Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Can Oral Zinc Supplementation Reduce Relapses in Childhood Steroid-Sensitive Nephrotic Syndrome? A Systematic Review
Authors Mbanefo NR, Uwaezuoke SN , Eneh CI , Odimegwu CL, Chikani UN, Muoneke UV , Nwolisa CE, Odo KE, Ogbuka FN, Akwue AT
Received 5 January 2023
Accepted for publication 31 March 2023
Published 20 April 2023 Volume 2023:16 Pages 143—153
DOI https://doi.org/10.2147/IJNRD.S403699
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pravin Singhal
Ngozi R Mbanefo,1 Samuel N Uwaezuoke,1 Chizoma I Eneh,2 Chioma L Odimegwu,1 Ugo N Chikani,1 Uzoamaka V Muoneke,1 Charles E Nwolisa,3 Kenneth E Odo,1 Francis N Ogbuka,2 Anthony T Akwue4
1Department of Pediatrics, the University of Nigeria Teaching Hospital Ituku-Ozalla Enugu, Enugu, Nigeria; 2Department of Pediatrics, Enugu State University Teaching Hospital Enugu, Enugu, Nigeria; 3Department of Pediatrics, Federal Medical Centre, Owerri, Imo State, Nigeria; 4Emergency Department, ASEER Field Hospital, Al Rabwah, Kingdom of Saudi Arabia
Correspondence: Samuel N Uwaezuoke, Tel +2348033248108, Email [email protected]
Introduction: Frequent relapses and steroid dependence are common treatment challenges of steroid-sensitive nephrotic syndrome (SSNS) in children. Acute respiratory infection (ARI) is the most frequently reported trigger of relapse. Given the role of zinc supplementation in preventing ARI, some studies show that this targeted intervention may reduce relapses in childhood SSNS.
Aim: This systematic review aimed to determine if oral zinc supplementation can significantly reduce relapses in this disease.
Methods: We searched the PubMed and Google Scholar electronic databases for interventional and observational analytical studies without limiting their year or language of publication. We selected studies with primary data that met our inclusion criteria, screened their titles and abstracts, and removed duplicates. We used a preconceived structured form to extract data items from selected studies and conducted a quality assessment of randomized controlled trials (RCTs) and non-randomized studies with the Cochrane collaboration tool and the Newcastle Ottawa Scale, respectively. We qualitatively synthesized the extracted data to validate the review’s objective.
Results: Eight full-text articles were selected, comprising four RCTs and four observational analytical studies. Two of the RCTs had a high risk of bias in three parameters of the Cochrane collaboration tool, while three non-randomized studies had low methodological quality. A total of 621 pediatric patients with SSNS were investigated in the eight studies: six participants dropped out in one study. Three RCTs indicate that zinc supplementation may lead to sustained remission or reduction in relapse rate. Similarly, three observational analytical studies suggest a significant relationship between reduced serum zinc levels and disease severity.
Conclusion: Despite the association of zinc deficiency with increased morbidity in SSNS and the reduction of relapse rates with zinc supplementation, there is no robust evidence to recommend its use as a therapeutic adjunct. We recommend more adequately-powered RCTs to strengthen the current evidence.
