Back to Journals » Research and Reports in Urology » Volume 15
Basal Cell Carcinoma of the Prostate Misdiagnosed as High-Grade Urothelial Cancer – A Case Report of a Diagnostic Pitfall
Authors Taskovska M , Frelih M, Smrkolj T, Volavšek M
Received 17 February 2023
Accepted for publication 12 May 2023
Published 9 June 2023 Volume 2023:15 Pages 187—192
DOI https://doi.org/10.2147/RRU.S391558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Video abstract of "Prostate BCC misdiagnosed as urothelial cancer" [ID 391558].
Views: 43
Milena Taskovska,1 Maja Frelih,2 Tomaž Smrkolj,1 Metka Volavšek2
1Chair of Surgery, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia; 2Institute of Pathology, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Correspondence: Metka Volavšek; Milena Taskovska, Email [email protected]; [email protected]
Purpose: Basal cell carcinoma of the prostate is rare. Usually, it is diagnosed in elderly men with nocturia, urgency, lower urinary tract obstruction and normal PSA.
Case Presentation: We report on a case of a 56-years-old patient who presented at the emergency ward with weight loss, nausea and vomiting. The diagnostic evaluation showed acute renal failure due to a bladder tumor. After admission to the urology ward and subsequent contrast-enhanced CT urography and contrast-enhanced chest CT, a non-metastatic bladder tumor that infiltrated the right side of the bladder and seminal vesicles was found. High-grade muscle-invasive urothelial carcinoma was diagnosed from TURBT specimens, followed by radical cystoprostatectomy with pelvic lymphadenectomy and formation of ureterocutaneostomy sec. Bricker. The histopathological examination of the resection specimen surprisingly revealed the presence of prostatic basal cell carcinoma pT4N0M0 and not urothelial cancer. Due to renal failure, the patient required hemodialysis. The recommendation of the multidisciplinary oncological meeting was to follow up with the patient by the surgeon-urologist. On imaging six months after surgery, it was suspicious for recurrence. Patient was considered for adjuvant oncological treatment.
Conclusion: Although rare, basal cell carcinoma of the prostate should be considered in patients with lower urinary tract symptoms, hematuria and normal PSA. Transurethral resection of bladder tumor is indicated in patients presenting with hematuria and bladder tumor. In evaluation of such cases rare histological types should be included in the differential diagnosis.
Keywords: prostate cancer, basal cell carcinoma of the prostate, renal failure, bladder tumor, transurethral resection of bladder tumor, cystoprostatectomy
Introduction
Basal cell carcinoma (BCC) of the prostate is a rare neoplasm. It usually presents with a normal prostate-specific antigen (PSA). Visceral metastases are more common than bone metastases.1,2 Rare case reports and case series in the literature describe different clinical outcomes of prostatic BCC and there is no standard treatment.1 To our knowledge, BCC of the prostate infiltrating a high proportion of the urinary bladder including both orificia and resulting in renal failure has not yet been described. In this paper, we present a patient with BCC of the prostate who presented with renal failure as a leading symptom and the challenges we faced during histopathological evaluation.
Case Presentation
A 56-year-old patient presented in the emergency room with weight loss, nausea, vomiting and loss of appetite. He was previously healthy, without medications, and a smoker for 30 years, 10–15 cigarettes/day. Clinical examination was within normal limits. Complete blood work was taken – creatinine 2673 µmol/L, urea 76.6 mmol/L, hemoglobin 73 g/L, leukocytes 8×10^9/L, thrombocytes 352×10^9/L, C-reactive protein 78 mg/L, S-potassium 5.3 mmol/L, S-sodium 140 mmol/L. An emergency abdominal ultrasound was done, which showed normal kidney size, reduced renal parenchyma, especially on the right side and bilateral hydronephrosis. Inside the bladder, there was a tumor infiltrating both ostia.
