glucocorticoid absorption but may also macerate in occluded areas (skin folds, high hair density, and interdigital spaces).102 Creams are mixes of water suspended in oil. They are usually moisturizing and cosmetically acceptable but are generally less potent than ointments. Whatever delivery vehicle is chosen, it is not generally recommended that a topical steroid be applied more than twice a day.102 Hydrocortisone was the first to be used and is most commonly found in commercial formulations.69 Previous studies have noted adrenal suppression in formulations containing betamethasone, triamcinolone, and fluocinonide.69,103 When choosing a steroid, it is best to choose a less potent steroid such as hydrocortisone 1% or a soft steroid to avoid overuse causing undesirable side effects. Soft steroids include a new generation of diester topical glucocorticoids: hydrocortisone aceponate, mometasone furoate, and prednicarbate. These are metabolized at skin level into inactive ingredients, thereby dramatically reducing unwanted systemic effects.104 Triamcinolone acetonide and betamethasone are typically found in veterinary prescribed formulations of topical glucocorticoids. These are moderate-to-high potent glucocorticoids, and side effects are common with misuse. In the United States, triamcinolone acetonide is commonly found as a cream or ointment and combined with neomycin and nystatin and betamethasone is found as a topical spray combined with gentamicin. Triamcinolone acetonide spray (Genesis®; Virbac, Carros, France) has been shown to be very efficacious in the treatment of CAD if applied for 4 weeks then tapered in the absence of clinical adverse effects. Of note, there is a commonly used product in the United States that contains neomycin sulfate, isoflupredone acetate, and tetracaine hydrochloride (Neo-predef®; Zoetis, Parsippany-Troy Hills, NJ). It is popular for desired effects of drying out moist lesions, but owners should be counseled as even mild overuse can quickly lead to undesired adverse effects. For all glucocorticoids, caution should be taken when applying on glabrous regions of the body, notably the abdomen, because undesired effects of dermal atrophy, scaling, comedones, or even calcinosis cutis, can occur quickly and most commonly in those locations.69
Pramoxine hydrochloride is a topical anesthetic and is used as an antipruritic. It is an effective surface anesthetic and is well tolerated by skin and mucous membranes with extremely low rates of toxicity.69 Pramoxine exerts its anesthetic and anti-pruritic effect by preventing excitation of slow C fibers that signal itch and pain. This is achieved by reversibly binding voltage-gated sodium channels preventing membrane depolarization, and subsequently inhibiting the generation of action potentials in these peripheral neurons.69 Rarely, contact reactions are seen. One crossover open clinical trial evaluated two formulations of pramoxine cream rinse and concluded that after 4 weeks, there was at least 50% reduction in pruritus from 41% of the owners and antipruritic effects lasted 48 hour.104,105 Unfortunately, with frequent and repeated use, duration of effect and efficacy wanes.106 In veterinary medicine, it is usually found in the form of a shampoo, rinse, and spray and often in combination with ceramides or hydrocortisone, and combination formulations serve dual moisturizing and antipruritic effects.
Tacrolimus has been explored for the treatment of localized lesions of atopic dogs.104 Three studies have concluded that although expensive, tacrolimus is promising for managing localized CAD lesions with nominal side effects. Most dogs were able to reduce at least 50% of clinical signs from baseline after 4- to 12-week treatments.107 Adverse effects of mild irritation were noted in a minority of cases: 0.1% ointment formulation is recommended due to 0.3% tacrolimus causing a fourfold increase in serum tacrolimus concentrations.108
Emollients and Moisturizers
Since CAD patients have skin barrier defects, replenishing the oils can be beneficial in controlling clinical signs. Emollients are vehicles that soften, lubricate, or soothe the skin. Moisturizers aide by reducing TEWL via hygroscopic molecules or blocking agents. There are many options:
Fatty acids are important in hydration and serving as a barrier in controlling TEWL. Commercial products are direct topical applications of fatty acids to the skin. Dermoscent Essential 6® Spot-on (Nextmune, Phoenix, AZ) contains a combination of fatty acids and emollients that restore hydrolipidic film on the skin, maintain hydration, and maintain epidermal barrier function. In addition, inflammation is controlled with antioxidant and anti-free radicals. There have been several studies proving the efficacy of these essential fatty acids in reducing TEWL and pruritus scores with no side effects.109,110 An induction dosing of one pipette every week for 2 months followed by maintenance dosing of one pipette every 2 weeks for as long as necessary according to manufacturer’s instructions. No massage is required, and it is recommended not to bathe 2 days before and after application. The amount of product per pipette depends on the size of the pet: up to 10 kg, 10–20 kg, and 20–40 kg. The fragrance is a fresh herbal scent. Another product, Atopivet® Spot-on (Dechra, Cheshire, CT) contains Biosfeen®, a sphingomyelin-rich sphingolipid, and Dermial®, a glycosaminoglycan, specifically hyaluronic acid. The fragrance is a lavender scent. It comes in 2mL pipettes and can be applied to affected areas or between the shoulder blades and down the back twice weekly for at least 5–8 weeks before modifying the application as needed. It also comes in a mousse and skin care collar. The collar only contains Biosfeen® and can help the skin barrier for up to 2 months.
