Back to Journals » ClinicoEconomics and Outcomes Research » Volume 6
Budget impact analysis of the fentanyl buccal tablet for treatment of breakthrough cancer pain
Authors Darbà J , Kaskens L, Sánchez-de la Rosa R
Received 31 July 2013
Accepted for publication 24 September 2013
Published 16 December 2013 Volume 2014:6 Pages 1—9
DOI https://doi.org/10.2147/CEOR.S52273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Josep Darbà,1 Lisette Kaskens,2 Rainel Sánchez-de la Rosa3
1University of Barcelona, Barcelona, 2BCN Health Economics and Outcomes Research SL, Barcelona, 3Medical and HEOR Department, TEVA Pharmaceutical Industries Ltd, Madrid, Spain
Background: The purpose of this study was to assess the economic impact of the fentanyl buccal tablet for the management of breakthrough cancer pain (BTcP) in Spain.
Methods: A 4-year budget impact model was developed for the period 2012–2015 for patients with BTcP from the perspective of the Spanish National Health System. BTcP products included in this model were rapid-onset opioids containing fentanyl (buccal, sublingual, or nasal transmucosal). Prevalence data on cancer, BTcP, opioid use, and number of BTcP episodes were obtained from the literature. Input data on health care resources associated with opioid use and opioid-induced side effects were obtained by consulting experts in oncology from different Spanish hospitals. Resources used included drugs, medical and emergency visits, other nonpharmacologic treatments, and treatment of opioid-induced side effects. Unit costs were obtained from the literature, and a 3% discount rate was applied to costs. Based on the unit costs for drugs and health care resources, the annual BTcP treatment costs per patient associated with each fentanyl product were determined to estimate the overall budget impact based on the total treatment population and the percentage of drug utilization associated with each product. One-way sensitivity analyses were conducted to test the robustness of the model.
Results: Patients treated with oral opioids for BTcP were estimated at 23,291 in 2012, with an increase up to 23,413 in 2015. The average annual budget savings, with an increase of fentanyl buccal tablets, fentanyl sublingual tablets, and intranasal fentanyl spray, and a decrease in oral transmucosal fentanyl citrate, was estimated at €2.6 million, which represents a 0.5% decrease in the total costs of BTcP over the next 4 years. Results of the sensitivity analysis showed that the model was most sensitive to drug cost per day for the fentanyl buccal tablet. A 50% decrease in the daily cost of the fentanyl buccal tablet resulted in the largest overall decrease in budget impact of €5.4 million.
Conclusion: The increase in use of the fentanyl buccal tablet leads to overall savings in the budget impact for the Spanish National Health System. Although the economic impact of treatment for BTcP was shown to increase over 4 years due to population growth, the average annual cost per patient was reduced by €29 with increased use of the fentanyl buccal tablet.
Keywords: cancer, breakthrough pain, economic analysis, costs, rapid onset opioids
Introduction
Pain is consistently one of the most feared consequences of cancer for both patients and their families.1 However, despite well controlled chronic pain in 80%–90% of patients with cancer,2 these patients may experience cancer-related breakthrough pain (BTcP). BTcP can be defined as an acute transient worsening of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger in patients who have relatively stable and adequately controlled baseline pain.3,4 While there are slight differences in the definitions of BTcP,5–7 all stress its transient nature against a background of stable and otherwise controlled baseline pain. BTcP is characterized by a typical temporal pattern which includes a short onset (generally a few minutes) and a short duration (30–90 minutes).8
BTcP is highly prevalent, occurring in 33%–65% of patients with chronic cancer pain, with the incidence varying widely according to the population surveyed, the setting, and the definition of BTcP used.9–12 Severe BTcP is reported in 60% of patients, with 40% of patients having mild to moderate BTcP, and almost all patients having mild background pain.1
BTcP is associated with a significant negative impact on both quality of life (including activities of daily living, sleep, social relationships, and mood) and functional status,6,13 and may also increase levels of anxiety and depression, increase the perception of pain severity, and lead to patients becoming dissatisfied with their overall pain management.14 BTcP also increases the economic burden placed on patients and the health care system.15,16 In Spain, patients receiving hospital treatment, as for the treatment of BTcP, are fully reimbursed and do not have to make any additional copayments.
