Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Benefits of Preserving Residual Urine Output in Patients Undergoing Maintenance Haemodialysis
Authors Dopierała M, Schwermer K , Hoppe K, Kupczyk M , Pawlaczyk K
Received 24 July 2023
Accepted for publication 1 September 2023
Published 17 October 2023 Volume 2023:16 Pages 231—240
DOI https://doi.org/10.2147/IJNRD.S421533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Mikołaj Dopierała, Krzysztof Schwermer, Krzysztof Hoppe, Małgorzata Kupczyk, Krzysztof Pawlaczyk
Department of Nephrology, Transplantology and Internal Medicine, Poznan University of Medical Sciences, Poznan, Poland
Correspondence: Krzysztof Pawlaczyk, Email [email protected]
Introduction: Chronic kidney disease is a widespread medical problem that leads to higher morbidity, mortality, and a decrease in the overall well-being of the general population. This is especially expressed in patients with end-stage renal disease (ESRD) undergoing maintenance haemodialysis. Several variables could be used to evaluate those patients’ well-being and mortality risk. One of them is the presence of residual urine output.
Materials and Methods: The study was conducted on 485 patients treated with maintenance haemodialysis. After enrollment in the study, which consisted of medical history, physical examination, hydration assessment, and blood sampling, each patient was followed up for 24 months. We used residual urine output (RUO) as a measure of residual renal function (RRF). The entire cohort was divided into 4 subgroups based on the daily urinary output (<=100mL per day, > 100mL to <=500mL, > 500mL to <=1000mL and > 1000mL).
Results: The data show that the mortality rate was significantly higher in groups with lower RUO, which was caused mainly by cardiovascular events. Also, patients with higher RUO achieved better sodium, potassium, calcium, and phosphate balance. They were also less prone to overhydration and had a better nutritional status. Preserved RRF also had a positive impact on markers of cardiovascular damage, such as NT-proBNP as well as TnT.
Conclusion: In conclusion, preserving residual urine output in ESRD patients undergoing maintenance haemodialysis is invaluable in reducing their morbidity and mortality rates and enhancing other favourable parameters of those patients.
Keywords: end stage renal disease, haemodialysis, residual urine output, cardiovascular risk, mortality
Introduction
Chronic kidney disease is a widespread medical problem that leads to higher morbidity, mortality, and a decrease in the overall well-being of the general population. This is especially evident in patients with end-stage renal disease (ESRD) undergoing maintenance haemodialysis (HD). The main cause of death in this population are cardiovascular (CV) events.1–4 As for the year 2022, chronic kidney disease affects more than 10% of the population across the world, which amounts to over 800 million individuals. It has emerged as one of the leading causes of mortality worldwide, and it is one of a small number of non-communicable diseases that have shown an increase in associated deaths over the past 2 decades. Chronic kidney disease poses a significant challenge, particularly in low- and middle-income countries, where resources and infrastructure to address its impact are limited. These countries face a disproportionate burden due to their relatively fewer capabilities in managing the consequences of this disease.5 Nearly 4 million people in the world are living on renal replacement therapy (RRT), and haemodialysis (HD) remains the most typical form of RRT, accounting for approximately 69% of all RRT and 89% of all dialysis.6 In Poland, at the end of the year 2022, there were 20,198 patients undergoing dialysis, 19,389 on haemodialysis and 809 on peritoneal dialysis.7 Several variables help evaluate the general condition and prognosis in haemodialysis patients, including nutritional status, fluid balance, the presence of chronic low-grade inflammation, kidney-related anaemia, oxidative stress, soft tissue calcifications and residual renal function (RRF).8–10
Residual renal function is the capacity of native kidneys to remove water and uremic toxins in patients with ESRD undergoing renal replacement therapy.11 Measuring the daily urine volume is the simplest way to evaluate RRF. Many studies have shown that residual urine output (RUO) correlates with residual glomerular filtration rate (GFR).12–14 Other methods of assessing RRF require laboratory testing, including isotope, inulin, creatinine and urea clearance analysis. Additionally, measuring plasma concentration of solutes that are too large to be removed by dialysis, such as cystatin C or beta-2 microglobulin, can be used as surrogate markers of kidney function.11,15–17
