Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Bardet-Biedl Syndrome: Current Perspectives and Clinical Outlook
Authors Melluso A , Secondulfo F , Capolongo G, Capasso G, Zacchia M
Received 2 November 2022
Accepted for publication 20 January 2023
Published 30 January 2023 Volume 2023:19 Pages 115—132
DOI https://doi.org/10.2147/TCRM.S338653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Andrea Melluso,1 Floriana Secondulfo,1 Giovanna Capolongo,1 Giovambattista Capasso,1,2 Miriam Zacchia1
1Department of Translational Medical Sciences, University of Campania “Luigi Vanvitelli”, Naples, Italy; 2Biogem Scarl, Ariano Irpino, AV, 83031, Italy
Correspondence: Miriam Zacchia, Via Pansini 5, Naples, 80131, Italy, Tel +39 081 566 6650, Fax +39 081 566 6671, Email [email protected]
Abstract: The Bardet Biedl syndrome (BBS) is a rare inherited disorder considered a model of non-motile ciliopathy. It is in fact caused by mutations of genes encoding for proteins mainly localized to the base of the cilium. Clinical features of BBS patients are widely shared with patients suffering from other ciliopathies, especially autosomal recessive syndromic disorders; moreover, mutations in cilia-related genes can cause different clinical ciliopathy entities. Besides the best-known clinical features, as retinal degeneration, learning disabilities, polydactyly, obesity and renal defects, several additional clinical signs have been reported in BBS, expanding our understanding of the complexity of its clinical spectrum. The present review aims to describe the current knowledge of BBS i) pathophysiology, ii) clinical manifestations, highlighting both the most common and the less described features, iii) current and future perspective for treatment.
Keywords: Bardet-Biedl syndrome, ciliopathies, chronic kidney disease, genetics, metabolic disorders
Introduction
The Bardet-Biedl syndrome (BBS) is an inherited disorder affecting multiple organs and systems.
It is a rare condition, and its frequency varies among different geographic areas. The distribution of the syndrome is in fact not homogeneous, as it was noted by Klein and Ammann in Switzerland, in 1969.1 In North America and Europe, it ranges between 1:120.000 and 1:160.000 individuals.2 In some isolated communities, due to increased marriages among consanguineous, it is far higher: 1:36,000 among the mixed Arab population in Kuwait, 1:13,500 among Bedouins, 1:6900 in Jahra district, 1 in 18,000 in Newfoundland and 1:3700 in Faroe Islands.3–5
The BBS is considered a model of non-motile ciliopathy. The latter includes a heterogenous spectrum of conditions characterized by the dysfunction of the primary cilium (PC). At least 35 different ciliopathies have been described; their clinical complexity ranges from multiorgan disorders, even with embryonic lethality, to non-syndromic and late onset forms.6–8
For decades, the PC has been considered only a vestigial organelle; however, in the recent years, it has been shown to play a pivotal role in several signalling pathways regulating important cellular functions, including cellular division, polarity and metabolism. Accordingly, emerging evidence demonstrated a crucial role in several human diseases.9
The PC is a dynamic organelle that appears as a subcellular component extruding from the cell surface and acting as an antenna sensing external stimuli. Its structure consists of a microtubule-based axonema emerging from a basal body and surrounded by a ciliary membrane (Figure 1). The basal body derives from the mother centriole, the major organizing center for mitotic spindles during cell division: in quiescent cells, it serves as the anchoring structure of the PC. Proteomic studies have demonstrated that the PC contains at least 600 different proteins;10,11 there is no evidence of protein synthesis within cilia, thus ciliary proteins reach and leave the PC through sophisticated mechanisms of transport that have been a hot topic in the field.12
Clinical Diagnosis and Natural History of BBS
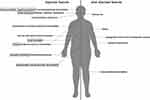
The diagnosis of BBS is based on clinical criteria published by Beales et al and requires the presence of at least four primary features or three primary features and two secondary features (Table 1) (Figure 2).13 Negative family history for BBS is common, as shown in other autosomal recessive disorders. Moreover, early diagnosis is further difficult, due to the progressive onset of all clinical signs over time.
 |
Table 1 Clinical Signs of BBS Patients |
One of the earliest signs is polydactyly; its association with genito-urinary anomalies on prenatal ultrasound, when present, should induce the clinical suspicious.14 Hyperechoic kidneys, cysts, pelvic dilation have been described in infants. These findings are clearly non-specific. Pre/peri-natal hyperechoic kidneys can also be found in other conditions as autosomal recessive polycystic kidney disease (ARPKD) and sometimes in autosomal dominant polycystic kidney disease (ADPKD), besides other syndromic ciliopathies, as Meckel-Gruber syndrome.15
Obesity is another quite early sign; while patients have normal body weight at the birth, they often develop obesity during the first year of life.16
Other major symptoms and signs emerge later, generally during the first decade of life; thus, the phenotype very commonly does not meet the criteria for diagnosis early in life. The average age at diagnosis is 9 years, according to Beales et al.13 In the Clinical Registry Investigation of BBS (CRIBBS), the median age at diagnosis is 5.8 years.17 Of note, phenotypic variability of BBS patients is quite high, even in the same family, making difficult the clinical diagnosis. Paucisymptomatic forms have been also described.18
Another difficulty in making the clinical diagnosis is the phenotypic overlap with other syndromic ciliopathies. Adult BBS patients often show clinical overlapping with Senior-Løken and Alström Syndromes.