Keywords: acute respiratory infection, frequent relapses, nephrotic syndrome, childhood, zinc supplementation
Introduction
Nephrotic syndrome is children’s most frequent glomerular disease and usually presents as massive proteinuria, hypoalbuminemia, and generalized edema.1 Minimal change nephropathy (MCN) is the most common histopathologic subtype of childhood idiopathic nephrotic syndrome worldwide.2 Most cases of MCN are steroid-sensitive and assume the nomenclature of steroid-sensitive nephrotic syndrome (SSNS). Although SSNS has a good prognosis, frequent relapses, and steroid dependence are common treatment challenges.3–5 A recent paradigm has proffered therapeutic strategies to mitigate these challenges. For frequently relapsing nephrotic syndrome (FRNS), the latest guidelines from the International Pediatric Nephrology Association (IPNA) recommend the use of steroid-sparing agents such as calcineurin inhibitors (cyclosporine A, tacrolimus), cyclophosphamide, levamisole, and mycophenolate mofetil.6 The caveat for using these immuno-suppressive/immuno-modulating drugs is either non-response of infrequently relapsing nephrotic syndrome (IRNS) to conventional steroid therapy or complicated relapse. More importantly, nephrologists should individualize this treatment strategy by considering the risk-benefit profile of these drugs.6
Trigger factors of relapse in childhood SSNS are now well documented.7 These triggers include acute respiratory infection (ARI), urinary tract infection (UTI), diarrhea, peritonitis, and skin infection.8–12 Interestingly, ARI appears to be the most frequent trigger factor in most series.8–10 Two studies in West Africa reported uncomplicated malaria as a possible trigger,13,14 while other authors in Japan noted school events and imminent hospital visits as non-infectious triggers besides the established infectious triggers.15 Targeted interventions against these infectious triggers are possible strategies for reducing disease-associated morbidity and mortality.
Data have accumulated over the years on the association between serum zinc levels and the risk of relapse in SSNS. Zinc deficiency occurs in children with nephrotic syndrome during remission or relapse, irrespective of serum albumin levels and possibly because of urinary loss.16 One of the critical functions of zinc is its influence on the immune system. Zinc is required to develop functional immune cells (T-lymphocytes and phagocytes) in the innate and adaptive immune systems. Thus, given the role of zinc supplementation in preventing ARI,17 some studies showed that the intervention reduced relapses in children with SSNS and was more likely to result in sustained remission.18,19
Although a previous systematic review of randomized controlled trials (RCTs) reported zinc as a helpful adjunct in treating SSNS, the authors concluded that the available evidence had “very low quality.”20 Their assessment was predicated on software that utilized five parameters for rating the quality of evidence, namely limitations to the design of RCTs, inconsistency of results or unexplained heterogeneity, indirectness of evidence, imprecision of results, and publication bias; these parameters were rated as “no”, ‘serious’ and ‘very serious’.20 Specifically, the authors noted “very low quality” evidence for outcomes such as frequency of relapses in twelve months and sustained remission/no relapse.20
Given their inconclusive findings, a repeat systematic review was deemed necessary to validate the effectiveness of zinc supplementation in reducing relapse rates, with a focus on well-conducted studies. With the availability of additional data, we initiated the present systematic review to determine if oral zinc supplementation can significantly reduce relapses in childhood SSNS. We conducted and reported the review in conformity with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines.21
Methods
Literature Search Strategy
We searched the PubMed and Google Scholar electronic databases for interventional and observational analytical studies without limiting their year of publication (Date of the last search: 31st October 2022). Based on the title of the systematic review, we obtained the following search details in PubMed in multiple combinations (as MeSH terms or not) with Boolean operators (AND/OR): (“zinc” [MeSH Terms] OR “zinc” [All Fields]) AND supplementation [All Fields] AND reducing [All Fields] AND (“recurrence” [MeSH Terms] OR “recurrence” [All Fields] OR “relapses” [All Fields]) AND “childhood” [All Fields] AND (“nephrotic syndrome” [MeSH Terms] OR (“nephrotic” [All Fields] AND “syndrome” [All Fields]) OR “nephrotic syndrome” [All Fields]).
Eligibility and Exclusion Criteria
We set the following eligibility criteria for papers for review: full-text articles of RCTs and observational analytical studies (case-controlled, cohort, and cross-sectional studies) on human subjects published in or translated into English. We excluded abstracts, reviews (narrative and systematic reviews/meta-analyses), letters to the Editor, commentaries/editorials, conference proceedings, papers on RCTs conducted on animal models, and studies with secondary data.
Study Selection
We screened the titles and abstracts of the articles retrieved from the two electronic databases and independently assessed full-text articles for potential eligibility for inclusion. We resolved possible inter-rater disagreements on selected studies by consensus. We excluded duplicates and studies with primary data whose objectives were not consistent with the aim of the present systematic review before arriving at the final list of papers.