Coconut oil has been a very popular remedy with self-directed owners in recent years. Virgin coconut oil is preferred since it is colorless, odorless, and cosmetically elegant, and has antibacterial activity. For coconut oil to be clinically effective, it must be applied twice daily for at least 4 weeks.111 This was adapted from human literature, and for dogs, the challenge is reaching skin level with hair being a barrier.
Mineral oil/liquid paraffin (highly refined mineral oil) baths also aid in reducing TEWL. In this protocol, the first step is to bathe the patient with a shampoo. The chosen shampoo should be decided based on the patient’s presentation. If a patient tends to present with secondary infections, then an antiseptic shampoo should be recommended. If the patient tends to present with scale, then the clinician can prescribe a keratolytic or keratoplastic shampoo. It may be prudent to warn the client that for the first couple of baths, alopecia may be expected from dislodging affected hairs. The second step is to formulate a 50:50 mixture of water and mineral oil. This step replaces the lipid matrix of the stratum corneum. Soak the patient for 1 hour, to allow the oils to penetrate. In the final step, the patient should be washed with dishwashing soap to break down excess oils. Another option is to use large puppy pads to soak up the excess oil. The authors prefer the brands Alpha Keri and Patterson Medical unscented paraffin oil.
Wet wrapping is a protocol adapted from human literature to control atopic eczema in children.112,113 This protocol is effective for rehydrating and calming the skin, as well as helping topical medications work better. Fill the bathtub with lukewarm water, then add liquid paraffin. Immerse the pet minus the head for 5 min. Remove the pet and pat (not rub) dry. Cover the skin with a thick moisturizer or emollient. For severely pruritic pets, mometasone furoate 0.1% ointment is advised on pruritic areas as this is a soft steroid and theoretically will not be absorbed systemically. Next, place fitted clothes and/or socks in hot water and wring them out, then dress the patient. Apply a second layer of fitted clothes and/or socks, leave on for 40 minutes or until the dressing is dry. Perform once daily until the patient is less pruritic or two to three times a week if steroids are used. Clients will commonly need to make adjustments to the fitted clothes and socks for desired effects. Socks should extend to above the second joint to avoid slippage. Human studies have repeatedly demonstrated the efficacy of wet wrapping with topical corticosteroids, resulting in an improved quality of life and avoiding systemic therapy. It is prudent to warn caretakers of this laborious and time-consuming task, but relief is almost immediate and can avoid systemic therapy.112,114,115 The authors often reach for this therapy for patients who have had adverse effects to systemic CAD medication.
Nutrition
The most important essential fatty acids (EFAs) in cutaneous homeostasis in dogs are linoleic acid (18:2N-6) and alpha-linoleic acid (18:3N-3). The proposed mechanism of how EFAs function in controlling pruritus includes inhibition of arachidonic acid metabolism in favor of dihomo-gamma-linolenic acid (DGLA). The final product of DGLA metabolism is prostaglandin E1, which is thought to have anti-inflammatory effects.69 There are unsatisfactory evidence-based studies supporting the use of EFAs; ultimately, results depend on the source, amount, and duration of supplementation.116,117 It has been proposed that an adequate therapeutic trial might necessitate 3–4 months of use69 and that EFAs may have a steroid sparing effect.117 Side effects are rarely reported but can consist of vomiting, diarrhea, weight gain, unpleasant odor, or “fish breath”.
Probiotics have recently gained attention. Many studies have shown a significant and synergistic connection between the gastrointestinal and dermatological systems, and scientific evidence link gastrointestinal homeostasis to cutaneous manifestations.118–120 Knowledge as to the exact mechanisms of the link are still being investigated, but it is thought that interactions between the microbiome and the intestinal immune system results in modulation of immunity with effects on many other systems beyond the gut. Many skin diseases, including atopic dermatitis, seem to be related to gut dysbiosis. Modulation of canine intestinal microbiota is getting more attention as a taking part of multimodal CAD management. The exact mechanism of action of probiotics still needs further investigation, but like immunotherapy, it appears that the immune response might shift towards a Th1 mediated response, instead of Th2, which is detrimental in AD.121 In a recent study, probiotics played a role in CAD management as a complimentary or steroid sparing therapy.122
Zinc plays a role in skin health and proper immune function and in the management of some skin diseases.123 In a 24-week, randomized, double-blinded controlled crossover study with 27 dogs, atopic dogs receiving glucocorticoids and zinc methionine supplementation may benefit in both clinical response and pruritus levels.124
Vitamin D has been a hot topic as part of multimodal therapy for many diseases including atopic dermatitis. Vitamin D is involved with skin barrier function and immune response, including the production of antimicrobial peptides. It has been associated with severe allergic diseases in humans, but the role of vitamin D in CAD is still in development. It has been shown that CAD has been associated with low vitamin D and that supplementation CADESI and pruritus has been associated with increased serum levels.125 There is also evidence that canine mast cell tumor growth is inhibited by vitamin D because in one study, Labrador Retriever dogs with low vitamin D levels had an increased chance of developing mast- cell tumors.126 Overall, at this time, there is insufficient evidence to determine vitamin D’s role in the development or pathogenesis of CAD.