The Senior-Løken syndrome (SLS) is an autosomal recessive disease characterized by development of retinal degeneration and a progressive tubulointerstitial kidney disease, leading to the end-stage kidney disease. In SLS obesity, polydactyly, hypogonadism and genitourinary malformations are uncommon.19
Alström syndrome is an autosomal recessive disorder characterized by cone-rod dystrophy, hearing loss, childhood truncal obesity, type 2 diabetes, hypertriglyceridemia, short stature, dilated cardiomyopathy and progressive pulmonary, hepatic and renal dysfunction.20 In AS, liver fibrosis and dilated cardiomyopathy are prevalent but show late onset (in ~ 60%); polydactyly is not reported.19
Retinal Dystrophy
Retinal dystrophy is the most penetrant feature, affecting up to 100% of individuals.13,21 The most common reported pattern is a rod–cone dystrophy with early macular involvement.21 Visual impairment has a quite early onset. The most frequent clinical presentation is nyctalopia, around the age of 4 to 8 years, followed by progressive peripheral vision loss and, lastly, decline of color vision and visual acuity. Sometimes, instead, patients lose first cones and then rods. Most patients reach legal blindness between the second and third decade of life.16,22 Fundus examination shows atypical pigmentary retinal dystrophy with macular involvement.23 Electroretinography could detect signs of retinopathy before clinical presentation, more likely after the age of five years, but early changes could be recognized within two years of life.16 Physicians should consider that some patients develop refractive errors or strabismus (minor features) before retinal dystrophy becomes evident.13 Denniston et al proposed a retinopathy grading system which reflected the stage of disease and the photoreceptors affected for standardization purposes.21 Understanding the pathogenesis of retinal degeneration (RD) in ciliopathies is a prerequisite to develop targeted therapy. The retina is affected in almost all ciliopathies. In fact, photoreceptors light-sensing outer segments are specialized cilia, thus highly vulnerable in this setting.24 Defective Rhodopsin trafficking is considered a common feature of RD in multiple ciliopathy models; moreover, in BBS animal models, endoplasmic reticulum stress response has been described.25
Obesity
BBS patients commonly manifest with obesity, in 72–92% of cases. Patients typically show normal body weight at birth, but in 90% of cases they gain weight in the first year of life and obesity becomes evident during the first 3 years of life. In adulthood, obesity is mainly truncal but in childhood it is usually described as diffuse.16,26,27
Its pathogenesis is multifactorial and includes both central and peripheral control of energy expenditure. The mechanisms are mainly based on the role of BBS proteins into the trafficking of proteins to the 1) PC or 2) to the plasma membrane. Proopiomelanocortin (POMC)-neurons of mice lacking Bbs1 showed reduced plasma membrane abundance of serotonin (5-HT2C) receptor, with abnormal cytoplasmic accumulation.28 Some studies suggest that cilia are necessary for leptin signaling in the hypothalamus.27 Mice models of BBS, namely Bbs2-/-, Bbs4-/- and Bbs6-/- mice, showed leptin resistance and hyperleptinemia and BBS1 has been shown to physically interact with leptin receptor, suggesting a central role of BBS proteins in the controls of body weight.29 Moreover, a peripheral dysfunction of adipogenesis has been described, since a PC is present in differentiating preadipocytes and contains receptors for Wnt and hedgehog pathways.30
Abnormal leptin signaling has been confirmed in patients. Plasma leptin levels were higher in BBS individuals than in controls, suggesting leptin resistance. Targeting leptin signalling for treating obesity in BBS is an emerging therapeutical approach under study. Finally, BB1 and BBS2 are required for insulin receptor trafficking to the membrane and Bbs2, Bbs4 and Bbs6 null mice show insulin resistance, indicating further extra ciliary functions of BBS proteins and their role in metabolism.31
Postaxial Polydactyly
Postaxial polydactyly is common (63–81%). BBS1 interacts with components of the Hedgehog Pathway as the smoothened, frizzled family receptor (Smo) and the human patched 1 (Ptch1), involved in limb formation. The loss of Bbs in mice result in a reduced Shh response that might cause polydactyly.32 The majority of the patients have fully formed additional digit on the lateral border of the foot (more frequently) or hand; polydactyly can be present in all four limbs (21%), only on the hands (8%) or only on the feet (21%).13
Interestingly, the uncommon mesoaxial polydactyly is associated to mutations in BSS17 (LZTFL1) gene.33 Secondary features are: brachydactyly (46%), syndactyly (8%; usually between second and third toes), fifth finger clinodactyly, thumb placed proximally, “sandal gap” between I and II toes.13
Renal Impairment