Oral opioids like morphine have traditionally been the only available drugs for BTcP.8,17,18 A typical episode of BTcP of high intensity, rapid onset, and short duration fits poorly with the pharmacology of short-acting opiates, such as morphine and oxycodone.8,19 Different technologies have been developed to provide rapid pain relief with potent opioid drugs or rapid-onset opioids such as fentanyl, delivered by noninvasive routes (buccal, sublingual, or nasal transmucosal).1,8 These fentanyl preparations include oral transmucosal fentanyl citrate (OTFC), the fentanyl buccal tablet (FBT), sublingual fentanyl (FSL), intranasal fentanyl spray (INFS), and fentanyl-pectin nasal spray (FPNS).8,17,20 The emergence of multiple, fast-acting fentanyl preparations in the market place over recent years has significantly improved the options available to clinicians treating BTcP.21 In a mixed-treatment meta-analysis which indirectly compared fentanyl preparations with morphine and placebo, it was shown that fentanyl preparations provide superior pain relief compared with placebo in the first 30 minutes after dosing (FBT provided an 83% probability of superior pain relief, FSL 66%, and OTFC 73% compared with placebo), with oral morphine performing little better than placebo (probability 56%).21
In the current study, we evaluated the expected economic impact from the increase in market share of FBT for the management of BTcP from the perspective of the Spanish National Health System using a budget impact model.
Materials and methods
Model development and structure
The budget impact model was developed in Microsoft Excel to estimate the economic impact of treatment for BTcP from the perspective of the Spanish National Health System. The treatment options for BTcP included in this analysis were the following fentanyl products: OTFC, FSL, FBT, INFS, and FPNS. The model analyzed drug and health care resource utilization for each patient based on their mean number of days with BTcP episodes annually and average number of drug doses taken on days with BTcP episodes. The differences in drug and medical costs associated with each treatment option, as well as the overall budget impact of the increasing market share of FBT, were calculated over a 4-year period from 2012 to 2015.
The model included prevalent (existing) cancer patients being treated with rapid-onset opioids for their BTcP episodes. Patients were assumed to be treated for an average of 3.8 days per week or 198 days per year based on the study reported by Di Palma et al in 2004.22 Patients would be at risk for experiencing one up to a maximum of four BTcP episodes per day according to the literature.23 The average number of BTcP episodes for a day on which a patient experiences BTcP with a maximum of four were estimated from clinical practice by a panel of four clinical experts in oncology from different Spanish hospitals. The model included drug and health care resource costs associated with opioid therapy and opioid-induced side effects. Drug costs included titration, maintenance, and rescue treatment costs. All cost estimates were reported in EUR 2012 and a discount rate of 3% was applied.
Opioid treatment for BTcP episodes was based on national sales data for Spain from the Intercontinental Marketing Services (IMS).24 These sales data were also the source for assumptions regarding future estimations on the market share distributions for each BTcP drug treatment. To maintain consistency with the sales data and to best reflect current prescribing and dosing patterns, when shifting market share from one product to another, the assumptions for the relative distribution of prescriptions according to dosage strength and daily average number of dosages for each BTcP episode were maintained. As a result, this base case analysis estimated the potential budget impact of shifting the number of prescriptions filled by one product, while keeping the proportion of product prescriptions according to dose strength and their daily average number of doses constant.
We assumed that the use of FBT would stem from patients switched from other BTcP products. At the time of this analysis, national sales data reported that the BTcP fentanyl product with the greatest market share of the BTcP market was OTFC. The model generated estimates for the costs per patient and the total direct costs of treatment including drug and medical costs based on market shares and other input parameters. All patients were assumed to receive treatment during a whole year, independent of having switched to another BTcP product. Sensitivity analyses were conducted for the base case analyses.