Medline indexes over 5000 articles on RRF. Many studies underline the importance of its preservation in ESRD patients undergoing dialysis. Residual renal function has been shown to correlate directly with higher survivability.8,16–20 One of the most significant benefits of RRF is middle molecule clearance, such as B2-macroglobulin,21 reducing the likelihood of dialysis-associated amyloidosis.22 Furthermore, it helps to control the hydration status, blood pressure and reduces the risk of left ventricular hypertrophy, improves cardiac index and reduces the total peripheral resistance index.16,17,19,20,23–27 Anuric patients have a greater risk of developing secondary hyperparathyroidism, vascular calcifications, and arterial stiffening, as well as hyperkalemia and hyponatremia.14,16,17,19,20,24,28,29 They are also more prone to restless leg syndrome.30 On the other hand, patients with preserved RUO benefit from less profound inflammation,31–34 malnutrition,35,36 anaemia and present with fewer hypotensive episodes during HD procedures.18,28,34,37 As shown above, RRF has a positive impact on reducing many of the morbidity and mortality risk factors in ESRD patients.16
Patients treated with dialysis suffer from declining renal function.8,10,36 The loss of RRF progresses more rapidly in patients undergoing maintenance haemodialysis than in those on peritoneal dialysis.38 Preserving RUO should be an important goal for physicians involved in the care of patients on dialysis. It can be achieved in many ways, including using biocompatible dialysis membrane and ultra-pure dialysate, avoiding nephrotoxins, prescribing ACE inhibitors and/or calcium blockers, avoiding volume depletion and hypotension. Incremental treatment starting with fewer HD sessions per week can also be a viable method of preserving residual renal function.21,37
This study aims to show how the presence of RUO impacts the frequency of CV events, CV and all-cause mortality, as well as other variables influencing the prognosis of ESRD patients undergoing maintenance haemodialysis.
Materials and Methods
Study Design
We gathered a group of 485 patients 18 years and older (191 females, 294 males) suffering from ESRD and undergoing maintenance HD with renal replacement therapy vintage lasting at least 4 weeks. This was a prospective, observational, multicentric study conducted in 7 separate dialysis centers located in western part of Poland. The period of recruitment lasted 5 years. Patients with temporary HD catheter access were not eligible for participation. We also excluded subjects with poor short-term prognosis, implantable cardioverter defibrillators and other subcutaneous devices, amputees, and patients with prosthetic joint replacement. Each patient was informed about the risks and benefits of participating in the study and signed a consent form. The study protocol was approved by the Poznan University of Medical Sciences Bioethical Committee (approval number 423/13) and complies with the Declaration of Helsinki. The study was initiated with the qualification, which included medical history analysis, blood sampling, physical examination, and hydration assessment. Then the follow-up started, which lasted for 24 months or was concluded earlier in case of a kidney transplant, HD therapy disqualification, conversion to peritoneal dialysis or death. In cases of patients receiving a kidney transplant the medical centers that carried through the procedure adhered to the highest ethical standards, and the transplants were conducted with the utmost consideration for patient well-being and informed consent, and in accordance with the principles outlined in the Declaration of Istanbul.
The main objective of the study was to assess the impact of residual urine output on cardiovascular and overall prognosis in patients with ESRD undergoing maintenance HD. The secondary objective was to evaluate the influence of residual urine output on other parameters that have a direct or indirect impact on the prognosis and wellbeing in patients with ESRD, such as electrolyte balance, hydration status, occurrence of malnutrition, mineral-bone disorders, anaemia and markers of cardiovascular damage.
Residual Renal Function Assessment
In our analysis, we used residual urine output (RUO) as a measure of RRF. To evaluate the volume of urine output, every patient was asked to perform three 24-hour urine collections during non-dialysis days; then, the results were averaged.
Fluid Overload Assessment
For hydration assessment, whole-body bioimpedance spectrum analysis (BIA) was conducted using Body Composition Monitor (BCM, Fresenius Medical Care Deutschland GmbH, Bad Homburg, Germany). The measurements were performed shortly before the mid-week HD session in the supine position using four disposable electrodes attached to a patient’s hand and foot contralateral to the location of the arteriovenous shunt (when present), as per the device manual.