Palmitoylethanolamide PEA, a type of cannabinoid receptor agonist, has been used in CAD management as part of multimodal therapy. PEA-Cannabinoid receptors (CB1, CB2) are expressed on endothelial cells, mast cells, and canine keratinocytes. Immunohistochemical staining indicated increased cannabinoid receptors in the dermis of atopic dogs, compared to their healthy counterparts. Treatment with cannabinoid receptor agonists, such as Palmitoylethanolamide or PEA, has been shown to decrease mast cell degranulation, histamine-related pruritus, and vasodilation. It has also been demonstrated that skin levels of endogenous PEA are higher in atopic dogs.127
In recent years, commercial diets aimed specifically for canine atopic dermatitis are available. Examples include Hill’s Derm Complete® and Royal Canin’s Skintopic®. Hill’s Derm Complete is an egg-based diet that has the HistaGuard Complex, which contains bioactives and phytonutrients that help reduce allergic response. Phytonutrients help decrease inflammatory cytokines, mast cell degranulation, and interfere with dendritic function and maturation. Royal Canin’s Skintopic features a patented Dermauxillium Complex, a unique blend of nutrients and antioxidants to aid in supporting skin and coat health. In a double-blinded placebo-controlled study, pVAS and CADESI-04 scores were improved by at least 30–50%, and drug-sparing effect could be observed within 3–6 months after starting the new diet.128
Environmental Control
Allergen avoidance should be considered whenever possible, although this is frequently difficult to accomplish. Bathing can help minimize cutaneous exposure as well as wiping the patient after outside exposure to minimize allergen exposure contact. Patients with pollen and plant allergies should not be outside when grass is mowed or on windy days. Ideally, inciting plants should be removed from the patient’s environment whenever possible. For house dust and molds, high efficiency particulate air (HEPA) filters air purifiers and frequent housekeeping should be implemented, such as increased frequency of vacuuming and changing bedding. Dogs should be removed from the property during these activities to limit exposure.
Conclusion
Considering the complexity of CAD pathophysiology, treatment needs to be on an individual basis, taking into consideration all factors, such as genetics, nutrition, and environment. However, further research is needed, as no treatment is 100% successful, and in some cases even with a combination of treatments, relief is not achieved. None of the current drugs are curative and will require lifelong use in some cases, putting a financial burden on patients or clients. There are many more therapeutics in the pipeline specifically in human medicine, which can translate to new therapies for dogs, albeit at a high cost, as many newer treatments are biologicals. Finding therapies that can successfully address the root cause and disrupt the cycle of insult and inflammation will require additional research and probably a wider approach looking at cells and pathways previously not investigated. With increased advances in technology, further elucidating the complex interactions of cells in the skin, this goal is achievable.
Acknowledgments
The authors would like to thank the library staff at Western University of Health Sciences, specifically Kelly Hines for help with Endnote.
Disclosure
Dr Charli Dong reports occasional contracted speaker engagements for Zoetis and Hill’s. The authors report no other conflicts of interest in this work.
References
1. Bizikova P, Pucheu‐Haston CM, Eisenschenk MN, Marsella R, Nuttall T, Santoro D. Role of genetics and the environment in the pathogenesis of canine atopic dermatitis. Vet Dermatol. 2015;26(2):95–e26. doi:10.1111/vde.12198
2. Marsella R. Advances in our understanding of canine atopic dermatitis. Vet Dermatol. 2021;32(6):547–e151. doi:10.1111/vde.12965
3. Outerbridge CA, Jordan TJ. Current knowledge on canine atopic dermatitis: pathogenesis and treatment. Adv Small Anim. 2021;2:101–115. doi:10.1016/j.yasa.2021.07.004
4. Grattan CE. Urticaria, Angioedema, and Atropy. In: Reedy LM, Miller WH, Willemse T, editors. Allergic Skin Diseases of Dogs and Cats.
5. Hillier A, Griffin CE. The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Vet Immunol Immunopathol. 2001;81(3–4):147–151. doi:10.1016/S0165-2427(01)00296-3
6. Lund EM, Armstrong PJ, Kirk CA, Kolar LM, Klausnor J. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc. 1999;214:1336–1341. doi:10.2460/javma.1999.214.09.1336
7. Scott DW, Paradis M. A survey of canine and feline skin disorders seen in a university practice: small animal clinic, University of Montreal, Saint-hyacinthe, Quebec (1987–1988). Can Vet J. 1990;31(12):830.