Model input variables: base case
Target population
To estimate the target population with cancer experiencing BTcP episodes treated with fentanyl products, the following algorithm was applied, as shown in Figure 1. A literature review was performed to identify the prevalence of cancer (364 per 100,000 inhabitants) among the Spanish adult population.25 The estimate for cancer prevalence has been extrapolated to the Spanish adult population obtained from the population projections conducted by the National Institute of Statistics.26 The number of adult patients in Spain with cancer suffering from BTcP in 2002 was estimated at 41% by Gómez-Batiste et al.27 The percentage of patients with BTcP episodes treated with opioids was estimated at 71%, and 58% were estimated to be oral or nasal opioids based on consultation of medical experts in oncology.
  | Figure 1 Target study population. |
Drug treatments and costs
The allocation percentages for the most frequently used dosage strength for each treatment were obtained from the literature.23,28 In the case of FBT and FSL, the unit costs for all dosage strengths were similar and the percentage of dosage utilization did not influence the total drug costs per dose. The daily average numbers of doses associated with each BTcP product were estimated based on data from clinical practice by a panel of four clinical experts in oncology from different Spanish hospitals (Table 1). Based on these percentages if applicable, the average number of doses on a day with BTcP according to the average number of episodes and the cost per dose in EUR, the average treatment cost for a day with BTcP episodes was calculated (Table 2). The maximum doses per day were based on the technical characteristics of each drug.29–33 The cost for each drug was obtained from the Spanish Medication Database34 and expressed in EUR 2012. A discount to the drug price was applied when applicable due to the mandatory rebate imposed by the Spanish Ministry of Health since May 2010 (Table 1).35
  | Table 1 Opioids, presentations, costs per dose, and allocation percentages |
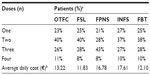  | Table 2 Average number of drug doses and costs in EUR on a day with BTcP episodes |
Medical resource utilization and costs
Medical resource utilization and costs included in the model were medical and emergency visits to hospital, nonpharmacologic treatments, and other interventions associated with treating opioid-induced side effects. The costs for medical resources associated with each drug were estimated based on consultation of an expert panel of oncologists and unit costs were obtained from the literature (Table 3).
  | Table 3 Drug costs, medical resource utilization, unit costs, and annual mean cost per patient |
Not all patients seek medical care for opioid-induced side effects, so these costs were only assigned to the proportion of patients who received treatment due to an opioid-induced side effect. The incidence rates for common opioid-induced side effects associated with each drug were derived from consultation of the expert panel of oncologists (Table 2). In the model, only incident patients were considered at risk for experiencing an opioid-induced side effect.
Budgetary impact analysis
Based on the annual drug cost and health care resource use per patient, the total treatment cost per patient was estimated in EUR 2012. With the annual average cost per patient for each treatment option, the target population, and the actual market shares for the fentanyl products included in this study, the overall economic impact of the management of BTcP for 2012–2015 was obtained. The base case scenario was based on actual market share data for 2012 obtained from IMS24 on the actual distribution of fentanyl products (Table 4), with expected increases in FBT, FSL, FPNS, and INFS and decreases in OTFC over 4 years. This scenario was designated as the “current” scenario.
The current scenario was compared with an “alternative” scenario in which we assumed that the market share of FBT would increase annually by 2%, FPNS and INFS would show a slower increase of their market share than in the base case scenario, and a decrease in market share was assumed for OTFC (8.3%). In the base case analysis, the potential budget impact was estimated based on the difference between the current scenario and the alternative scenario with an expected increase in the use of FBT after switching from BTcP treatment.
Sensitivity analysis
In order to test the robustness of the results, a one-way sensitivity analysis was performed with the main variables. The base case value for each parameter was varied from the default value within reasonable lower and upper limits as defined in the published literature and by expert consultation, with variations of ±50% applied to parameters for which no ranges were identified in the literature.
Results
In our model based on the prevalence of cancer, BTcP, and opioid use it would be expected that 23,291 in 2012 that experience cancer BTcP annually receive treatment with oral or nasal opioids. Based on population growth, this number increases slightly to 23,413 in 2015 (Table 5).
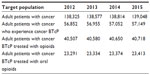  | Table 5 Target population for BTcP treatment |
In the base case analysis, in the current scenario, the total economic impact of treatment with oral opioids for patients with BTcP was estimated at €119 million, €122 million, €127 million, and €133 million for the years 2012, 2013, 2014, and 2015, respectively (Table 5).