Quality Assessment
We evaluated the methodological quality of each study using the Newcastle-Ottawa Scale and the Cochrane collaboration tool for observational analytical studies and RCTs, respectively. The Newcastle Ottawa Scale comprises the following criteria for assessing case-control or cross-sectional studies: “selection” (maximum of 5 stars), “comparability” (maximum of 2 stars), and “exposure/outcome” (maximum of 3 stars).22 We rated the quality of each study “high” if the total score was ≥ 7 stars or “low” if the total score was ≤ 7 stars. The Cochrane collaboration’s tool assesses the risk of bias in RCTs based on seven parameters: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.23 For each parameter, we assessed a study as having a low risk of bias (+), high risk of bias (-), or unclear risk of bias (?).
Data Extraction and Data Items
We extracted specified data items from the selected papers using a preconceived structured form. These items were the authors’ names, year of publication, country of study, study setting, study design, study population, sample size, and participants’ demographics such as age and sex. Other extracted items were the interventions in the form of oral zinc supplementation and placebo, the estimation of serum zinc levels in SSNS patients, and the assessment of relapse risk or frequency of relapses. We also retrieved the risk of bias in each of the RCTs.
Data Synthesis
We qualitatively synthesized the extracted data to determine if there were differences in the frequency of relapses between the intervention (zinc supplementation) and the control (placebo) groups. We also assessed the relationship between participants’ serum zinc levels and the number of relapses (as a basis for justifying zinc supplementation in SSNS patients with zinc deficiency). The significant heterogeneity of the eight included studies precluded a meta-analysis (quantitative synthesis).
Results
Study Selection
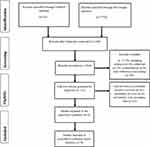
The search of the PubMed and Google Scholar databases yielded 82 and 7730 articles, respectively: giving a total of 7812 records. Removal of duplicates and unrelated articles produced 3592 records. Screening for their relevance to the present systematic review resulted in the further exclusion of other records (n=3540), including abstracts (n=8), editorials (n=4), commentaries (n=2) and conference proceedings (n=16), which scaled down the number of papers to 52. Following the assessment of the 52 full-text articles for eligibility, further exclusion of narrative reviews (n=31), systematic review/meta-analysis (n=3), and studies with secondary data (n=10) yielded eight papers. We finally selected eight full-text articles for the present systematic review (Figure 1).
Study Characteristics
As indicated in Table 1, the eight selected full-text articles consist of four RCTs,18,19,24,25 one case-control study,16 a prospective, non-comparative study,26 a cross-sectional study,27 and an observational study.28 The studies were conducted in Iraq16 and Iran26,27 and in Asian countries, namely Pakistan,18,25 Bangladesh,24 and India.19,28 The study settings were all hospital-based. The total number of participants in the eight studies was 627. However, six dropped out during the course of one study.18 Thus, 621 participants completed the eight studies. They presented variable age and sex distributions. For instance, different age ranges were reported: < 5–10 years,28 2–10 years,24 2–15 years,18 2–14 years,16 and 1–16 years.19 Other studies documented the participants’ mean ages as 6.38 ± 3.42 years,25 6.5 ± 3.7 years,26 and 6.2 ± 3.2 years.27 The majority of the studies reported male predominance18,24–28 while one study showed a female predominance.16
In Table 2, the quality assessment of four of the eight studies using the Newcastle-Ottawa Scale shows a star rating of ≥ 7 (high quality) for one study16 while the remaining studies are rated < 7 (low quality).26–28 The latter had no study comparators or controls. Thus, assessment parameters like selection and comparability of cases and controls did not apply to these studies: precluding star rating on items under these parameters. The study that was adjudged high-quality was case-control in design. As shown in Table 3, the methodological quality of the four RCTs reveals a low risk of bias for random sequence generation,18,19,24,25 allocation concealment,19,24 blinding of participants and personnel,18,19,24 blinding