8. Favrot C, Steffan J, Seewald W, Picco F. A prospective study on the clinical features of chronic canine atopic dermatitis and its diagnosis. Vet Dermatol. 2010;21(1):23–31. doi:10.1111/j.1365-3164.2009.00758.x
9. Tarpataki N, Pápa K, Reiczigel J, Vajdovich P, Vörös K. Prevalence and features of canine atopic dermatitis in Hungary. Acta Vet Hung. 2006;54(3):353–366. doi:10.1556/avet.54.2006.3.6
10. Meury S, Molitor V, Doherr M, et al. Role of the environment in the development of canine atopic dermatitis in Labrador and golden retrievers. Vet Dermatol. 2011;22(4):327–334. doi:10.1111/j.1365-3164.2010.00950.x
11. Harvey ND, Shaw SC, Craigon PJ, Blott SC, England GC. Environmental risk factors for canine atopic dermatitis: a retrospective large‐scale study in Labrador and golden retrievers. Vet Dermatol. 2019;30(5):396–e119. doi:10.1111/vde.12782
12. Wilhem S, Kovalik M, Favrot C. Breed‐associated phenotypes in canine atopic dermatitis. Vet Dermatol. 2011;22(2):143–149. doi:10.1111/j.1365-3164.2010.00925.x
13. Couceiro GA, Ribeiro SMM, Monteiro MM, Meneses AMC, Sousa SKS, Coutinho LN. Prevalence of canine atopic dermatitis at the veterinary hospital of the “universidade federal rural da Amazônia” in Belém/Pará, Brazil. Pesquisa Veterinária Brasileira. 2021;41. doi:10.1590/1678-5150-pvb-6778
14. Mazrier H, Vogelnest LJ, Thomson PC, Taylor RM, Williamson P. Canine atopic dermatitis: breed risk in Australia and evidence for a susceptible clade. Vet Dermatol. 2016;27(3):167–e42. doi:10.1111/vde.12317
15. Nødtvedt A, Bergvall K, Sallander M, Egenvall A, Emanuelson U, Hedhammar Å. A case–control study of risk factors for canine atopic dermatitis among boxer, bull terrier and West Highland white terrier dogs in Sweden. Vet Dermatol. 2007;18(5):309–315. doi:10.1111/j.1365-3164.2007.00617.x
16. Noli C. Assessing quality of life for pets with dermatologic disease and their owners. Vet Clin North Am Small Anim Pract. 2019;49(1):83–93. doi:10.1016/j.cvsm.2018.08.008
17. Linek M, Favrot C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Vet Dermatol. 2010;21(5):456–462. doi:10.1111/j.1365-3164.2010.00899.x
18. McAuliffe LR, Koch CS, Serpell J, Campbell KL. Associations between atopic dermatitis and anxiety, aggression, and fear-based behaviors in dogs. J Am Anim Hosp Assoc. 2022;58(4):161–167. doi:10.5326/jaaha-ms-7210
19. Pegram C, Gray C, Packer RMA, et al. Proportion and risk factors for death by euthanasia in dogs in the UK. Sci Rep. 2021;11(1):9145. doi:10.1038/s41598-021-88342-0
20. Facheris P, Jeffery J, Del Duca E, Guttman-Yassky E. The translational revolution in atopic dermatitis: the paradigm shift from pathogenesis to treatment. Cell Mol Immunol. 2023;20(5):448–474. doi:10.1038/s41423-023-00992-4
21. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–246. doi:10.1111/j.1600-065X.2011.01027.x
22. He H, Suryawanshi H, Morozov P, et al. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol. 2020;145(6):1615–1628. doi:10.1016/j.jaci.2020.01.042
23. Hightower K, Marsella R, Flynn-Lurie A. Effects of age and allergen exposure on transepidermal water loss in a house dust mite-sensitized beagle model of atopic dermatitis. Vet Dermatol. 2010;21(1):88–95. doi:10.1111/j.1365-3164.2009.00839.x
24. Kim JE, Kim HS. Microbiome of the skin and gut in atopic dermatitis (AD): understanding the pathophysiology and finding novel management strategies. J Clin Med. 2019;8(4):444. doi:10.3390/jcm8040444
25. Irvine AD, McLean WI, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–1327. doi:10.1056/NEJMra1011040
26. Paternoster L, Standl M, Chen C-M, et al. Meta-analysis of genome-wide association studies identifies three new risk loci for atopic dermatitis. Nature Genet. 2012;44(2):187–192. doi:10.1038/ng.1017
27. Tengvall K, Sundström E, Wang C, et al. Bayesian model and selection signature analyses reveal risk factors for canine atopic dermatitis. Commun Biol. 2022;5(1):1348. doi:10.1038/s42003-022-04279-8
28. Shaw SC, Wood JL, Freeman J, Littlewood JD, Hannant D. Estimation of heritability of atopic dermatitis in labrador and golden retrievers. Am J Vet Res. 2004;65(7):1014–1020. doi:10.2460/ajvr.2004.65.1014
29. Vilson Å, Bonnett B, Hansson‐Hamlin H, Hedhammar Å. Disease patterns in 32,486 insured German shepherd dogs in Sweden: 1995–2006. Vet Rec. 2013;173(5):. doi:10.1136/vr.101577
30. Combarros D, Cadiergues MC, Simon M. Update on canine filaggrin: a review. Vet Q. 2020;40(1):162–168. doi:10.1080/01652176.2020.1758357
31. Bibel DJ, Greenberg JH, Cook JL. Staphylococcus aureus and the microbial ecology of atopic dermatitis. Can J Microbiol. 1977;23(8):1062–1068. doi:10.1139/m77-159
32. Akiyama H, Ueda M, Toi Y, Kanzaki H, Tada J, Arata J. Comparison of the severity of atopic dermatitis lesions and the density of Staphylococcus aureus on the lesions after antistaphylococcal treatment. J Infect Chemother. 1996;2(2):70–74. doi:10.1007/BF02350843
33. Fazakerley J, Nuttall T, Sales D, et al. Staphylococcal colonization of mucosal and lesional skin sites in atopic and healthy dogs. Vet Dermatol. 2009;20(3):179–184. doi:10.1111/j.1365-3164.2009.00745.x
34. Bradley CW, Morris DO, Rankin SC, et al. Longitudinal evaluation of the skin microbiome and association with microenvironment and treatment in canine atopic dermatitis. J Invest Dermatol. 2016;136(6):1182–1190. doi:10.1016/j.jid.2016.01.023
35. Findley K, Oh J, Yang J, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498(7454):367–370. doi:10.1038/nature12171