In the alternative scenario, with the market share of FBT increased by 2% annually from 2012, matched by a reduction in the share of OTFC, the total economic impact was estimated at €119 million, €121 million, €126 million, and €132 million for 2012, 2013, 2014, and 2015, respectively (Table 6). Overall, the total budget savings with the revised market shares with an annual increase of FBT, FSL, and INFS and decrease of OTFC in the alternative scenario was expected to save €2.6 million over the next 4 years (Table 6). Overall, total direct costs represented 51% and drug costs 49% of the total budget.
At the patient level, the average annual cost per patient in the current scenario increases from 2012 to 2015 from €5,127 to €5,685 due to demographic changes in the population, although the increase would be less with €5,640 in the alternative scenario. The average cost per patient over the period 2012–2015 with the increase in market share of FBT was €29 lower in the alternative scenario with an annual per patient cost of €5,336 than in the current scenario with an annual per patient cost of €5,366 (Table 6).
Sensitivity analysis
One-way sensitivity analyses were conducted to examine how changes in key model parameters might affect the results of the base case analysis. The parameters that were varied included the percentage of patients with BTcP episodes, percentage of patients treated with opioids, percentage of patients treated with oral opioids, days with BTcP episodes, market share projection of FBT, and cost per day of FBT. The results of varying each parameter are shown in Table 7. The model was most sensitive to drug cost per day of FBT. A decrease of 50% in the daily drug cost of FBT resulted in the largest overall decrease in budget impact of €5.4 million.
  | Table 7 One-way sensitivity analysis for the most influential variables in the budget impact model |
Discussion
This study compares the costs of fentanyl products and estimates the budget impact of treatment for BTcP in Spain for the period 2012–2015. Results of the budget impact analysis suggest that the increasing use of FBT would result in 4-year adjusted total budget savings for the Spanish National Health System of €2.6 million, which represents a 0.5% decrease in total BTcP. BTcP causes a significant economic burden to the Spanish National Health System, is a leading cause of disability, and has a negative impact on an individual’s functional status and quality of life. Direct health care costs in our study were shown to be a high part of the total costs for BTcP. These results are in line with previous reports15,16 on the relationship between BTcP and use of health care resources in a cancer population with pain. Eligible patients were questioned about the occurrence of BTcP and pain-related hospitalizations, emergency department visits, and physician office visits. The results show that patients with BTcP sustain higher costs of care than cancer patients without BTcP as a result of pain-related hospitalizations and physician office visits.
Our analyses suggest that increased use of FBT may reduce both drug and health care costs due to a shift in market share from other fentanyl products, such as OTFC. Based on these analyses, the increase in market share of FBT may yield cost savings to the Spanish National Health System. It is important to mention that in this study the most frequent drug doses were taken into account when calculating the drug cost per episode because unit costs for different drug doses in the case of OTFC or two nasal sprays doubled the cost per episode of FPNS and INFS.23,28 Different doses of FSL and FBT had similar unit costs, for which the unit costs for each dose would not affect the final costs per episode.
The pharmacology of fentanyl is suitable for treatment of short-lived BTcP episodes and amenable to production of transmucosal and nasal formulations, which have been shown to reduce first-pass metabolism, allowing for a rapid onset of action of 5–10 minutes.20 However, it is important to note that not all fentanyl products are indicated for every BTcP patient. Although nasal sprays are convenient, easy to administer and noninvasive, with few local side effects, contraindications exist for nasal fentanyl products, such as INFS and FPNS. These products are prone to indiscriminant use as a result of abuse and addiction,36 so use of these products requires prior screening and appropriate training.
The population of interest included patients with BTcP episodes who received treatment with an oral or nasal opioid to manage their pain. As with any model, there are several limitations that should be noted. First, the model was developed to estimate the potential budget impact when increasing the market share of FBT, FPNS, and INFS due to reduced utilization of OTFC. This outcome may not fully reflect real world changes. However, the current study included only the oral and nasal fentanyl products available on the Spanish market. Therefore, the effect of possible uptake of fentanyl buccal soluble film, which was approved in the European Union in 201018 and is expected to enter the Spanish market in the coming years, was not considered in the current study.