of outcome assessment24 and incomplete outcome data.19,24,25 On the other hand, a high risk of bias was noted for allocation concealment, blinding of participants and personnel, and blinding of outcome assessment in one study.25 Another study had a high risk of bias for incomplete outcome data; some participants (n=6) did not complete the trial, giving an attrition rate of 10%.18 Additionally, there was an unclear risk of bias for allocation concealment,18 blinding of outcome assessment,18,19 and selective reporting.18,19,24,25 Overall, none of the RCTs had a low risk of bias for the seven parameters, suggesting that the studies had either moderate methodological quality19,24 or low methodological quality.18,25
 |
Table 2 The Methodological Quality of the Observational Analytical Studies Using the Rating of the Newcastle-Ottawa Scale |
 |
Table 3 The Methodological Quality of the Randomized Controlled Trial (RCT) Using the Cochrane Collaboration’s Tool to Assess the Risk of Bias |
Study Findings
The major findings of the four RCTs are summarized in Table 4. The study by Hashmi et al evaluated and compared the effectiveness of zinc and vitamin B-complex supplementations in preventing the recurrence of SSNS in 192 study participants.25 The investigators administered oral zinc sulfate (10 mg/day) to 96 participants (the zinc group) for four months and oral vitamin B-complex to the remaining 96 participants (the vitamin B-complex group) for the same duration. They assessed disease remission and relapse in the two groups every 2–4 months. They observed a sustained remission rate of 49% in the zinc group compared to 22.9% in the B-complex group: a difference they reported as statistically significant (p< 0.001). In another study, Haque et al assessed the effect of zinc supplementation in 60 patients with idiopathic nephrotic syndrome and evaluated its association with the serum albumin level, relapse rate, and infection frequency.24 The patients were divided into two equal groups; 30 patients received oral elemental zinc at 2 mg/kg/day for fourteen days (the zinc group) while 30 patients received a placebo for the same duration (the placebo group). The study outcomes/endpoints were serum zinc levels during relapse and fourteen days after zinc/placebo supplementation. The authors found significantly lower mean serum zinc levels during relapse in both groups (0.54±0.18µg/dl in the zinc group vs 0.56± 0.22µg/dl in the placebo group, p=0.02). However, there was no significant difference between the numbers of patients that developed relapse in both groups (63.3% in the zinc group vs 50% in the placebo group). Additionally, there was a non-significant differential infection rate in both groups after zinc supplementation (73.3% in the intervention group vs 63.3% in the placebo group).
 |
Table 4 Significant Findings of the RCTs Reporting the Effect of Zinc Supplementation on Relapses in Steroid-Sensitive Nephrotic Syndrome (SSNS) |
In a related study by Sherali et al, the authors determined if zinc supplementation could reduce the relapse rate in 60 children with idiopathic nephrotic syndrome.18 Following the non-completion of the trial by six participants, 25 and 29 children were eventually evaluated in the zinc and placebo groups, respectively. The participants were administered oral zinc and a placebo for six months. Pre-study serum zinc levels and the number of infections and relapses in both groups were estimated. The study endpoint was chosen as the post-study relapse rate after one year. The major findings were lower post-study relapses in the zinc group compared to the placebo group (28% vs 34.5%); significantly lower post-study mean relapse rate in the zinc group of 1.14 ± 0.37 per patient per year compared to a pre-study mean relapse rate of 2.71 ± 1.11 per patient per year (p=0.005); and relapse rate reduction of 43% after zinc supplementation compared to 27% reduction after placebo. Finally, Arun et al determined the efficacy of zinc supplementation in reducing relapse rates in 81 patients with SSNS who were enrolled in the zinc group (n=40) and placebo group (n=41).19 The participants received either treatment with the recommended dietary allowance (RDA) of zinc (10 mg/day) or with placebo for twelve months. The authors used the relapse rate after the duration of zinc/placebo intervention as the study outcome. Patients with frequent relapses who received zinc supplementation showed a 28% reduction in relapse.