36. Bajwa J. Canine Malassezia dermatitis. Can Vet J. 2017;58(10):1119.
37. Frazier W, Bhardwaj N. Atopic dermatitis: diagnosis and treatment. Am Fam Physician. 2020;101(10):590–598.
38. Chermprapai S, Ederveen THA, Broere F, et al. The bacterial and fungal microbiome of the skin of healthy dogs and dogs with atopic dermatitis and the impact of topical antimicrobial therapy, an exploratory study. Vet Microbiol. 2019;229:90–99. doi:10.1016/j.vetmic.2018.12.022
39. Leung DYM. Can antibiotics be harmful in atopic dermatitis? Br J Dermatol. 2018;179(4):807–808. doi:10.1111/bjd.17023
40. Jaworek AK, Szafraniec K, Zuber Z, Wojas-Pelc A, Jaworek J. Interleukin 25, thymic stromal lymphopoietin and house dust mites in pathogenesis of atopic dermatitis. J Physiol Pharmacol. 2020;71(2). doi:10.26402/jpp.2020.2.14
41. Liew FY, Girard J-P, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16(11):676–689. doi:10.1038/nri.2016.95
42. Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–2950. doi:10.1084/jem.20130351
43. Mastraftsi S, Vrioni G, Bakakis M, et al. Atopic dermatitis: striving for reliable biomarkers. J Clin Med. 2022;11(16):4639. doi:10.3390/jcm11164639
44. Weidinger P, Foyer G, Kock S, Gnauert J, Kumme R. Procedure for torque calibration under constant rotation investigated on a nacelle test bench. VDE. 2018;2018:1–4.
45. Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33–independent innate lymphoid cell responses to promote skin inflammation. Sci Trans Med. 2013;5(170):170ra16–170ra16. doi:10.1126/scitranslmed.3005374
46. Früh SP, Saikia M, Eule J, et al. Elevated circulating Th2 but not group 2 innate lymphoid cell responses characterize canine atopic dermatitis. Vet Immunol Immunopathol. 2020;221:110015. doi:10.1016/j.vetimm.2020.110015
47. Soumelis V, Liu Y-J. Human thymic stromal lymphopoietin: a novel epithelial cell-derived cytokine and a potential key player in the induction of allergic inflammation. Springer. 2004;2004:325–333.
48. Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell–mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680. doi:10.1038/ni805
49. Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell–mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5(4):426–434. doi:10.1038/ni1048
50. Klukowska‐Rötzler J, Chervet L, Müller EJ, Roosje P, Marti E, Janda J. Expression of thymic stromal lymphopoietin in canine atopic dermatitis. Vet Dermatol. 2013;24(1):54–e14. doi:10.1111/j.1365-3164.2012.01096.x
51. Dubin C, Del Duca E, Guttman-Yassky E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. 2021;17(8):835–852. doi:10.1080/1744666x.2021.1940962
52. Tominaga M, Takamori K. Peripheral itch sensitization in atopic dermatitis. Allergol Int. 2022;71(3):265–277. doi:10.1016/j.alit.2022.04.003
53. Olivry T, Mayhew D, Paps JS, et al. Early activation of Th2/Th22 inflammatory and pruritogenic pathways in acute canine atopic dermatitis skin lesions. J Invest Dermatol. 2016;136(10):1961–1969. doi:10.1016/j.jid.2016.05.117
54. Marsella R, Olivry T. The ACVD task force on canine atopic dermatitis (VII): mediators of cutaneous inflammation. Vet Immunol Immunopathol. 2001;81(3–4):205–213. doi:10.1016/S0165-2427(01)00300-2
55. Bao L, Shi VY, Chan LS. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: implication for atopic dermatitis. Mol Immunol. 2012;50(1–2):91–97. doi:10.1016/j.molimm.2011.12.008
56. Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–1354. doi:10.1016/j.jaci.2012.07.012
57. Spidale NA, Malhotra N, Frascoli M, et al. Neonatal-derived IL-17 producing dermal γδ T cells are required to prevent spontaneous atopic dermatitis. Elife. 2020;9:e51188. doi:10.7554/eLife.51188
58. Pucheu‐Haston CM, Bizikova P, Marsella R, Santoro D, Nuttall T, Eisenschenk MN. Lymphocytes, cytokines, chemokines and the T‐helper 1–T‐helper 2 balance in canine atopic dermatitis. Vet Dermatol. 2015;26(2):124–e32. doi:10.1111/vde.12205
59. Nomura T, Honda T, Kabashima K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int Immunol. 2018;30(9):419–428. doi:10.1093/intimm/dxy015
60. Tokura Y, Hayano S. Subtypes of atopic dermatitis: from phenotype to endotype. Allergol Int. 2022;71(1):14–24. doi:10.1016/j.alit.2021.07.003
61. Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol. 2019;122(5):449–455. doi:10.1016/j.anai.2018.11.015
62. Ko KI, Merlet JJ, DerGarabedian BP, et al. NF-κB perturbation reveals unique immunomodulatory functions in Prx1+ fibroblasts that promote development of atopic dermatitis. Sci Trans Med. 2022;14(630):eabj0324. doi:10.1126/scitranslmed.abj0324
63. Sparling BA, Moss N, Kaur G, Clark D, Hawkins RD, Drechsler Y. Unique Cell Subpopulations and Disease Progression Markers in Canines with Atopic Dermatitis. J Immunol. 2022;209(7):1379–1388. doi:10.4049/jimmunol.2200304