The base case analysis was based on certain assumptions that may not reflect fully the actual treatment of BTcP in the Spanish setting. For instance, the model estimates the pharmacologic costs, health care resource utilization costs associated with each drug, and the costs of treating opioid-induced side effects associated with the oral and nasal formulations; although it does not consider the costs associated with poor adherence as observed in several studies,37,38 accidental intoxication caused by abuse,36 titration costs to adjust for the correct dose, or rescue medication. We have not taken into account the possible effect of adherence or abuse of oral or nasal opioids in our analysis and their possible effect on medical resource utilization and costs or on treatment of opioid-induced side effects. Abuse of nasal opioids has been reported and can lead to accidental intoxication, as well as associated hospitalization and rescue medication costs.39 Titration costs for each patient considers extra doses that are necessary for each patient to reach the optimal effective dosage, and rescue medication is also reported in different clinical trials with the different fentanyl products17 including the costs for an extra dose of the same strength in the event that a patient does not obtain adequate pain relief. In both cases, these extra drug costs per patient due to titration or rescue medication are likely to have an effect on total drug costs and, therefore, the overall costs per patient treated with fentanyl products.
Large variations have been observed in the prevalence of patients with BTcP episodes in the published literature.9–12 In this study, we used the 41% BTcP prevalence reported for a Spanish group of patients by Gomez-Batiste et al in 2002,27 and although variations were explored in sensitivity analysis, it has to be acknowledged that any variations in BTcP prevalence have a high impact on the costs associated with its management.
Few head-to-head comparisons of oral opioids exist that could provide input data on resources, safety, and cost when these agents are used to treat BTcP episodes, although some attempts have been made using mixed treatment comparisons.22,40,41 However, not all input parameters necessary for our model were compared in these studies. One of the strengths of our analysis is that, because of the lack of published clinical input data on drug use, medical resource utilization, and treatment of opioid-induced side effects, it was based on real-world use of these treatments, given that our input data was based on the expert opinion of clinical and radiotherapeutic oncologists working in Spanish hospitals.
Finally, the model did not consider the efficacy of oral opioids. It was observed in a recent publication22 that FBT was superior in the probability of pain relief when compared with FSL, OTFC, and oral morphine. The results of our budget impact analysis suggest that increased use of FBT results in cost savings to the Spanish National Health System. Therefore, in addition to the clinical evidence, it can be concluded that our budget impact model provides important information to payers for evaluation of the potential economic impact resulting from oral opioids on medical and pharmacy budgets beyond just forecasting utilization.
Conclusion
The results of our analysis suggest that when FBT use grows annually with an increase of 2%, while the use of OTFC decreases and use of FPNS and IFNS increases more slowly, there would be a €2.6 million decrease in the overall budget for the period 2012–2015, which represents a 0.5% decrease in total expected costs of BTcP, due to lower drug and health care resource utilization costs. Although the economic impact of BTcP treatment was shown to increase over 4 years due to population growth, the increased use of FBT for treatment of BTcP decreased the average annual cost per patient by an average of €29 over 4 years.
Acknowledgment
The authors wish to thank our oncologic experts, Rafael Moleron Mancebo, Dulce Rodríguez Mesa, César A Rodríguez Sánchez, and Juan A Virizuela, for their participation in this study.
Author contributions
The contributions of JD and LK included designing the study, extracting data, conducting the analysis, and writing the draft and final manuscript. JD is guarantor of the manuscript. The contributions of RS included designing the study, defining the study objective and analysis strategy, and reviewing the draft and final manuscript.
Disclosure
This study was sponsored by TEVA Pharmaceutical Industries Ltd. JD is employed by the University of Barcelona and was involved as an external advisor hired by TEVA Pharmaceutical Industries Ltd. LK is an employee of BCN Health Economics and Outcomes Research SL, Barcelona, Spain, an independent contract health economic organization that has received research funding from TEVA Pharmaceutical Industries Ltd. RS is employed by TEVA Pharmaceutical Industries Ltd and works in the Medical and HEOR Department.