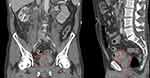
The patient was admitted to the urology ward. Bilateral percutaneous nephrostomies were inserted upon admission. The patient had regular hemodialysis and his creatinine level decreased. Six days after admission, contrast-enhanced CT urography and contrast-enhanced CT of the thorax were done. They showed a large, right-sided bladder tumor that infiltrated about half of the bladder and the seminal vesicles (Figure 1). The enlarged para-aortic aortocaval lymph nodes looked reactive. There were no signs of metastases in the thorax.
On the 8th day after admission, transurethral resection of bladder tumor (TURBT) was performed. During the procedure, we found a large superficially necrotic bladder tumor, which infiltrated both ostia; the right side of the bladder was completely infiltrated. Samples were taken from the area of the trigonum and the right side of the bladder.
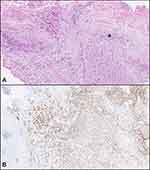
Histopathological examination of TURBT specimens with extensive cautery artifacts showed poorly differentiated carcinoma (Figure 2A). Small tumor cells with hyperchromatic nuclei and scant cytoplasm, arranged in irregular nests or as single cells were invading the lamina propria and muscularis propria. GATA3 was the only immunohistochemical marker used and because of its positivity (Figure 2B), the diagnosis of invasive high-grade urothelial carcinoma pT2 was made.
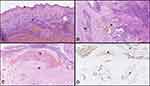
During hospitalization, the patient received antibiotic therapy for urinary tract infection based on urine culture, followed by radical cystoprostatectomy with pelvic lymphadenectomy and Bricker ileocutaneous derivation on the 17th day of admission. After radical cystoprostatectomy, the tumor was extensively sampled. Histopathological examination revealed a kind of dual growth pattern. The tumor cells in luminal portions of the bladder were growing in small irregular nests and as single cells (Figure 3A), but in deeper portions of the bladder wall, the tumor cells were growing in solid nests and cords accompanied by eosinophilic basement membrane-like material. In other areas, the microcystic architectural pattern was also present (Figure 3B). There were fibromyxoid stromal reaction, extensive intratumoral hemorrhage in superficial portions of the tumor, and massive perineural invasion. The pattern of growth of small basaloid cells was indicating basal cell carcinoma.
On thorough histopathological examination, it was evident that the tumor was originating in the transition zone of the prostate (Figure 3C), spreading to the bladder neck and occupying almost the whole bladder, more on the right side as seen on the CT scan. In the bladder, it was infiltrating muscularis propria, both ostia and perivesical fat, through which it spread downwards to fat surrounding seminal vesicles and prostate. From the periprostatic fat, it was “returning to the prostate” by infiltrating its outer portions. No urothelial carcinoma in situ was identified (Figure 3A). Immunohistochemically, the tumor was positive for CK34βE12, CK7, CK 5/6, p63, GATA3 and negative for PSA, NKX3.1, AMACR, CK20, Bcl2, and neuroendocrine markers. Ki-67 proliferative index was uneven, in some areas up to 40%. The immunohistochemical profile of the tumor was therefore consistent with basal cell carcinoma of the prostate.
Postoperative PSA was 0.05mcg/l (10th postoperative day). The patient was discharged from the ward on the 14th postoperative day (the 30th day after admission). Before discharge, percutaneous nephrostomies and ureteric catheters were removed. The wound was healing per primam without signs of infection. During hospitalization, he was enrolled in an education programme for urostomy care. After discharge, the patient continued with the hemodialysis programme, AV fistula was formed.
Patients´ documentation was presented at the uro-oncological multidisciplinary meeting, which recommended follow-up by the surgeon – urologist. Postoperative follow-up of six months was within expectations. PSA was undetectable, and the patient was hemodialysis dependent, without local recurrence. After six months, contrast enhanced CT urography was performed which showed enlarged lymph node in the right iliac fossa. He also reported pain in the right inguinal region. The lymph node was inaccessible for needle biopsy. We did not decide on surgical removal of the enlarged lymph node since the risk for complications was high. PSA was undetectable, cytology was negative. Patient was presented at the urology-oncology multidisciplinary meeting. Since the patient had end-stage renal disease and was dialysis dependent, he was not a candidate for chemotherapy, but considered for further molecular diagnostics and targeted immunotherapy. During the manuscript processing, the patient died of metastatic disease 17 months after disease presentation. The autopsy was not performed.