Bloodwork
Blood samples were taken before the mid-week HD session. The panel included: a complete blood count with haemoglobin [Hb, g/dL], albumin [Alb, g/dL], total cholesterol [TC, mg/dL], serum N-terminal pro-brain natriuretic peptide (NT-proBNP, Elecsys® assay, Roche Diagnostics, Basel, Switzerland) and cardiac troponin T (cTnT, Elecsys® Troponin T fourth generation assay, Roche Diagnostics, Basel, Switzerland). HD efficacy was assessed with Kt/V, ultrafiltration rate (UFR, [mL]) and weekly HD dose expressed as the total weekly timespan of HD (HDD, [h/week]).
Subgroups
The entire cohort (n=485) was divided into four groups based on the RUO volume. The first group (n=121) consisted of anuric patients (defined as less or equal to 100mL per day). The second group consisted of patients with daily RUO higher than 100mL up to 500mL (n=118), the third group contained patients with daily urine volume from over 500mL up to 1000mL (n=121) and the fourth group was made up of patients with RUO over 1000mL (n=125) respectively. The threshold between subgroups was chosen based on previous publications on this matter.8,9,18,23,24,28,29,33,34
Statistical Evaluation
Statistical analysis was performed using StatSoft, Inc. (2014) STATISTICA (data analysis software system) version 12 (www.statsoft.com). Since most of the data did not show normal distribution (assessed with the Shapiro–Wilk test), the analysis employed non-parametric tests only. The presence of statistically significant differences between the studied groups was tested using the Kruskal–Wallis test. The chi-square test (Χ2) was employed to test qualitative data, whereas the significance of correlations was verified with the Spearman test. Survival analysis was based on Kaplan-Meier curves which were tested with long rank test. A p-value lower than 0.05 was considered significant. The values throughout the paper are presented using medians (first quartile-third quartile) unless noted otherwise.
Results
Group Characteristics
The median age of the entire cohort was 67.2 (58.2–76.3) years. The most common cause of ESRD in the studied cohort was diabetic kidney disease (n=111, 22.9%), ischemic nephropathy (n=57, 11.6%), chronic glomerulonephritis (n=54, 11.1%) and hypertensive nephropathy (n=53, 10.9%). Patient demographics and median values for studied parameters in the entire cohort can be found in Table 1. The median observation time lasted 24.0 (11.0–24.0) months, with a median HD therapy vintage of 52.2 (33.0–78.8) months. During the study, 223 patients (46.0%) reached one of the designed endpoints: 171 of them died (35.3%), 34 received kidney transplants (7.0%), 4 subjects were disqualified from HD therapy (0.8%), 12 patients (2.5%) were transferred to another HD centre, and 2 patients (0.4%) were converted to peritoneal dialysis. Residual urine output was not correlated with age (Spearman r=0.046, p=0.310) but was significantly lower in patients with longer HD vintage (Spearman r=−0.139, p=0.002).
 |
Table 1 Patient Demographics and Mean Values for Studied Parameters in the Entire Cohort and Urine Output Subgroups |
Subgroups
The median values of tested parameters in subgroups are also summarised in Table 1. Statistical analysis showed no significant differences with respect to patients’ age between the subgroups. There was a male dominance of patients with residual urine output above 500mL (p=0.0113), but there were also more males in the entire cohort (298 vs 191). Electrolyte disturbances, including hyperkalaemia and hyperphosphatemia, were most significant in the anuric group. The patients with daily RUO volume under 100mL also had the highest concentration of cardiac damage markers (NTproBNP and cTnT). ECW/TBW and ICW/TBW ratio was most favourable in the group with the highest RUO and most detrimental in the second group (RUO volume between 100 and 500mL per day).
Correlations
The correlation analysis has been performed on the entire cohort (n=485). The data showed a positive correlation between RUO and total cholesterol (p=0.0008), sodium (p=0.02757) and calcium levels (p=0.01096). There was a negative connection between RRF and overhydration (p=0.0295), NTproBNP (p<0.0001, TnT (p<0.0001), osteocalcin (p=0.00477), potassium (p=0.00037) and phosphates (p=0.00144) levels (for more details see Table 2).