Table 5 summarizes the findings of the four observational analytical studies. Jaiswal et al estimated serum zinc levels during the initial episode/relapse and remission in 49 children with idiopathic nephrotic syndrome and correlated these variables with time to remission.28 The authors measured serum zinc levels at confirmation of the initial episode/relapse and repeated the estimation at remission: using serum zinc level at remission as the study endpoint. They found that serum zinc level was inversely correlated to the time of remission, although the relationship was not significant. In a prospective non-comparative study, Ghafoorimehr et al evaluated 43 patients with idiopathic nephrotic syndrome to determine if serum zinc levels were related to the number of relapses.26 The researchers estimated the patients’ baseline and remission serum zinc levels and used the number of relapses within one year as the study outcome. They observed a significant relationship between baseline and remission serum zinc levels (<78 µg/dl vs 78–110 µg/dl) and the number of relapses (35 vs 7, p=0.005). Another study by Taherahmadi et al investigated the serum zinc levels in 102 children with idiopathic nephrotic syndrome.27 The authors used serum zinc levels in different severities of the disease as the study outcome.
 |
Table 5 Significant Findings of the Observational Analytical Studies Reporting the Relationship Between Zinc Status/Serum Zinc Level and Steroid-Sensitive Nephrotic Syndrome (SSNS) |
They found significantly lower serum zinc levels in severe nephrotic syndrome (31.48 ± 6.8 µg/dl) than in moderate (59.12 ± 5.4 µg/dl) and mild forms (78.82 ± 4.1 µg/dl) of the disease (p<0.001). These findings suggest an inverse relationship between disease severity and serum zinc level. Finally, Ali et al evaluated changes in serum zinc levels in 40 children with idiopathic nephrotic syndrome in comparison with an equal number of healthy controls.16 The investigators estimated serum zinc levels in 24 patients with relapse (Group A patients) and 16 patients with remission (Group B patients) as well as 40 healthy controls. The study outcome was the serum zinc levels following steroid or non-steroid therapy. The major study findings included significantly lower mean serum zinc level in Group A patients than in controls (57.2 ± 15.286 µg/dl vs 96.2 ± 7.501 µg/dl) and an elevated mean serum zinc level in Group B patients, although the value was significantly lower than that of controls.
Discussion
Previous studies confirm that zinc deficiency frequently occurs in childhood nephrotic syndrome.29–33 Importantly, it is associated with worse outcomes in nephrotic syndrome because it increases relapse rates: an effect linked to an immunologic dysfunction that puts the child at risk of infectious triggers of relapse such as ARI.7 A previous systematic review on using zinc supplementation to reduce relapse rates had provided “very low quality” evidence.20 Thus, we conducted a repeat systematic review to re-assess pooled data from recently published studies.
In the present systematic review, three RCTs showed that zinc supplementation may be beneficial in nephrotic syndrome by leading to sustained remission,25 or reduction in relapse rate.18,19 The remaining RCT failed to demonstrate any significant difference in the number of patients that developed relapses in the zinc and placebo groups.24 However, the studies that reported a significant relapse rate reduction of 43%18 and significant sustained remission25 appear to have low methodological quality, with a high and unclear risk of bias for several parameters of the Cochrane collaboration tool. On the other hand, the RCTs that documented a 28% reduction in relapse rate19 and insignificant differential relapses between zinc and placebo supplements24 are adjudged to have better methodological quality, with a low risk of bias for several of these parameters. Our findings are in tandem with those of a previous systematic review of RCTs which showed that zinc supplementation reduced the frequency of relapses, resulted in sustained remission, and reduced the proportion of infection episodes associated with relapse compared to placebo.20 The authors reviewed four RCTs, two of which were co-incidentally included in our systematic review.18,19 Given that the methodological quality of these two studies is assessed as either low or moderate, it is not surprising that the authors of the previous systematic review considered the evidence from their review as having “very low quality”- based on their use of the GRADE Profiler software.34 Despite the weak evidence from this previous systematic review, the pathophysiologic basis for zinc supplementation as an adjunct therapy in nephrotic syndrome has been well established. It is believed that zinc deficiency is associated with T-helper 1 (Th1)-T-helper 2 (Th2) cytokine imbalance with Th2 bias, leading to an increased risk of infection and relapses in SSNS.35,36 Zinc supplementation restores Th1 immune response, and thus corrects the Th1-Th2 perturbation, by augmenting the gene expression of interleukin-2 (IL-2) and interferon- γ (IFN- γ).37,38 This zinc-mediated immunologic outcome results in reduced infections and consequently reduced episodes of relapse.