Discussion
BCC of the prostate is a rare tumor. The first case of prostatic BCC was published in 1974; to date, there are no more than 150 cases reported in the literature.3,4 Usually, it is detected in patients aged 50 to 70 years, mostly arises in the transitory zone and the patients present with nocturia, urgency and urinary obstruction.3,5,6 PSA is usually not elevated. Patients with elevated PSA usually have concomitant adenocarcinoma of the prostate.5 Gleason grading for BCC is not applicable, as by its unique nature BCC is not a prostatic adenocarcinoma.7 In our case, the tumor was growing from the transitory zone of the prostate to the trigonum of the bladder and was infiltrating both ostia and posteriorly the seminal vesicles.
Due to its rarity, BCC is treated as adenocarcinoma of the prostate.3 It can present as an indolent tumor with long-term survival with non-curative treatment or as an aggressive tumor, prone to metastasis or recurrence.3,8 High proliferative activity by Ki67 staining may suggest aggressive behavior.5 The predominant basaloid component may be associated with a poor prognosis.3,8
BCC of the prostate is morphologically diverse. It can be considered as 1) an adenoid cystic carcinoma, which is histologically similar to a salivary gland tumor; 2) basaloid cell carcinoma, which is histologically similar to a basal cell carcinoma of the skin; or 3) mixed (contains both components), which is encountered in most cases, ours as well.3,8 The adenoid cystic pattern is characterized by cylinders of hyalinized or mucinous stroma, surrounded by nests of small epithelial cells, which have a perforated, sieve-like or cribriform appearance.5 The basaloid pattern is characterized by irregular solid clumps, trabeculae and large cellular masses of basaloid cells. Tumor cell nuclei are small and hyperchromatic, cytoplasm is scant, forming small nests in a peripheral palisading pattern.5,9 In the latest 5th edition of World Health Organization (WHO) classification of genitourinary tumors, the term adenoid-cystic (basal cell) carcinoma of the prostate has been introduced for these tumors.10 Characteristics of BCC of the prostate are infiltrative permeation, extraprostatic extension, perineural invasion, necrosis and stromal desmoplasia.
Immunohistochemically, most BCC of the prostate are positive for Bcl-2, CK34βE12, p63, and CK5/6.9 Our case was also positive for HMWCK and p63 but was negative for Bcl2. To the best of our knowledge, there is no data on GATA3 positivity in basal cell carcinoma of the prostate in the literature. It is known, and we also proved it in our case, that some normal prostatic basal cells are GATA3 positive (Figure 3D).11 So, it is therefore expected that basal cell carcinoma of the prostate can be GATA3 positive. GATA3 positivity was very misleading in our case. First, because the patient presented with a bladder mass and second, the histological pattern of tumor cells in the TURBT specimen corresponded with invasive high-grade urothelial carcinoma. Although no IHC markers distinguish BCC from urothelial carcinoma, uroplakin IHC might be helpful, if available.
Mutations in MYB proto-oncogene (MYB), phosphatase and tensin homolog (PTEN), epidermal growth factor receptor (EGFR), and erb-b2 receptor tyrosine kinase 2 (HER-2) genes, ATM, SMARCB1, and PIK3R1 can be present.9
Differential diagnosis of BCC includes »benign« basal cell hyperplasia, poorly differentiated adenocarcinoma and poorly differentiated squamous cell carcinoma. Basal cell carcinoma differs from basal cell hyperplasia by the invasive growth pattern, extensive infiltration, perineural invasion, extraprostatic extension, the presence of necrosis, and elevated Ki67 labelling index. In poorly differentiated adenocarcinoma, negative staining for high-molecular-weight cytokeratin helps in distinguishing it from BCC. Primary squamous cell carcinoma has its characteristics, including epithelial keratinization, obvious intercellular bridges, and lack of acinar structures, which help in differentiation from BCC of the prostate.5 According to data in the literature, BCC of the prostate is more aggressive than acinar adenocarcinomas. The extraprostatic extension is reported in 44–71%, distant metastasis in 14–29% and disease-associated death in 50% of the patients. BCC of the prostate metastasizes most commonly in visceral organs (liver, lungs), rarely in bones.4 In our case, the tumor was limited to the prostate and bladder. During the follow-up after six months, there was an enlarged lymph node in the right iliac fossa. Patient was considered for further molecular diagnostics. Considering his comorbidities, he was not a candidate for chemotherapy. Further oncological treatment is a challenge because of the tumor rarity and patients’ comorbidities. The patient died of metastatic disease 17 months after presentation.