Kidney abnormalities in BBS are indeed both anatomical and functional and include fetal lobulation, cystic dysplasia, small kidneys, horseshoe, ectopic/duplex/absent kidneys, calyceal clubbing or blunting, tubular and interstitial nephritis, glomerulosclerosis, polyuria and urine concentrating defects.34 Low urinary tract defects, as neurogenic bladder, bladder outflow obstruction or vesicoureteral reflux have been reported in 5–10% of adults.19
The prevalence of kidney disease among patients varies among studies of the literature; one of the reason is the definition of kidney disease, as some studies consider structural kidney abnormalities and some others loss of renal function.35–37
The study conducted by Forsythe et al in 350 BBS patients showed that 31% of children and 42% of adults had chronic kidney disease (CKD), stage 2–5; the prevalence of latest CKD stages was 6% and 8% in pediatric and adult patients, respectively. However, the comparison between adult and children is made difficult for the low number of subjects older than 30 year-old.38 Meyer et al have recently analyzed the presence of kidney failure (KF) in a cohort of 607 BBS patients of the Clinical Registry Investigation of BBS (CRIBBS); 364 individuals had genetic confirmation of BBS. KF was present in 44 (7.2%) of individuals; when considering the three most common genotypes, namely BBS1,2 and 10, the authors found a significantly increased risk of KF in patients carrying BBS2 and BBS10 mutations. BBS10, the second most common gene in the CRIBBS cohort, was present in 32.4% of the KF population with genetic confirmation, accounting for 26.6% of genotyped patients.
The pathogenesis of kidney disease is largely unknown. Lower urinary tract dysfunction, when present, can cause upper renal tract sequelae.38 Hypertension, obesity and diabetes, frequently observed in these patients, are known risk factors for kidney disease progression; however, their contribution into the progression of kidney disease in this setting requires deeper analysis; based on Forsythe et al, CKD stage 2–5 correlated with hypertension and urinary tract abnormalities.38–40
We have recently shown that renal hyposthenuria is associated with a faster decline of the estimated glomerular filtration rate (eGFR) in a population of 54 Italian BBS patients; urine concentrating defect could represent a marker of tubulointerstitial defect.36,41 Consistent with this hypothesis, we have recently show that BBS patients with still preserved eGFR show abnormal functional Magnetic Resonance parameters, especially in the medulla, supporting the fact that kidney disease is mainly a primitive tubulointerstitial disorder.42
The expression of BBS proteins in the kidney suggests that at least in part local factors may contribute to kidney disease;43 in consistency with this hypothesis, we have recently shown that the absence of one of major BBS gene, namely BBS10, caused several metabolic aberrations, in a renal-epithelial-derived cell line (IMCD3-Bbs10-/-); these abnormalities included increased aerobic glycolysis, abnormal cytoplasmic lipids accumulation and mitochondrial dysfunction, possible factors contributing to kidney disease progression.44,45 These biological aberrations are known to correlate with the onset of chronic kidney dysfunction in the general population and might contribute to the high incidence of kidney dysfunction in this setting.
Learning Disabilities
Cognitive impairment is present in 60–66% of subjects. Magnetic resonance imaging shows a reduction in volume of hippocampus grey matter, white matter volume loss in the right inferior longitudinal fasciculus, volume loss in the anterior temporal lobes and in the medial orbitofrontal cortex.32,46,47
In 2011, Mockel et al observed that intellectual performance of 4% of patients was in the higher range of abilities for the normal population. Developmental delay is often global but sometimes is specific for some areas, like motor and/or language. Speech is nasal, slow, with misarticulations, substitutions and omissions, the voice high pitched and/or of breathy quality. Speech delay is often responsive to speech therapy.13,19,48 Kerr et al reported that the mean intellectual functioning of patients was 1.5 standard deviations below normal expectations, but only 20–25% of patients met diagnostic criteria (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, DSM-5) for intellectual disability. 22–40% of patients had severe impairment in verbal fluency and auditory rote learning, 53% in perceptual reasoning, 69% in attention capacity, 74% in functional independence.41
Hypogonadism and Genitourinary Abnormalities
Hypogonadism or genitourinary abnormalities are present in 59% of BBS subjects.19 Patients can manifest delayed onset of secondary sexual characteristics, cryptorchidism (9%), micropenis buried in adipose tissue, small volume testes, malformed uterus, hydrometrocolpos, vaginal atresia and other genital anomalies. Female have often irregular menstrual cycle and polycystic ovaries (14.7%; a minor feature).18,19 Mujahid reported that 19.5% of male BBS patients were hypogonadal, but pituitary anomalies were uncommon.49
Recently, using CRIBBS registry, Meyer et al identified Eagle Barrett syndrome in four children.50 Reproduction is difficult but some patients gave birth to children.