64. DeBoer DJ. The future of immunotherapy for canine atopic dermatitis: a review. Adv Vet Dermatol. 2017;8:26–31.
65. Gutermuth J, Grosber M, Pfaar O, Bergmann KC, Ring J. 111 years of allergen-immunotherapy: a long and successful history of the only available disease-modifier in allergic diseases. Allergol Select. 2022;6:248. doi:10.5414/ALX02330E
66. Ring J, Gutermuth J. 100 years of hyposensitization: history of allergen-specific immunotherapy (ASIT). Allergy. 2011;66(6):713–724. doi:10.1111/j.1398-9995.2010.02541.x
67. Wittich FW. Spontaneous allergy (atopy) in the lower animal: seasonal hay fever (fall type) in a dog. J Allergy. 1941;12(3):247–251. doi:10.1016/S0021-8707(41)80008-2
68. Olivry T, DeBoer DJ, Favrot C, et al. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet Res. 2015;11(1):1–15. doi:10.1186/s12917-015-0514-6
69. Miller WH, Griffin CE, Campbell KL, Muller GH, Scott DW. Muller and Kirk’s Small Animal Dermatology.
70. Fennis EE, van Damme CM, Schlotter YM, et al. Efficacy of subcutaneous allergen immunotherapy in atopic dogs: a retrospective study of 664 cases. Vet Dermatol. 2022;33(4):321–e75. doi:10.1111/vde.13075
71. Ramió‐Lluch L, Brazís P, Ferrer L, Puigdemont A. Allergen‐specific immunotherapy in dogs with atopic dermatitis: is owner compliance the main success‐limiting factor? Vet Rec. 2020;187(12):. doi:10.1136/vr.106024
72. Mueller RS, Zablotski Y, Baumann K, et al. A randomised, double‐blinded comparison between subcutaneous rush and intralymphatic allergen immunotherapy induction in atopic dogs. Vet Dermatol. 2023;34(2):91–98. doi:10.1111/vde.13138
73. Elenburg S, Blaiss MS. Current status of sublingual immunotherapy in the United States. World Allergy Organ J. 2014;7(1):1–7. doi:10.1186/1939-4551-7-24
74. DeBoer DJ, Verbrugge M, Morris M. Clinical and immunological responses of dust mite sensitive, atopic dogs to treatment with sublingual immunotherapy (SLIT). Vet Dermatol. 2016;27(2):82–e24. doi:10.1111/vde.12284
75. Fischer NM, Rostaher A, Favrot C. A comparative study of subcutaneous, intralymphatic and sublingual immunotherapy for the long‐term control of dogs with nonseasonal atopic dermatitis. Vet Dermatol. 2020;31(5):365–e96. doi:10.1111/vde.12860
76. Fischer N, Rostaher A, Favrot C. Intralymphatic immunotherapy: an effective and safe alternative route for canine atopic dermatitis. Schweizer Archiv fur Tierheilkunde. 2016;158(9):646–652. doi:10.17236/sat00085
77. Dell DL, Griffin CE, Thompson LA, Griffies JD. Owner assessment of therapeutic interventions for canine atopic dermatitis: a long‐term retrospective analysis. Vet Dermatol. 2012;23(3):228–e47. doi:10.1111/j.1365-3164.2012.01054.x
78. Bäumer W, Stahl J, Sander K, et al. Lack of preventing effect of systemically and topically administered histamine H1 or H4 receptor antagonists in a dog model of acute atopic dermatitis. Exp Dermatol. 2011;20(7):577–581. doi:10.1111/j.1600-0625.2011.01268.x
79. Hsiao Y-H, Chen C, Willemse T. Effects of cetirizine in dogs with chronic atopic dermatitis: a randomized, double blind, placebo-controlled trial. J Vet Sci. 2016;17(4):549–554. doi:10.4142/jvs.2016.17.4.549
80. DeBoer D, Griffin C. The ACVD task force on canine atopic dermatitis (XXI): antihistamine pharmacotherapy. Vet Immunol Immunopathol. 2001;81(3–4):323–329. doi:10.1016/S0165-2427(01)00306-3
81. Tani E, Shiosaka S, Sato M, Ishikawa T, Tohyama M. Histamine acts directly on calcitonin gene-related peptide- and substance P-containing trigeminal ganglion neurons as assessed by calcium influx and immunocytochemistry. Neurosci Lett. 1990;115(2–3):171–176. doi:10.1016/0304-3940(90)90450-n
82. Muether PS, Gwaltney JM Jr. Variant effect of first-and second-generation antihistamines as clues to their mechanism of action on the sneeze reflex in the common cold. Clin Infect Dis. 2001;33(9):1483–1488. doi:10.1086/322518
83. Eichenseer M, Johansen C, Mueller R. Efficacy of dimetinden and hydroxyzine/chlorpheniramine in atopic dogs: a randomised, controlled, double-blinded trial. Vet Rec. 2013;173(17):. doi:10.1136/vr.101907
84. Philpot EE. Safety of Second Generation Antihistamines. OceanSide Publications; 2000:15.
85. Archer T, Boothe D, Langston V, Fellman C, Lunsford K, Mackin A. Oral cyclosporine treatment in dogs: a review of the literature. J Veterinary Internal Med. 2014;28(1):1–20. doi:10.1111/jvim.12265
86. Guaguère E, Steffan J, Olivry T. Cyclosporin A: a new drug in the field of canine dermatology. Vet Dermatol. 2004;15(2):61–74. doi:10.1111/j.1365-3164.2004.00376.x
87. Thelen A, Mueller R, Linek M, Peters S, Stechmann K, Steffan J. Influence of food intake on the clinical response to cyclosporin A in canine atopic dermatitis. Vet Rec. 2006;159(25):854.