References
Mercadante S. Cancer pain. Curr Opin Support Palliat Care. 2013;7:139–143. | |
Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59:850–856. | |
Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13:331–338. | |
Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain. Part 1: clinical considerations. J Pain Symptom Manage. 2001;21:144–150. | |
Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–281. | |
Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain 1999; 81:129-34. | |
Mercadante S, Radbruch L, Caraceni A, et al; Steering Committee of the European Association for Palliative Care (EAPC) Research Network. Episodic (breakthrough) pain: consensus conference of an expert working group of the European Association for Palliative Care. Cancer. 2002;94:832–839. | |
Mercadante S. Pharmacotherapy for breakthrough cancer pain. Drugs. 2012;72:181–190. | |
Caraceni A, Martini C, Zecca E, et al. Breakthrough pain characteristics and syndromes in patients with cancer pain: an international survey. Palliat Med. 2004;18:177–183. | |
Portenoy RK, Bruns D, Shoemaker B, Shoemaker SA. Breakthrough pain in community-dwelling patients with cancer pain and noncancer pain. Part 1: prevalence and characteristics. J Opioid Manag. 2010;6:97–108. | |
Mercadante S, Costanzo BV, Fusco F, et al. Breakthrough pain in advanced cancer patients followed at home: a longitudinal study. J Pain Symptom Manage. 2009;38:554–560. | |
Mercadante S, Zagonel V, Breda E, et al. Breakthrough pain in oncology: a longitudinal study. J Pain Symptom Manage. 2010;40:183–190. | |
Portenoy RK, Bennett DS, Rauck R, et al. Prevalence and characteristics of breakthrough pain in opioid-treated patients with chronic noncancer pain. J Pain. 2006;7:583–591. | |
Dickman A. Basics of managing breakthrough cancer pain. Pharm J. 2009;283:213e216. | |
Fortner BV, Okon TA, Portenoy RK. A survey of pain-related hospitalizations, emergency department visits, and physician office visits by cancer patients with and without breakthrough pain. J Pain. 2002;3:38–44. | |
Fortner BV, Demarco G, Irving G, et al. Description and predictors of direct and indirect costs of pain reported by cancer patients. J Pain Symptom Manage. 2003;25:9–18. | |
Smith H. A comprehensive review of rapid-onset opioids for breakthrough pain. CNS Drugs. 2012;26:509–535. | |
Zeppetella G. Opioids for the management of breakthrough cancer pain in adults: a systematic review undertaken as part of an EPCRC opioid guidelines project. Palliat Med. 2010;25:516–524. | |
Zeppetella G. Dynamics of breakthrough pain vs pharmacokinetics of oral morphine: implications for management. Eur J Cancer Care. 2009;18:331–337. | |
McWilliams K, Fallon M. Fast-acting fentanyl preparations and pain management. QJM. 2013;106:887–890. | |
Jandhyala R, Fullarton JR, Bennett MI. Efficacy of rapid-onset oral fentanyl formulations vs oral morphine for cancer-related breakthrough pain: a meta-analysis of comparative trials. J Pain Symptom Manage. February 1, 2013. [Epub ahead of print.] | |
Di Palma M, Poulain P, Pichard E. [What’s new in the treatment of cancer pain?]. Bull Cancer. 2004;91:95–98. French. | |
Mercadante M. A comparison of intranasal fentanyl spray with oral transmucosal fentanyl citrate for the treatment of breakthrough cancer pain: an open-label, randomised, crossover trial. Curr Med Res Opin. 2009;25:2805–2815. | |
IMS Health. National sales data July 2011–January 2012 for rapid onset opioids, oral morphine and oxycodone for Spain. Data on file. | |
International Association for Cancer Research. GLOBCAN cancer incidence, mortality and prevalence worldwide in 2008. Available from: http://globocan.iarc.fr/summary_table_site_prev.asp?selection=280&title=All+cancers+excl.+non. Accessed May 8, 2013. | |