These patients are generally female, but rare cases of male patient with descendants are reported.13
Secondary Features
Several secondary clinical features have been described: they include speech delay, behavioral anomalies, developmental delay, metabolic syndrome, diabetes mellitus, hypothyroidism, polycystic ovary syndrome, dental anomalies, facial dysmorphism, congenital heart disease, laterality defects, brachy- and syndactyly, strabismus, cataract, astigmatism, mild spasticity, ataxia/poor coordination, anosmia/hyposmia, hepatic fibrosis/disease and other gastrointestinal disease (Hirschsprung disease, inflammatory bowel disease, celiac disease).
Insulin resistance is a common feature in BBS patients; however, the progression to type 2 diabetes mellitus is not strikingly high, based on the literature. Diabetes prevalence is: 15.8% in patients with a mean age of 33 years; 6% and 48% in patients aged 26.3 and 43 years, respectively.
Hypothyroidism is present in 19.4% of patients (vs 4.6% of the control subjects). In Mujahid’s study, the prevalence of metabolic syndrome (using International Diabetes Federation criteria) is 54.3%: all metabolic syndrome parameters were higher in BBS group compared with control group, except for HDL-C. Polycystic ovary syndrome is present in 14.7% of female with BBS.49
Facial dysmorphism is often subtle; some individuals show hypertelorism, downward slanting palpebral fissures, large ears, enophthalmos, retrognathia, flat nasal bridge, anteverted nares long philtrum, thin upper lip, early frontal balding in adult male.13,16
Orodental anomalies are common, with a prevalence >50%; the most frequently reported anomalies are: hypodontia, high-arched palate (35%), malocclusion, microdontia, crowding of the teeth (46%), short dental roots.51
Anosmia/hyposmia has been reported in 50% of BBS patients, but the percentage could be higher because of the difficulties in its evaluation in clinical practice.52 MRI evaluation of olfactory bulb can help physicians to assess olfactory dysfunction.53
Olfaction impairment is related to defects in olfactory cilia or in the olfactory bulb.54
Cardiovascular disease is another secondary feature sometimes observed in BBS. The frequency of heart anomalies reported by Niederlova et al was 29.8% and included congenital heart disease as atrial septal defect and other anomalies. Previously, Beales reported that congenital heart defects, including aortic stenosis, patent ductus arteriosus and unspecified cardiomyopathy, were present in 7% of patients.13,18 Several types of heart and vascular (eg, bilateral persistent superior vena cava, interrupted inferior vena cava, hemiazygos continuation, right sided aorta or left sided vena cava) anomalies have been described and males seem to be more affected than female.40,55 Olson et al used the CRIBBS registry to determine the prevalence of thoraco-abdominal abnormalities and midlines defects that was 1.6%. Prevalence in BBS patients may be underestimated, since comprehensive imaging was available only for some patients. Cilia are fundamental for the correct development of laterality and several types of anomalies for various organs have been reported. In some cases, physicians should consider and investigate the presence of two ciliopathies: Olson et al reported the presence of clinical criteria for both BBS and primary cilium dyskinesia in the same patient.56 It has been suggested that atrioventricular canal defects (AVCD) and laterality defects can be an important signal for a possible early diagnosis of BBS and ciliopathies with atypical features. Digilio et al reported that 7.7% of patients with AVCD and ciliopathy had BBS.57
Liver disease, defined as abnormalities on liver imaging and/or high plasma transaminase enzymes level, occurs in 30% of patients; additional gastrointestinal abnormalities are more common than expected in the general population and include Hirschsprung disease (2.8%), celiac disease (1.5%), inflammatory bowel disease (1.1%), anal stenosis and other anatomic anomalies.19 Obesity and metabolic impairment may contribute to liver disease, especially for non-alcoholic fatty liver disease.58
Neuropsychiatric disorders are not uncommon. Autism-related signs were present in 77% of participants.59 Behavioral anomalies have an incidence of 33% and include obsessive compulsive and ritualistic behavior, anxiety, emotional immaturity, labile behavior, disinhibition, hyperactivity.13 Depression can present when the subject realizes the impact of the syndrome. BBS proteins are normally expressed in human hippocampus and brain; primary cilia seem to be important for hippocampal neurogenesis.60 Other neurological aspects include epilepsy (9.6%); ataxia has been documented in BBS and seems to affect lower limbs to a greater degree than upper limbs.13 Seizures/epilepsy has been reported in some patients in the CRIBBS registry but, in most of the cases, it is resolved before adulthood.19 Mild spasticity has also been reported.