88. Denti D, Caldin M, Ventura L, De Lucia M. Prolonged twice‐daily administration of oclacitinib for the control of canine atopic dermatitis: a retrospective study of 53 client‐owned atopic dogs. Vet Dermatol. 2022;33(2):149–e42. doi:10.1111/vde.13053
89. Zoetis Inc. Apoquel. Package insert. Kalamazoo, MI: Zoetis Inc; 2020.
90. Gadeyne C, Little P, King VL, Edwards N, Davis K, Stegemann MR. Efficacy of oclacitinib (Apoquel®) compared with prednisolone for the control of pruritus and clinical signs associated with allergic dermatitis in client‐owned dogs in Australia. Vet Dermatol. 2014;25(6):512–e86. doi:10.1111/vde.12166
91. Panteri A, Strehlau G, Helbig R, Prost C, Doucette K. Repeated oral dose tolerance in dogs treated concomitantly with ciclosporin and oclacitinib for three weeks. Vet Dermatol. 2016;27(1):22–e7. doi:10.1111/vde.12278
92. Little PR, King VL, Davis KR, Cosgrove SB, Stegemann MR. A blinded, randomized clinical trial comparing the efficacy and safety of oclacitinib and ciclosporin for the control of atopic dermatitis in client‐owned dogs. Vet Dermatol. 2015;26(1):23–e8. doi:10.1111/vde.12186
93. Fleck T, Norris L, King V, Lesman S, Gonzales AJ. Speed of onset of a new chewable formulation of oclacitinib maleate (Apoquel®) in a canine model of IL‐31‐induced pruritus. J Vet Pharmacol Ther. 2022;45(4):380–384. doi:10.1111/jvp.13065
94. Michels GM, Ramsey DS, Walsh KF, et al. A blinded, randomized, placebo‐controlled, dose determination trial of lokivetmab (ZTS‐00103289), a caninized, anti‐canine IL‐31 monoclonal antibody in client owned dogs with atopic dermatitis. Vet Dermatol. 2016;27(6):478–e129. doi:10.1111/vde.12376
95. Zoetis Inc. Cytopoint. Package insert. Kalamazoo, MI: Zoetis Inc; 2016.
96. Moyaert H, Van Brussel L, Borowski S, et al. A blinded, randomized clinical trial evaluating the efficacy and safety of lokivetmab compared to ciclosporin in client-owned dogs with atopic dermatitis. Vet Dermatol. 2017;28(6):593–e145. doi:10.1111/vde.12478
97. Souza CP, Rosychuk RA, Contreras ET, Schissler JR, Simpson AC. A retrospective analysis of the use of lokivetmab in the management of allergic pruritus in a referral population of 135 dogs in the western USA. Vet Dermatol. 2018;29(6):489–e164. doi:10.1111/vde.12682
98. Fleck TJ, Norris LR, Mahabir S, et al. Onset and duration of action of lokivetmab in a canine model of IL‐31 induced pruritus. Vet Dermatol. 2021;32(6):681–e182. doi:10.1111/vde.12943
99. Marsella R, Ahrens K, Wilkes R, Trujillo A, Dorr M. Comparison of various treatment options for canine atopic dermatitis: a blinded, randomized, controlled study in a colony of research atopic beagle dogs. Vet Dermatol. 2020;31(4):284–e69. doi:10.1111/vde.12849
100. Singh SK, Dimri U, Saxena SK, Jadhav RK. Therapeutic management of canine atopic dermatitis by combination of pentoxifylline and PUFAs. J Vet Pharmacol Ther. 2010;33(5):495–498. doi:10.1111/j.1365-2885.2009.01146.x
101. Xanthine Derivatives. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
102. Sauvé F. Use of topical glucocorticoids in veterinary dermatology. Can Vet J. 2019;60(7):785.
103. Helfer EL, Rose LI. Corticosteroids and adrenal suppression. Characterising and avoiding the problem. Drugs. 1989;38(5):838–845. doi:10.2165/00003495-198938050-00008
104. Santoro D. Therapies in canine atopic dermatitis: an update. Vet Clin. 2019;49(1):9–26.
105. Scott D, Rothstein E, Miller W Jr. A clinical study on the efficacy of two commercial veterinary pramoxine cream rinses in the management of pruritus in atopic dogs. Canine Practice. 2000;25(2):15–17.