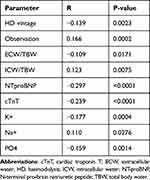 |
Table 2 Correlations Between Studied Parameters and Residual Urine Output |
Mortality
During the 2-year follow-up, 171 patients died (35.3%), and the remaining 262 patients (54%) were still under observation at the end of the study. During the first year of observation, 61 patients died, which sets the mortality rate at 12.6%; in the second year, the mortality rate reached 22.9% (n=111). Most fatalities occurred secondary to cardiovascular events (60.7% of all deaths). The mortality rate was significantly higher in groups with lower RRF (p=0.00163). The highest all-cause mortality has been noted as expected in the first – anuric group (total mortality rate of 35.5%). There were no evident differences between the second (22.5%) and the third group (24.6%). The lowest mortality rate was present in the group with the highest RUO (14.4%). For more details, see Table 1. For Kaplan-Meier survival analysis, see Figures 1 and 2.
 |
Figure 1 Kaplan-Meier curves for all-cause mortality in the studied cohort. Long rank p<0.00039. |
 |
Figure 2 Kaplan-Meier curves for cardiovascular mortality in the studied cohort. Long rank p<0.00067. |
Discussion
Preserving residual renal function plays an important role in achieving therapeutic goals in ESRD patients undergoing RRT and reducing their morbidity and mortality rates. Many studies conducted thus far prove multiple positive results of maintaining RUO. Our study showed a significant mortality rate increase following the reduction of RUO volume. Other authors ended up with similar results.8,16–20 In their study, Shafi et al18 analysed the difference in mortality rate in patients starting their HD treatment based on urine output preservation during a one-year observation. They proved that baseline urine output was not associated with a lower mortality rate, but the presence of RUO at one year mark, meaning RRF preservation, correlated significantly with lower mortality. Shemin et al8 highlighted an interesting fact of a more rapid decline in residual renal function in women compared to men.
CV events are the most common cause of death in ESRD patients, as was proven multiple times. In their article, Ma and Ding24 presented the negative correlation between RUO and the concentration of serum BNP, homocysteine and left ventricular hypertrophy in patients undergoing maintenance HD. The results also indicated that patients with preserved RRF have better left ventricular function. Similar conclusions could be found in the article by Araujo et al,23 where they described a negative correlation between RUO and the total peripheral resistance index and stated that patients in the group with preserved residual urine output (RUO+) exhibited a significantly higher cardiac index. Similarly, our results showed that RRF was negatively correlated with both serum NTproBNP and TnT. The highest concentration of both of those markers was present in the anuric subgroup. In their study, Wang et al39 assessed the efficacy of 10 biomarkers in predicting mortality and major cardiovascular events in a cohort of around 3000 individuals monitored for up to 10 years. They observed that the most informative biomarker for both predicting death and major cardiovascular events was B-type natriuretic peptide. What is also interesting from a nephrological point of view is that one of the most informative biomarkers in both groups was the urinary albumin-to-creatinine ratio. Daniels et al investigated the predictive significance of detectable cardiac troponin T (TnT) and elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels in a sample of older adults. In the examined cohort, higher TnT was associated with higher NT-proBNP levels and the concentration of both those markers was positively correlated with reduced creatinine clearance. Subjects who exhibited detectable cardiac troponin T (TnT) levels had a higher mortality rate. Elevated levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) were found to be predictive of both all-cause mortality and cardiovascular mortality. Notably, participants with both high NT-proBNP levels and detectable TnT levels experienced the most significant increase in mortality risk.40 In their works, de Lemos et al, de Filippi et al, and Saunders et al came to similar conclusions. They showed that cTnT detected with a highly sensitive assay in the general population was associated with structural heart disease, incident heart failure, incident coronary heart disease, the subsequent risk of cardiovascular death and all-cause mortality.41–43
Hyperphosphatemia is considered to be one of the risk factors for increased cardiovascular mortality among dialysis patients. Iwasawa et al,14 in their study conducted on a group of HD patients with preserved RRF, presented evidence that urinary phosphate excretion strongly correlated with GFR, which in turn correlated with urine volume. They also showed that mean serum phosphate and FGF-23 were significantly lower in the group with higher GFR. Furthermore, serum intact PTH, urine phosphate, tubular reabsorption of phosphate and tubular maximum reabsorption for phosphate corrected for GFR were more elevated in the group with lesser RRF. Penne et al28 made similar observations where patients with RRF suffered from hyperphosphatemia significantly less often despite using lower doses of phosphate-binding agents. Ma and Ding24 showed that patients with RUO had lower serum phosphate and parathormone levels while maintaining higher serum calcium levels. In their study Almeida et al29 also showed that patients with RRF have lower phosphatemia values. Moreover, they presented that the group without RRF had significantly higher potassium levels increase during the long interdialytic interval. Those patients also exhibited lower sodium levels, and a greater percentage of patients with natremia below 137 mmol/l was present in the anuric group. Almedia et al29 also suggested that patients without RRF are more likely to have lower bicarbonate levels and inadequate respiratory response to metabolic acidosis. These patients also showed higher interdialytic weight gain, which could be an explanation for the inadequate respiratory response. In