Additional Clinical Manifestations
Other clinical features have been described in BBS subjects that are not listed among clinical diagnostic criteria. Hawks et al found cutaneous dermatoses, such as keratosis pilaris, seborrheic dermatitis and obesity-related dermatologic disorders, in all subjects with BBS. This study suggests the presence of defect of keratinization and keratinocyte function, highlighting the importance of dermatological care as part of clinical management.61
Hearing loss was detected in 21% of patients and in most of them was conductive and associated with chronic otitis media in childhood (and no more present in adulthood). A smaller percentage of patients, instead, show sensorineural hearing loss.13 Forsyth and Gunay-Aygun reported a high rate of musculoskeletal abnormalities registered in CRIBBS, including joint laxity (27.6%), scoliosis (16%), leg length discrepancy (9.6%), club foot (1.8%), Blount disease (0.9%).19
It has been reported that BBS patients have a high prevalence of neonatal respiratory distress at birth (12% vs 3% UK population) asthma (21% vs 8–10% UK population) and rhinitis (36% vs 18.25 UK population).62
Interestingly, autoimmune diseases have a higher prevalence in BBS. A recent study of Tsyklauri et al revealed the possible connection between BBS and dysregulated immune and hematopoietic systems; in this study, BBS patients showed decreased platelet and red blood counts, increased total white blood cells, neutrophils, and eosinophils.63
The incidence of cancer has never been systematically assessed in BBS patients. Three independent reports have described four cases of endometrial carcinoma in BBS patients.64–66 The association between the BBS and this cancer may be correlated with the presence of risk factors as hyper-estrogenism due to truncal obesity, hyperinsulinemia and ovulatory dysfunction.
The study by Beales et al analyzed the incidence of cancer in unaffected relatives of 109 BBS patients, thus, in the setting of heterozygote BBS mutation.67 The authors found that three out of 180 relatives had renal cell carcinoma, estimating an increased risk of RCC in BBS gene carriers compared with the general population. A subsequent study does not confirm this finding. Hjortshøj et al performed a population-based study to examine the incidence of cancer in 116 BBS patients and 428 relatives.68 The study excluded the presence of an increased risk of renal cancer among family members of BBS patients, confuting previous data. Additional studies are needed to better understand the possibility of this relationship. Perhaps further understanding of the role of the PC in renal pathophysiology will clarify this issue.
Genetics of BBS and Genotype-to-Phenotype Correlation
The BBS has an autosomal recessive inheritance, although some authors suggested a oligogenic inheritance.69–72 Currently, thanks to the progress in genetics, twenty-six genes have been associated with the syndrome, an increasing number over years (Table 2).50 However, mutations in BBS1 to BBS18 account for about 70–80% of cases worldwide73 and about 50% of diagnosis in the western countries are due to mutations in three genes: BBS1, BBS2 and BBS10.19,74 In Caucasian populations, indeed, mutations in BBS1 and BBS10 are detected in 21–30% of patients,75 a percentage rising to 40–50% in Northern European patients.76 A founder effect is responsible for the two most common mutations in Northern Europe: p.M390R (BBS1, accounting for 50% of BBS1 cases) and p.C91Lfs*5 (BBS10).77
 |
Table 2 Known Causative Genes of Human Bardet–Biedl Syndrome88,144,145 |
In Indian population, instead, BBS1 and BBS10 mutations represent only 7% and 10% of diagnosis, respectively, while BBS3 (14%), BBS9 (10%), and BBS6 (10%) mutations are more frequent.78
BBS1, BBS3, and BBS4 mutations are commonly reported in Saudi Arabia,72,79 while pathogenic variants in BBS1, BBS2 and BBS8 are common in Tunisia;80 Middle Eastern and North African individuals have a high frequency of BBS4, BBS5, and TTC8 variants.81 A possible bias is the underdiagnosis and scarcity of large studies from some areas of the world;74,82–84in isolated populations characterized by a high frequency of marriages among consanguineous, the frequency of the disease is higher and founder effects are common; the Faroe Islands (c.1091 + 3G>C in BBS1),5 Tunisia (p.R189* in BBS2 and c.459 + 1G>A in BBS8)80,85,86 and the Hutterite population (c.472–2A>G in BBS2) are some examples.32,73,87 Similarly, we have recently found a possible BBS4 (c.332+1G>GTT) founder mutation in a restricted geographic area of the province of Naples.74
Most BBS genes have a low tissue specificity. Few exceptions are BBIP1, which is mainly expressed in the testis, LZTFL1, expressed in lymphoid tissue and NPHP1, in the skeletal muscle.88