106. Agarwal A, Das A, Hassanandani T, Podder I, Panda M. Topical pramoxine in chronic pruritus: where do we stand? Indian J Dermatol. 2021;66(5):576. doi:10.4103/ijd.ijd_1_21
107. Bensignor E, Olivry T. Treatment of localized lesions of canine atopic dermatitis with tacrolimus ointment: a blinded randomized controlled trial. Vet Dermatol. 2005;16(1):52–60. doi:10.1111/j.1365-3164.2005.00419.x
108. Marsella R, Nicklin C, Saglio S, Lopez J. Investigation on the clinical efficacy and safety of 0.1% tacrolimus ointment (Protopic®) in canine atopic dermatitis: a randomized, double‐blinded, placebo‐controlled, cross‐over study. Vet Dermatol. 2004;15(5):294–303. doi:10.1111/j.1365-3164.2004.00397.x
109. Tretter S, Mueller RS. The influence of topical unsaturated fatty acids and essential oils on normal and atopic dogs. J Am Anim Hosp Assoc. 2011;47(4):236–240. doi:10.5326/JAAHA-MS-5607
110. Blaskovic M, Rosenkrantz W, Neuber A, Sauter-Louis C, Mueller R. The effect of a spot-on formulation containing polyunsaturated fatty acids and essential oils on dogs with atopic dermatitis. Vet J. 2014;199(1):39–43. doi:10.1016/j.tvjl.2013.10.024
111. Verallo-Rowell VM, Dillague KM, Syah-Tjundawan BS. Novel antibacterial and emollient effects of coconut and virgin olive oils in adult atopic dermatitis. DERM. 2008;19(6):308–315.
112. Andersen RM, Thyssen JP, Maibach HI. The role of wet wrap therapy in skin disorders–a literature review. Acta Derm vener. 2015;95(8):933–939. doi:10.2340/00015555-2134
113. Devillers A, Oranje A. Efficacy and safety of ‘wet‐wrap’dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154(4):579–585. doi:10.1111/j.1365-2133.2006.07157.x
114. Janmohamed SR, Oranje AP, Devillers AC, et al. The proactive wet-wrap method with diluted corticosteroids versus emollients in children with atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2014;70(6):1076–1082. doi:10.1016/j.jaad.2014.01.898
115. Nicol NH, Boguniewicz M. Wet wrap therapy in moderate to severe atopic dermatitis. Immunol Allergy Clin. 2017;37(1):123–139.
116. Bensignor E, Morgan DM, Nuttall T. Efficacy of an essential fatty acid‐enriched diet in managing canine atopic dermatitis: a randomized, single‐blinded, cross‐over study. Vet Dermatol. 2008;19(3):156–162. doi:10.1111/j.1365-3164.2008.00670.x
117. Saevik B, Thoresen S, Taugbøl O. Fatty acid composition of serum lipids in atopic and healthy dogs. Res Vet Sci. 2002;73(2):153–158. doi:10.1016/S0034-5288(02)00043-7
118. Sinha S, Lin G, Ferenczi K. The skin microbiome and the gut-skin axis. Clin Dermatol. 2021;39(5):829–839. doi:10.1016/j.clindermatol.2021.08.021
119. Lee SY, Lee E, Park YM, Hong SJ. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10(4):354–362. doi:10.4168/aair.2018.10.4.354
120. Lunjani N, Hlela C, O’Mahony L. Microbiome and skin biology. Curr Opin Allergy Clin Immunol. 2019;19(4):328–333. doi:10.1097/aci.0000000000000542
121. Marchegiani A, Fruganti A, Spaterna A, et al. Impact of nutritional supplementation on canine dermatological disorders. Vet Sci. 2020;7(2):38. doi:10.3390/vetsci7020038
122. Ohshima‐Terada Y, Higuchi Y, Kumagai T, Hagihara A, Nagata M. Complementary effect of oral administration of L actobacillus paracasei K 71 on canine atopic dermatitis. Vet Dermatol. 2015;26(5):350–e75. doi:10.1111/vde.12224
123. Schwartz JR, Marsh RG, Draelos ZD. Zinc and skin health: overview of physiology and pharmacology. Dermatol Surg. 2005;31(7 Pt 2):837–47; discussion 847. doi:10.1111/j.1524-4725.2005.31729
124. McFadden RA, Heinrich NA, Haarstad AC, Tomlinson DJ. A double‐blinded, randomized, controlled, crossover evaluation of a zinc methionine supplement as an adjunctive treatment for canine atopic dermatitis. Vet Dermatol. 2017;28(6):569–e138. doi:10.1111/vde.12466
125. Klinger CJ, Hobi S, Johansen C, Koch HJ, Weber K, Mueller RS. Vitamin D shows in vivo efficacy in a placebo‐controlled, double‐blinded, randomised clinical trial on canine atopic dermatitis. Vet Rec. 2018;182(14):. doi:10.1136/vr.104492
126. Pucheu‐Haston CM, Santoro D, Bizikova P, Eisenschenk MN, Marsella R, Nuttall T. Innate immunity, lipid metabolism and nutrition in canine atopic dermatitis. Vet Dermatol. 2015;26(2):104–e28. doi:10.1111/vde.12199
127. Campora L, Miragliotta V, Ricci E, et al. Cannabinoid receptor type 1 and 2 expression in the skin of healthy dogs and dogs with atopic dermatitis. Am J Vet Res. 2012;73(7):988–995. doi:10.2460/ajvr.73.7.988
128. Watson A, Rostaher A, Fischer NM, Favrot C. A novel therapeutic diet can significantly reduce the medication score and pruritus of dogs with atopic dermatitis during a nine‐month controlled study. Vet Dermatol. 2022;33(1):55–e18. doi:10.1111/vde.13020
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.