our study, patients with preserved RUO had lower serum phosphate levels with higher calcium concentration. The group with daily urine output over 1000mL had the lowest PTH values; however, the correlation analysis between RUO and PTH levels showed no statistical significance. On the other hand, the negative correlation between RRF and serum potassium concentration was very strong. Not as strong, but still significant, was the positive correlation with serum sodium levels. The patients with the highest RUO also showed the highest sodium concentration compared to the three other groups. In other studies, lower sodium levels were associated with higher mortality.44 We have not examined the correlation of acid-base balance in our patients. However, similarly to other authors,29 we found that patients without RRF suffered from more severe overhydration. We have no statistically significant correlation between RUO and blood pressure values. However, a group of Polish authors45 described an elevated level of serum norepinephrine in haemodialysed patients, especially anuric ones, which in turn led to higher blood pressure values. Interestingly, de Sequera et al34 showed that patients with RRF suffered from fewer hypotensive episodes. They found no correlation between hypotensive episodes and intradialytic weight gain, the patients’ hydration status or the ultrafiltration amount during HD sessions. That is why in their opinion, the possible explanation for fewer hypotensive episodes may be the better condition of the vascular system in patients with RUO.
Patients with ESRD have elevated levels of inflammation markers which are independent predictors of increased mortality. Pecoits-Filho et al31 conducted a study on a group of patients with stage 5 chronic renal failure shortly before initiating RRT. The entire cohort was divided into 2 groups: the first included patients with GFR lower than 6.5mL/min/1.73m2, while the second included patients with GFR ranging from 6.5 to 16.5mL/min/1.72m2. They found that the first group had significantly greater hsCRP, neopterin and hyaluronian levels, while differences in S-albumin, IL-6 and TNF-a were not statistically significant. They also described significant negative correlations between GFR and hsCRP, IL-6, hyaluronian, and neopterin. Borges et al33 and de Sequera et al34 presented similar results, showing that patients with RRF undergoing haemodialysis had lower hsCRP levels. In our study, there were no significant differences in CRP levels between the groups as well as no significant correlation between RUO and CRP.
In nearly all research studies that have assessed the nutritional status of individuals with end-stage renal disease (ESRD), a consistent finding has been the presence of malnutrition to varying degrees.46 Malnutrition has been found to be linked with various negative consequences, including apathy, depression, fatigue, and diminished motivation to recover. Furthermore, the decline in muscle strength resulting from malnutrition can adversely affect respiratory function, making individuals more vulnerable to chest infections. Additionally, malnutrition can negatively impact cardiac function. Furthermore, impaired immune function associated with malnutrition raises the risk of infections. Ultimately, these complications contribute to a higher incidence of illness and death.47,48 RUO positively impacts nutritional status in ESRD patients undergoing maintenance haemodialysis, as shown in a study by Suda et al.34 In a group of over 100 patients, they proved that serum albumin was positively correlated with RRF and was not associated with dialysis Kt/V urea. Furthermore, they described a positive connection between RUO and body fat percentage and normalised protein catabolic rate, indicating an overall better nutritional status. In our group of patients, there was no significant correlation between serum albumin and RRF. However, RUO was positively associated with higher lean tissue mass (p=0.007629) and lean tissue index (p=0.048465). Several authors stated in their studies that lower LTI is correlated with higher morbidity and mortality in ESRD patients undergoing maintenance haemodialysis. On top of that, Marceli et al showed that not only LTI but likewise FTI falling within the 10th–90th percentile range of a healthy population matched for age and sex were associated with the most favourable survival outcomes. Conversely, individuals with low FTI and low LTI, particularly when both conditions were present together, exhibited higher mortality rates. Interesting observations were made by Trimarchi et al,49 who, in their study, stated that higher BMI correlated with higher urinary output and a slower decline in urine output. Subjects with high BMI displayed higher urine output, albumin, leptin and insulin levels. There was a notable relationship between higher urinary outputs and lower levels of cardiac biomarkers, specifically troponin T and pro-B-type natriuretic peptide. Moreover, there was an inverse and significant correlation between albumin levels and TnT and Pro-BNP levels. Naturally, in the studied cohort, insulin levels increased in parallel with BMI. They proposed that the ability of insulin to retain salt and water may trigger pressure-diuresis and a maintained urinary output. It is possible that higher levels of albumin further enhance this effect. On the other hand, Drechsler et al50 showed that during their observation of patients through the first 18 months after the initiation of dialysis therapy, obesity and overweight significantly increased the decline in kidney function. Kalantar-Zadeh et al, in a review article, stated that overweight (BMI: 25–30) or obesity (BMI: >30) in patients with chronic kidney disease undergoing maintenance hemodialysis or high BMI, in general, is associated with improved survival.51
Many authors also explored the connection between RRF and anaemia management in the ESRD patients undergoing RRT. It was proven that patients with RUO have lower requirements for erythropoiesis-stimulating agents.28,34 We found no significant correlation between RUO and haemoglobin levels or any other marker of the iron economy like serum Fe, ferritin, TIBC and TSAT.