Almost all BBS genes are related to the function of the PC and many of them have been described also in other ciliopathies; BBS15, BBS13 and SDCCAG8 mutations have been reported in Meckel syndrome89–91 and CEP290/NPHP6 mutations in Joubert, Senior–Løcken, Meckel–Gruber, nephronophthisis and Leber congenital amaurosis.45
Major BBS gene products have been detected on the base of the PC. Some of them have been detected along the cilium92; extracilia localization include the nucleus93 and the endoplasmic reticulum.34,94
Functional studies demonstrated that they form two multimeric complexes: the BBSome and chaperonin complex. The BBSome is a cargo adaptor involved into intra-ciliary trafficking; it mediates vesicular trafficking of membrane proteins to the primary cilium (Figure 1).34,95 BBSome is an octamer composed of BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, BBS9, and BBIP10, encoded by the respective genes.96
Chaperonin complex mediates BBSome assembly with a multistep process.17,97–99
The chaperonins-like proteins include MKKS/BBS6, BBS10 and BBS12.98
Genotype–phenotype correlation is made difficult by the unavailability of large cohort studies.100 Genetic heterogeneity further complicates the analysis.34
Nevertheless, several studies have discussed this topic, many of them describing a milder phenotype in the presence of BBS1 variants.101–106 This can be partially explained by the high prevalence of the hypomorphic BBS1M390R variant,105 but also by the minimal effect of loss of BBS1 function on BBSome formation and stability, according to in vitro studies.107 Some reports also suggested that mutations in BBS1, BBS2, BBS3, and BBS4 could be associated to specific ocular phenotypes and digital malformations.34,73,108
The meta-analysis recently published by Niederlova et al analyzed the largest cohort of BBS patients, with a total of 899 individuals, by assembling data from 85 articles focused on gene-phenotype correlation.14
The authors used a parameter, the syndromic score, to quantify disease severity. Patients with assumed loss of function mutations (as frameshifts or splicing mutations) turned out to have higher syndromic score than those with missense mutations and a higher frequency of the most severe form of the disease.18
The study also showed that patients with mutations in BBS3/ARL6 had a significantly lower syndromic score than patients with mutations in the BBSome or chaperonin BBS genes. In particular, BBS3/ARL6 deficiency was characterized by a lower penetrance of cognitive impairment, renal anomalies and heart anomalies. Conversely, mutations in the BBSome‐encoding and chaperonin‐encoding genes had similar effects.18
Among patients with mutations in BBSome components, those with mutations in BBS1 and BBS8/TTC8 showed the lowest mean syndromic score, while patients with mutations in BBS2 and BBS7 the highest. Renal anomalies showed a low frequency in patients with mutations in BBS1, BBS4, or BBS8/TTC8 and a high frequency in those with mutations in BBS2, BBS7, or BBS9.
The similar effect on phenotype in patients with BBS1 and BBS8/TTC8 could be explained by the direct interaction of the two subunits.109 On the other hand, BBS2, BBS7 and BBS9 were previously proposed to form the core of the BBSome.110 It is plausible that the BBSome core is specifically involved in the kidney disease. In contrast, BBS1, BBS4, and BBS8/TTC8 genes encode peripheral BBSome subunits probably less important in the overall BBSome function.111–113
Some studies suggested a milder obesity phenotype in BBS1 compared to other BBS genotypes, a difference that seems to shrink in adolescence. An independent study demonstrated that children with loss of function variants showed the highest risk for severe obesity, consistent with other BBS clinical traits.17
The meta-analysis did not take in account all secondary BBS clinical signs; additional studies are required to elucidate some still open questions.101,102
Current Knowledge on Patients’ Survival and Clinical Management
Nowadays, there is no targeted treatment available for the syndrome. Interestingly, the 22-year prospective cohort study of Moore et al4 on Newfoundland’s population described a median patients survival of 63 years. O’Dea reported that about 25% of patients died before reaching the age of 44, compared to the 2% of the unaffected siblings; 72% of dead patients had renal impairment, with chronic uremia as cause of death for 38% of them.35 In 1996, Riise highlighted the prominent role of kidney failure (50% of cases) and cardiovascular diseases as causes or contributing cause of death in BBS.114 Also, other previous studies confirmed the major role of kidney disease as cause of mortality115,116 (Table 3).
 |
Table 3 Causes of Death of BBS Patients |
Given its pleiotropic nature, a multidisciplinary management is required14.