Nevertheless, it is pertinent to establish if zinc dosing and its duration and specific serum levels may contribute to its effectiveness in ameliorating disease morbidity in nephrotic syndrome. In the present systematic review, we also found that the observational analytical studies suggest a significant relationship between subnormal serum zinc levels and disease severity. For instance, Ali et al reported significant differential serum zinc levels between nephrotic syndrome patients on relapse (57.2 ± 15.3 µg/dl) and their healthy controls (96.2 ± 7.501 µg/dl).16 Similarly, Taherahmadi et al noted a mean serum zinc level as low as 31.48 ± 6.8 µg/dl in severe nephrotic syndrome; disease severity was based on diagnostic criteria and response to treatment.27 Again, Ghafoorimehr et al confirmed that a significant inverse association occurred between baseline and remission serum zinc levels ((<78 µg/dl vs 78–110 µg/dl) and the frequency of relapses (n=35 vs n=7).26 The association of hypozincemia with nephrotic syndrome severity, therefore, suggests a possible beneficial role of zinc supplementation in improving disease morbidity. Zinc deficiency or hypozincemia refers to insufficiency of zinc to meet the body’s physiologic needs or serum zinc below the normal range of 70–120 µg/dl.39 A reduction in serum level is only detectable after long-term or severe depletion: making serum zinc level an unreliable marker for zinc status.40 It is plausible to assume that the dosing of zinc and duration of its supplementation may therefore be critical in normalizing serum levels and achieving good therapeutic outcomes in nephrotic syndrome. Also, zinc supplementation is known to produce a dose-dependent increase in the levels of inflammatory cytokines such as IL-2, IL-6, and tumor necrosis factor-α (TNF-α).41 Interestingly, a comparative analysis of zinc dosing/duration vs effects on relapse rates show a significant reduction in relapse rates in trial interventions with RDA and long duration of zinc,19 high dose and short duration of zinc,24 and RDA and moderate duration of zinc.25
The present systematic review has these limitations. Firstly, the heterogeneity of the eight included studies precluded a robust meta-analysis. Secondly, the four observational analytical studies did not evaluate the effect of zinc supplementation on nephrotic syndrome relapses although these studies assessed serum zinc levels and related them to disease severity. By extrapolation, the studies’ findings however suggest that zinc supplementation may be an effective therapeutic adjunct in nephrotic syndrome. Thirdly, all eight included studies were skewed toward the developing world (countries in the Middle East and south-east Asia) where the prevalence of premorbid zinc deficiency is relatively high. Thus, the causative factors of zinc deficiency may have appeared as confounding variables in these studies. Additionally, we speculate that the preponderance of the reviewed studies from south-east Asian countries is because of the epidemiologic pattern of childhood SSNS which shows a high disease prevalence among children of Asian and African ancestries. Finally, an assessment of the methodological quality of the four RCTs shows that they had either low quality,18,25 or moderate quality.19,24
Conclusions
The present systematic review has shown that oral zinc supplementation may reduce relapses in childhood SSNS. Whereas two of the included observational studies confirm that hypozincemia significantly increases the risk of relapses, three of the RCTs suggest that zinc supplementation results in a significant reduction in relapse rates when compared to placebo. Regarding its safety profile, zinc appears relatively safe as only one RCT reported metallic taste as its side effect.18 Given the absence of high-quality methodology in most of the eight reviewed studies, we conclude that there is currently no robust evidence to recommend zinc supplementation in SSNS. Nevertheless, given the established role of zinc in innate immunity (which reduces the incidence of infectious triggers of relapse, especially ARI), we suggest that zinc supplementation may be a useful therapeutic adjunct. We recommend more adequately-powered RCTs to strengthen the evidence about the supplement’s effectiveness in reducing relapses in SSNS. The global spread of such interventional studies should involve the developed western world to address the problem of confounders like premorbid zinc deficiency seen among the populace in developing countries.