Numerous approaches have been documented on how to preserve RUO in patients undergoing maintenance haemodialysis, including avoiding nephrotoxins, maintaining euvolemia and avoiding volume depletion and managing blood pressure. Medication such as angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin receptor blockers (ARBs), and calcium blockers may be prescribed to help manage blood pressure and preserve kidney function. Other important examples are using biocompatible dialysis membrane and ultra-pure dialysate and optimising dialysis prescription, including dialysis duration, frequency, and dose.21,37
Study Limitations
The main factor that could disturb the reliability of the measurements is the fact that the daily urine collections were performed by the patients themselves, without any medical supervision, which is considered a standard routine practice. However, this could lead to measurement errors and improper allocation of some patients into specific groups. Moreover, there was no re-assessment of the urinary output during the study’s follow-up period. The lack of correlation between RUO and inflammation in our study may be caused by the fact that the evaluation of systemic inflammation was limited to CRP and complete blood count.
Conclusions
The preservation of residual urine output in ESRD patients undergoing maintenance haemodialysis is invaluable in achieving therapeutic goals, reducing morbidity, and lowering mortality rates. Multiple studies have demonstrated the positive outcomes associated with maintaining RRF. RUO has shown beneficial effects on various aspects of cardiovascular health. It has been linked to lower levels of cardiac biomarkers, such as troponin T and pro-B-type natriuretic peptide, indicating improved cardiac function. Patients with preserved RRF have been found to have better left ventricular function and less frequent left ventricular hypertrophy. Hyperphosphatemia, a common risk factor for increased cardiovascular mortality in dialysis patients, has been less prevalent among individuals with RRF. These patients exhibited lower serum phosphate levels as well as higher serum calcium levels. Furthermore, RRF has been correlated with improved potassium and sodium homeostasis. Maintaining RRF has shown positive effects on nutritional status, anaemia management and limiting chronic inflammation. Overall, the evidence strongly supports the importance of preserving residual urine output in ESRD patients undergoing RRT.
Acknowledgments
We would like to extend our sincere gratitude to Dr Tomasz Paciorkowski for language editing.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. doi:10.1161/01.CIR.0000095676.90936.80
2. Go AS, McCulloch CE. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;10:1.
3. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–2648. doi:10.1681/ASN.2007020220
4. Baber U, Stone GW, Weisz G, et al. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S53–61.
5. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12:7–11. doi:10.1016/j.kisu.2021.11.003
6. Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18:378–395. doi:10.1038/s41581-022-00542-7
7. Dębska-ślizień A, Jagodziński P, Rutkowski P, et al. Current status of renal replacement therapy in Poland in 2022. NEFROL DIAL POL. 2022;2022:21–38.