Instituto Nacional de Estadístico. [National institure for statistics. Projections for the population on the short term.] Proyecciones de población a corto plazo. 2011–2021. Available from: http://www.ine.es/jaxi/menu.do?type=pcaxis&path=%2Ft20%2Fp269%2F2011-2021&file=pcaxis&L=. Accessed October 12, 2012. | |
Gómez-Batiste X, Madrid F, Moreno F, et al. Breakthrough cancer pain: prevalence and characteristics in patients in Catalonia, Spain. J Pain Symptom Manage. 2002;24:45–52. | |
Fallon M, Reale C, Davies A, et al. Fentanyl nasal spray study. Efficacy and safety of fentanyl pectin nasal spray compared with immediate-release morphine sulfate tablets in the treatment of breakthrough cancer pain: a multicenter, randomized, controlled, double-blind, double-dummy multiple-crossover study. J Support Oncol. 2011;9:224–231. | |
Agencia de Medicamentos y Productos Sanitarios Española. Ficha técnica de Actiq®. Available from: http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=buscar. Accessed June 25, 2013. | |
Agencia de Medicamentos y Productos Sanitarios Española. Ficha técnica de Abstral®. Available from: http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=buscar. Accessed June 25, 2013. | |
Agencia de Medicamentos y Productos Sanitarios Española. Ficha técnica de PecFent®. Available from: http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=buscar. Accessed June 25, 2013. | |
Agencia de Medicamentos y Productos Sanitarios Española. Ficha técnica de Instanyl®. Available from: http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=buscar. Accessed June 25, 2013. | |
Agencia de Medicamentos y Productos Sanitarios Española. Ficha técnica de Effentora®. Available from: http://www.aemps.gob.es/cima/fichasTecnicas.do?metodo=buscar. Accessed June 25, 2013. | |
Consejo General de Colegios Oficiales de Farmacéuticos. BOT plus web. 2012. Available from: https://botplusweb.portalfarma.com/. Accessed May 8, 2013. | |
Real Decreto-Ley 8/2010, May 20th where extraordinary measures were adopted for public deficit reduction. | |
Davis MP. Recent development in therapeutics for breakthrough pain. Expert Rev Neurother. 2010;10:757–773. | |
Davies AN, Vriens J, Kennett A, McTaggart M. An observational study of oncology patients’ utilization of breakthrough pain medication. J Pain Symptom Manage. 2008;35:406–411. | |
Ferrell BR, Juarez G, Borneman T. Use of routine and breakthrough analgesia in home care. Oncol Nurs Forum. 1999;26:1655–1661. | |
Ramos Gracia M, Catala Hortelano L, Sala Langa MJ, Gómez Sánchez D, Conejero Morant MA, Saneugenio Gregori J. [Accidental poisoning by intranasal fentanyl.] An Pediatr (Barc). May 15, 2013. [Epub ahead of print.] Spanish. | |
Vissers D, Stam W, Nolte T, Lenre M, Jansen J. Efficacy of intranasal fentanyl spray versus other opioids for breakthrough pain in cancer. Curr Med Res Opin. 2010;26:1037–1045. | |
Vissers DC, Lenre M, Tolley K, Jakobsson J, Sendersky V, Jansen JP. An economic evaluation of short-acting opioids for treatment of breakthrough pain in patients with cancer. Value Health. 2011;14:274–281. | |
Diari Oficial de la Generalitat de Catalunya (DOGC) Núm. 5325 – 24.2.2009. Available at: http://www20.gencat.cat/portal/site/portaldogc. Accessed June, 2013. | |
Diari Oficial de la Generalitat de Catalunya (DOGC) Núm. 5907 – 27.6.2011. Available at: http://www20.gencat.cat/portal/site/portaldogc. Accessed June, 2013. | |
Diario Oficial de Galicia. (DOG) Núm. 213 Martes, 8 de noviembre de 2011 Pág. 32557. Available at: http://www.xunta.es/diario-oficial-galicia/8. Accessed June, 2013. | |
Boletin Oficial de Cantabria. (DOC) Núm. 85 Jueves, 5 de mayo de 2011. Pág. 15393. Available at: http://boc.cantabria.es/boces/. Accessed June, 2013. |
 © 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