Abbreviations
ARI, acute respiratory infection; FRNS, frequently relapsing nephrotic syndrome; IPNA, International Pediatric Nephrology Association; IRNS, infrequently relapsing nephrotic syndrome; MCN, minimal change nephropathy; RCTs, randomized controlled trials; SSNS, steroid-sensitive nephrotic syndrome; UTI, urinary tract infection.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi:10.1016/S0140-6736(03)14184-0
2. International Study of Kidney Disease in Children. Nephrotic Syndrome in children. Prediction of histopathology from clinical and laboratory characteristics at the time of diagnosis. Kidney Int. 1978;13:159–165. doi:10.1038/ki.1978.23
3. Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018;392:61–74. doi:10.1016/S0140-6736(18
4. Carter SA, Mistry S, Fitzpatrick J, et al. Prediction of short- and long-term outcomes in childhood nephrotic syndrome. Kidney Int Rep. 2020;5:426–434. doi:10.1016/j.ekir.2019.12.015
5. Teeninga N, Kist-van Holthe JF, Nauta J. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol. 2012;24:149–159. doi:10.1681/ASN.2012070646
6. Trautmann A, Boyer O, Hodson E, et al. On behalf of the international pediatric nephrology association. Pediatr Nephrol. 2022. doi:10.1007/s00467-022-05739-3
7. Uwaezuoke SN. Steroid-sensitive nephrotic syndrome in children: triggers of relapse and evolving hypotheses on pathogenesis. Italian J Pediatr. 2015;41:19. doi:10.1186/s13052-015-0123-9
8. Moorani KN. Infections are a common cause of relapse in children with nephrotic syndrome. Pak Paed J. 2011;35:213–219.
9. Gulati A, Sinha A, Sreenivas V, Math A, Hari P, Bagga A. Daily corticosteroids reduce infection-associated relapses in frequently relapsing nephrotic syndrome: a randomized controlled trial. J Am Soc Nephrol. 2011;6:163–169. doi:10.2215/CJN.01850310
10. MacDonald N, Wolfish N, Mclaine P, Phipps P, Rossier E. Role of respiratory viruses in exacerbations of primary nephrotic syndrome. J Paediatr. 1986;108:378–382. doi:10.1016/s0022-3476(86)80876-9
11. Afroz S, Khan Hossain MA, Roy KD, Ahmed F. Urinary tract infection (UTI) is associated with a higher rate of relapse in children with nephrotic syndrome. DS Child H J. 2010;26:82–86.
12. Shenguttuvan P, Ravanan K, Prabhu N, Tamilarasi V. Infections encountered in childhood nephrotics in a pediatric renal unit. Indian J Nephrol. 2004;14:85–88.
13. Uwaezuoke SN, Okafor HU, Eneh CI, Odetunde OI. The triggers and patterns of relapse in childhood idiopathic nephrotic syndrome: a retrospective, descriptive study in a tertiary hospital, southeast Nigeria. J Clin Nephrol Res. 2016;3(1):1032.
14. Ademola AE, Ibraheem I. Patterns, triggers, and predictors of relapses among children with steroid-sensitive idiopathic nephrotic syndrome at the University of Abuja Teaching Hospital, Gwagwalada, Abuja, Nigeria. J Egypt Soc Nephrol Transplant. 2022;22:117–127. doi:10.4103/jesnt.jesnt_36_21
15. Takahashi S, Wada N, Murakami H, et al. Triggers of relapse in steroid-dependent and frequently relapsing nephrotic syndrome. Pediatr Nephrol. 2007;22:232–236. doi:10.1007/s00467-006-0316-y
16. Ali SH, Jabar RA, Sharba YF. Serum zinc level in children with nephrotic syndrome. J Arab Board Health Special. 2013;14:2–10.
17. Roth DF, Richard SA, Black R. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta-analysis and meta-regression of randomized trials. Int J Epidemiol. 2010;39:795–808. doi:10.1093/ije/dyp391
18. Sherali AR, Moorani KN, Chishty SH, Khan SI. Zinc supplement in reduction of relapses in children with steroid-sensitive nephrotic syndrome. J Coll Physicians Surg Pak. 2014;24:110–113. PMID: 24491005.