8. Shemin D, Bostom AG, Laliberty P, Dworkin LD. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38:85–90. doi:10.1053/ajkd.2001.25198
9. Brener ZZ, Thijssen S, Kotanko P, et al. The impact of residual renal function on hospitalization and mortality in incident hemodialysis patients. Blood Purif. 2011;31:243–251. doi:10.1159/000322252
10. Obi Y, Rhee CM, Mathew AT, et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol. 2016;27:3758–3768. doi:10.1681/ASN.2015101142
11. Lowenstein J, Grantham JJ. Residual renal function: a paradigm shift. Kidney Int. 2017;91:561–565. doi:10.1016/j.kint.2016.09.052
12. Moist LM, Port FK, Orzol SM, et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi:10.1681/ASN.V113556
13. Lemes HP, Araujo S, Nascimento D, et al. Use of small doses of furosemide in chronic kidney disease patients with residual renal function undergoing hemodialysis. Clin Exp Nephrol. 2011;15:554–559. doi:10.1007/s10157-011-0427-z
14. Iwasawa H, Nakao T, Matsumoto H, Okada T, Nagaoka Y, Wada T. Phosphate handling by end-stage kidneys and benefits of residual renal function on phosphate removal in patients on haemodialysis: phosphate handling by RRF in HD patients. Nephrology. 2013;18:285–291. doi:10.1111/nep.12039
15. Milutinovic J, Cutler RE, Hoover P, Meijsen B, Scribner BH. Measurement of residual glomerular filtration rate in the patient receiving repetitive hemodialysis. Kidney Int. 1975;8:185–190. doi:10.1038/ki.1975.98
16. Brener ZZ, Kotanko P, Winchester JF, Thijssen S, Bergman M. Clinical benefit of preserving residual renal function in dialysis patients: an update for clinicians. Am J Med Sci. 2010;339:453–456. doi:10.1097/MAJ.0b013e3181cf7d5b
17. Rădulescu D, Ferechide D, Davila C, Ioan S. The importance of residual renal function in chronic dialysed patients 2. J Med Life. 2009;2(2):199.
18. Shafi T, Jaar BG, Plantinga LC, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the choices for healthy outcomes in caring for end-stage renal disease (CHOICE) Study. Am J Kidney Dis. 2010;56:348–358. doi:10.1053/j.ajkd.2010.03.020
19. Wang AY-M, Lai K-N. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69:1726–1732. doi:10.1038/sj.ki.5000382
20. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009;53:1068–1081. doi:10.1053/j.ajkd.2009.02.012
21. Fernández-Lucas M, Teruel-Briones JL, Gomis-Couto A, Villacorta-Pérez J, Quereda-Rodríguez-Navarro C. Mantenimiento de la función renal residual en hemodiálisis: experiencia de 5 años de una pauta de diálisis incremental [Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen]. Nefrología. 2012;32(6):767–776. doi:10.3265/Nefrologia.pre2012.Jul.11517
22. Scarpioni R, Ricardi M, Albertazzi V, De Amicis S, Rastelli F, Zerbini L. Dialysis-related amyloidosis: challenges and solutions. Int J Nephrol Renov Dis. 2016;9:319–328. doi:10.2147/IJNRD.S84784
23. Araujo S, Lemes HP, Cunha DA, et al. Cardiac morphology and function in patients with and without residual diuresis on hemodialysis. Brazilian. 2011;8:74–81.
24. Ma T, Ding G. Effects of residual renal function on left ventricle and analysis of related factors in patients with hemodialysis. Ren Fail. 2013;35:198–203. doi:10.3109/0886022X.2012.745153
25. Artunc F, Mueller C, Breidthardt T, et al. Sensitive troponins – which suits better for hemodialysis patients? Associated factors and prediction of mortality ed W-H schunck. PLoS One. 2012;7:e47610. doi:10.1371/journal.pone.0047610
26. Bragg-Gresham JL, Fissell RB, Mason NA, et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the dialysis outcomes and practice pattern study (DOPPS). Am J Kidney Dis. 2007;49:426–431. doi:10.1053/j.ajkd.2006.12.012
27. Helal I, Belhadj R, Mohseni A, et al. Clinical significance of N-terminal Pro-B-type natriuretic peptide (NT-proBNP) in hemodialysis patients. Saudi J Kid Dis Transpl. 2010;21(2):262–268.