BCC of the prostate can be challenging to diagnose, especially when presenting as bladder mass as in our case. Awareness of this entity and broader immunohistochemical profile can aid in achieving the correct diagnosis.4
Conclusion
BCC of the prostate is a very rare neoplasm. It should be considered in the differential diagnosis of patients who present with normal PSA and have signs of the infiltration of the urinary bladder. Due to its rarity, there are no guidelines for treatment. Such cases should be discussed in multidisciplinary meetings including urologist, oncologist, pathologist and radiologist and patients should be offered the most optimal treatment depending on the extent of the disease.
Ethics and Consent Statement
Written informed consent for publication of his details was obtained from the patient.
No institutional approval was required for publishing the case report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grossman JE, Wu Y, Ye H, Bhatt RS. Case of basal cell carcinoma of the prostate successfully treated before and after a BRCA2 reversion mutation. JCO Precis Oncol. 2018;2:1–5. doi:10.1200/PO.18.00193
2. Begnami MD, Quezado M, Pinto P, Linehan WM, Merino M. Adenoid cystic/basal cell carcinoma of the prostate: review and update. Arch Pathol Lab Med. 2007;131:637–640. doi:10.5858/2007-131-637-ABCCOT
3. Shibuya T, Takahashi G, Kan T. Basal cell carcinoma of the prostate: a case report and review of the literature. Mol Clin Oncol. 2019;10(1):101–104. doi:10.3892/mco.2018.1754
4. He L, Metter C, Margulis V, Kapur P. A review leveraging a rare and unusual case of basal cell carcinoma of the prostate. Case Rep Pathol. 2021;2021:5520581. doi:10.1155/2021/5520581
5. Chang K, Dai B, Kong Y, et al. Basal cell carcinoma of the prostate: clinicopathologic analysis of three cases and a review of the literature. World J Surg Oncol. 2013;11(1):193. doi:10.1186/1477-7819-11-193
6. Pedersen V, Petersen KS, Brasso K, Østrup O, Loya AC. Basal cell carcinoma of prostate with MSMB–NCOA4 fusion and a probable basal cell carcinoma in situ: case report. Int J Surg Pathol. 2021;29(8):850–855. doi:10.1177/10668969211017321
7. Grignon D. Unusual subtypes of prostate cancer. Mod Pathol. 2004;17:316–327. doi:10.1038/modpathol.3800052
8. Ali TZ, Epstein JI. Basal cell carcinoma of the prostate: a clinicopathologic study of 29 cases. Am J Surg Pathol. 2007;31:697–705. doi:10.1097/01.pas.0000213395.42075.86
9. Dong S, Liu Q, Xu Z, Wang H. An unusual case of metastatic basal cell carcinoma of the prostate: a case report and literature review. Front Oncol. 2020;10:859. doi:10.3389/fonc.2020.00859
10. McKenney JK, Iczkowski KA, Parwani AV, van Leenders GJLH. Adenoid cystic (basal cell) carcinoma of the prostate. In: WHO Classification of Tumours. Urinary and Male Genital Tumours.
11. Miettinen M, McCue PA, Sarlomo-Rikala M, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38(1):13–22. doi:10.1097/PAS.0b013e3182a0218f
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.