Based on our experience and data from the literature, even if scarce, at baseline, medical assessment should include:16,19,117
- Family history
- Anthropometric assessment, vital signs and accurate clinical examination
- Neuropsychological testing adapted to age and low vision
- Ophthalmological evaluation: complete eye examination, visual acuity, visual field testing, fundus examination, electroretinogram (generally from 4–5 years of age) and, if necessary, visually evoked responses and optical coherence tomography (OCT)
- Orodental assessment
- Audiometry
- Echocardiogram, electrocardiogram (ECG)
- Abdominal ultrasound
- Analysis of renal function, including the estimation of the glomerular filtration rate (GFR), albuminuria, electrolytes and acid base balance; urine osmolality
- If neurological abnormalities are present, consider brain magnetic resonance (MRI)
- Laboratory tests: liver function tests, complete blood count, electrolytes, creatine, urea, lipid panel, blood glucose (HbA1c, oral glucose tolerance test for older children/adults and plasma insulin concentration), gonadotropins and sex hormones (if in age of puberty), thyroid hormones
- Genetic analysis and counseling.
Major clinical signs are treated by specific-disease specialists, including nephrologist, dental specialist, endocrinologist, psychologist/psychiatrist, dietitian, ophthalmologist, gastroenterologist, neurologist, urologist, gynecologist, dermatologist and others.19
There is no treatment to prevent deterioration of vision and early educational planning (eg: Braille, mobility training, dedicated software for electronic devices) is fundamental to reduce the impact of vision loss, developing independent living skills. Low vision aids are also important when vision begins to decrease, and tinted glasses can be used if photophobia is present. Sometimes correction of refractive errors is also needed. In case of cataracts, surgery should be considered.19
In the presence of cognitive impairment and/or developmental delay, it is pivotal an early, age-based and personalized treatment with special education, speech therapy and physiotherapy. A clinical psychologist or a psychiatrist could be necessary if the patient shows behavioral disorders.16,117
The screening at renal function at basal is mandatory, given the common presence of kidney dysfunction. Periodic follow-up, based on kidney disease stage, should be suggested.36,38,42 Specific intervention to slow the progression of CKD are unknown. Dervisoglu et al reported a mild improvement of creatinine clearance in two obese siblings affected by BBS, after two years of hypocaloric and low protein diet.118 For patients in end stage renal disease, organ transplantation can be considered, although obesity should be a limit, especially for adult subjects. In fact, outcomes are comparable to those of the general population. It is reported an increase of the median BMI of the renal transplant cohort compared to the non-transplant cohort.119
A low-calorie diet and practice aerobic exercises to try to control obesity; for high-risk obese patients, bariatric surgery has to be considered.120,121 There are few data on long-term safety and efficacy but a recent review reported that benefits may be less durable in hyperphagic disorders.122,123
Patients with autism-related symptoms can receive treatment of autism spectrum disorder, like ABA (applied behavior analysis).19 New studies suggest that more attention should be given to bone metabolism disorders.124 The risk of developing type 2 diabetes mellitus increases with age and it is desirable to try to prevent it with lifestyle modifications.49 In fact, it is fundamental that metabolic syndrome, diabetes and hypertension are well-controlled to avoid serious secondary damages on organs already affected by BBS.14 Thyroid function has to be controlled annually and, if laboratory values are abnormal, exams for thyroid autoimmunity should be requested.49
Surgery has a role in treatment of polydactyly (removal of accessory digits), genitourinary, orodental (eg, dental extraction for dental crowding), heart anomalies and other anatomical abnormalities. Imaging, such as CT angiography to detect possible vascular anomalies, is very important for preoperative planning.56 When anesthesia is required, it is necessary to have a multidisciplinary preoperative evaluation for the risk of airway obstruction and the risks related to cardiovascular, kidney and/or endocrine diseases.125 Appropriate planning (also for dental operations) is important to avoid perioperative and postoperative complications, considering concomitant medications and all the features of the syndrome, such as obesity, anxiety, structural cardiopathy (eg, risk of endocarditis), autistic-like behavior.51 In 2011, an interesting case report described the resolution/improvement of clinical abnormalities that was maintained at least for three years (when case report was published) after that a child was treated with dietary supplementation (based on detailed biochemical testing that showed multiple nutrient deficiencies). This report suggested that a nutritional status assessment could be useful; we did not find any similar new case reports in literature.126
Future Perspectives
Research on gene therapy is one of the main prospective in BBS.14,127
Gene therapy using a viral vector is generally used to target a single organ like the eye and has been attempted in animal models of BBS for treating retinal degeneration.128,129
The recent approval of voretigene neparvovec as gene therapy for another form of retinal dystrophy increases expectations on gene therapy also in this setting.127,130