28. Penne EL, van der Weerd NC, Grooteman MPC, et al. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:281–289. doi:10.2215/CJN.04480510
29. de Almeida LLS, Sette LH, Fonseca FLA, da Bezerra LS, Oliveira Júnior FH, Bérgamo RR. Metabolic and volume status evaluation of hemodialysis patients with and without residual renal function in the long interdialytic interval. Braz J Nephrol. 2019;41:481–491. doi:10.1590/2175-8239-jbn-2018-0171
30. Pizza F, Persici E, La Manna G, et al. Family recurrence and oligo-anuria predict uremic restless legs syndrome: clinical predictors of RLS in hemodialysis patients. Acta Neurol Scand. 2012;125:403–409. doi:10.1111/j.1600-0404.2011.01581.x
31. Pecoits-Filho R, Heimbürger O, Bárány P, et al. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis. 2003;41:1212–1218. doi:10.1016/S0272-6386(03)00353-6
32. Naskalski JW, Kapusta M, Fedak D, et al. Effect of hemodialysis on acid leukocyte-type ribonuclease, alkaline ribonuclease and polymorphonuclear elastase serum levels in patients with end-stage renal disease. Nephron Clin Pract. 2009;112:c248–54. doi:10.1159/000224791
33. Borges DL, Lemes HP, de Castro Ferreira V, Filho SRF. High-sensitivity C-reactive protein, apolipoproteins, and residual diuresis in chronic kidney disease patients undergoing hemodialysis. Clin Exp Nephrol. 2016;20:943–950. doi:10.1007/s10157-016-1230-7
34. de Sequera P, Corchete E, Bohorquez L, et al. Residual renal function in hemodialysis and inflammation: RRF in HD and inflammation. Ther Apher Dial. 2017;21:592–598. doi:10.1111/1744-9987.12576
35. Suda T, Hiroshige K, Ohta T, et al. The contribution of residual renal function to overall nutritional status in chronic haemodialysis patients. Nephrol Dial Transplant. 2000;15:396–401. doi:10.1093/ndt/15.3.396
36. Nechita AM, Rădulescu D, Peride I, et al. Determining factors of diuresis in chronic kidney disease patients initiating hemodialysis 8. J Med Life. 2015;8(3):371.
37. Merino JL, Domínguez P, Bueno B, Amézquita Y, Espejo B, Paraíso V. Application of model of incremental haemodialysis, based on residual renal function, at the initiation of renal replacement therapy. Nefrol Engl Ed. 2017;37:39–46.
38. Misra M, Vonesh E, Churchill DN, Moore HL, Van Stone JC, Nolph KD. Preservation of glomerular filtration rate on dialysis when adjusted for patient dropout. Kidney Int. 2000;57:691–696. doi:10.1046/j.1523-1755.2000.00891.x
39. Wang TJ, Tofler GH, Jacques PF, Rifai N, Benjamin EJ, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi:10.1056/NEJMoa055373
40. Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults. J Am Coll Cardiol. 2008;52:450–459. doi:10.1016/j.jacc.2008.04.033
41. de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503.
42. deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494. doi:10.1001/jama.2010.1708
43. Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the atherosclerosis risk in communities study. Circulation. 2011;123:1367–1376. doi:10.1161/CIRCULATIONAHA.110.005264
44. Hecking M, Karaboyas A, Saran R, et al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7:92–100. doi:10.2215/CJN.05440611
45. Zbroch E, Koc-Zorawska E, Malyszko J, Malyszko J, Mysliwiec M. Circulating levels of renalase, norepinephrine, and dopamine in dialysis patients. Ren Fail. 2013;35:673–679. doi:10.3109/0886022X.2013.778754
46. Ikizler TA, Hakim RM. Nutrition in end-stage renal disease. Kidney Int. 1996;50:343–357. doi:10.1038/ki.1996.323
47. Raslan M, Gonzalez MC, Gonçalves Dias MC, et al. Comparison of nutritional risk screening tools for predicting clinical outcomes in hospitalized patients. Nutrition. 2010;26:721–726. doi:10.1016/j.nut.2009.07.010
48. McWhirter JP, Pennington CR. Incidence and recognition of malnutrition in hospital. BMJ. 1994;308:945–948. doi:10.1136/bmj.308.6934.945
49. Trimarchi H. Residual urinary output in high body mass index individuals on chronic hemodialysis: a disregarded life vest? World J Nephrol. 2014;3:317. doi:10.5527/wjn.v3.i4.317
50. Drechsler C, de Mutsert R, Grootendorst DC, et al. Association of body mass index with decline in residual kidney function after initiation of dialysis. Am J Kidney Dis. 2009;53:1014–1023. doi:10.1053/j.ajkd.2008.11.027
51. Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi:10.1093/ajcn/81.3.543
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