19. Arun S, Bhatnagar S, Menon S, Saini S, Hari P, Bagga A. Efficacy of zinc supplements in reducing relapses in steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2009;24:1583–1586. doi:10.1007/s00467-009-1170-5
20. Bhatt GC, Jain S, Das RR. Zinc supplementation as an adjunct to standard therapy in childhood nephrotic syndrome - a systematic review. World J Clin Pediatr. 2016;5:383–390. doi:10.5409/wjcp.v5.i4.383
21. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097
22. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS). Disponibile all’indirizzo; 2000. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.
23. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing the risk of bias in randomized trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
24. Haque F, Hanif M, Choudhury TR. Role of zinc in patients with nephrotic syndrome. J Ped Nephrol. 2017;5(1):1–7.
25. Hashmi F, Ghazi SS, Mumtaz H, Amjad M, Zaman N, Ahmad S. Role of zinc supplementation in comparison to B complex for reducing the frequency of sustained remission in steroid-sensitive nephrotic syndrome among pediatric age group. Ann Pak Inst Med Sci. 2021;17(3):236–240. doi:10.48036/apims.v17i3.408
26. Ghafoorimehr F, Moghtaderi M, Bazargani B, Fahimi D, Abbasi A. Influence of zinc deficiency on one-year recurrence in children with nephrotic syndrome. J Renal Inj Prev. 2019;8:243–246. doi:10.15171/jrip.2019.46
27. Taherahmadi H, Yousefichaijan P, Rezagholizamenjany M, Rafiei M, Norozi S. Serum zinc level in recurrent nephrotic syndrome. Nephro-Urol Mon. 2019;11(4):e96628. doi:10.5812/numonthly.96628
28. Jaiswal R, Shrivastava A, Tiwari AD, Yadav RK, Maurya M, Mishra N. Varying zinc levels in pediatric nephrotic syndrome patients and its correlation with remission and relapse: an observational study. Panacea J Med Sci. 2022;12(1):91–96. doi:10.18231/j.pjms.2022.018
29. Mumtaz A, Anees M, Fatima S, Ahmed R, Ibrahim M. Serum zinc and copper levels in nephrotic syndrome patients. Pak J Med Sci. 2011;27(5):1173–1176.
30. Almosawi QM. Serum zinc level in children with relapsing nephrotic syndrome. Int J Curr Res. 2016;8(11):42328–42330.
31. Tumer N, Baskan S, Arcasory A, et al. Hypozincemia in nephrotic syndrome. Clin Nephrol. 1991;35:135–137. PMID: 2032400.
32. Mahajan SK, Speck J, Varghese G, et al. Zinc metabolism in nephrotic syndrome. Nutr Res. 1985;1(Suppl):360–362.
33. Lindeman RD, Baxter DJ, Yunice AA, King RW, Kraikit S. Zinc metabolism in renal disease and renal control of zinc excretion. Prog Clin Biol Res. 1977;14:193–209. PMID: 605138.
34. Schu¨nemann H, Brozek J, Oxman A. GRADE handbook for grading the quality of evidence and strength of recommendation. Version. 2008;3.2.
35. Prasad AS. Zinc: mechanisms of host defense. J Nutr. 2007;137:1345–1349. doi:10.1093/jn/137.5.1345
36. Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–463S. doi:10.1093/ajcn/68.2.447S
37. Bao B, Prasad AS, Beck FW, Godmere M. Zinc modulates mRNA levels of cytokines. Am J Physiol Endocrinol Metab. 2003;285:E1095–1102. doi:10.1152/ajpendo.00545.2002
38. Prasad AS. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J Infect Dis. 2000;182(1):62–68. doi:10.1086/315916
39. Smith JC, Butrimovitz GP, Purdy WC. Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clin Chem. 1979;25(8):1487–1491. PMID: 455691. doi:10.1093/clinchem/25.8.1487
40. Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bulletin. 2007;28(3 Suppl):S403–429. doi:10.1177/15648265070283S303
41. Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardio-metabolic disease. Nutrients. 2012;4(7):676–694. doi:10.3390/nu4070676
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.