Whether other organs, as the kidney, could be targeted using this approach is debated; clearly, gene delivery from the blood is affected by glomerular filtration and only few studies have been reported about gene therapy in other kidney diseases.131,132
The eye is easy to access, there is a control eye and patients typically develop symptoms only in mid-late childhood.14 Gene editing therapies and CRISPR/Cas9 tools could be of interest.127
Read-through therapy could be used for nonsense mutations (premature termination codons), responsible of approximately the 12% of Bardet Biedl cases127 and its efficacy has already been assessed at a preclinical stage on other ciliopathies.133–135
Recently, Eintracht and others demonstrated that PTC124 (ataluren) or amlexanox cause the recovery of full-length BBS2 expression and correction of ciliary defects in BBS2 mutated fibroblasts.136
Some BBS mutations influences splicing and could be targeted by splicing-correcting approaches like antisense oligonucleotides, snRNAs and RNA interference. It has also been demonstrated in vivo the potential therapeutic effects in fibroblasts with a BBS1 mutation.14,127,137
New therapies are emerging for BBS-related obesity. Pomeroy et al observed that strategies to increase sleep duration could be useful to mitigate obesity.138 In 2009, Seo et al demonstrated that intravenous melanocortin receptor agonist administration reduced body weight and food intake in Bbs knockout mice;29 interestingly, in November 2020 and July 2021, in USA and EU, respectively, a new drug named setmelanotide has been approved for the treatment of obesity due to mutations in POMC, PCSK1 and LEPR. Setmelanotide is a melanocortin-4 receptor (MC4R) agonist and according to a phase-two study, it mitigates hyperphagia in BBS, reducing weight and hunger.139 A phase-3 study is ongoing.140 Ganawa et al reported a successful use of GLP-1 agonists for reducing the body mass index (BMI) in a young woman with BBS that showed childhood-onset obesity and hyperphagia. It was necessary to maintain the drug in therapy, because it was observed a weight regain after dose reduction.141
Pre-clinical studies demonstrated that the use of roscovitine and rapamycin was able to rescue renal cysts in zebrafish models of BBS.142
A novel target for therapy in BBS is glycosphingolipid metabolism, in experimental models of disease. It has been shown that it is impaired in this syndrome, with consequent accumulation of monosialodihexosylganglioside in Bbs2-/- mice. Indeed, it has been demonstrated that the glucosylceramide synthase inhibitor Genz-667161 decreased obesity, liver disease, retinal degeneration and olfaction defect in Bbs2-/- mice and preserved ciliary structure and signaling.143
In addition, to reduce adverse effects of current symptomatic treatment predicting how patients will respond to drugs, pharmacogenomics profile of BBS patients could be studied. In some countries, private companies offer pharmacogenomics gene panels, even if evidence base is still unclear for many of them.14,127
Technological advances like data sharing in the Cloud or telemedicine, may be useful, especially for who lives far from reference centers and/or have a more severe phenotype.14
Conclusions
The BBS is a rare pleiotropic disorder considered a model of ciliopathy. Given its rarity, its genetic and clinical heterogeneity, our understanding of the pathophysiology of major clinical signs is limited and data on genotype to phenotype correlation are scanty. To date, there is no specific therapy and supportive treatment is the milestone of patients’ care. A multidisciplinary approach is mandatory in BBS, considering the presence of multiple organ dysfunction, and personalized follow-up is required. Recent investigations have provided insight into the understanding the function of BBS proteins, highlighting their ciliary and extraciliary functions. Dissecting their role in cellular biology is a prerequisite to develop specific therapy.
Acknowledgment
This work is generated within the European Reference Network of Rare Kidney Diseases (ERKNet). The authors acknowledge the association of Italian BBS families, ASBBI, for its support.
Disclosure
The authors declare no competing interests.
References
1. Klein D, Ammann F. The syndrome of Laurence-Moon-Bardet-Biedl and allied diseases in Switzerland. J Neurol Sci. 1969;9(3):479–513. doi:10.1016/0022-510X(69)90091-4
2. Beales PL, Warner AM, Hitman GA, Thakker R, Flinter FA. Bardet-Biedl syndrome: a molecular and phenotypic study of 18 families. J Med Genet. 1997;34(2):92–98. doi:10.1136/jmg.34.2.92
3. Farag TI, Teebi AS. High incidence of Bardet Biedl syndrome among the Bedouin. Clin Genet. 1989;36(6):463–464. doi:10.1111/j.1399-0004.1989.tb03378.x
4. Moore SJ, Green JS, Fan Y, et al. Clinical and genetic epidemiology of Bardet-Biedl syndrome in newfoundland: a 22-year prospective, population-based, cohort study. Am J Med Genet A. 2005;132A(4):352–360. doi:10.1002/ajmg.a.30406
5. Hjortshøj TD, Grønskov K, Brøndum-Nielsen K, Rosenberg T. A novel founder BBS1 mutation explains a unique high prevalence of Bardet-Biedl syndrome in the Faroe Islands. Br J Ophthalmol. 2009;93(3):409–413. doi:10.1136/bjo.2007.131110
6. Turkyilmaz A, Geckinli BB, Alavanda C, et al. Meckel-Gruber syndrome: clinical and molecular genetic profiles in two fetuses and review of the current literature. Genet Test Mol Biomarkers. 2021;25(6):445–451. doi:10.1089/gtmb.2020.0311
7. Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol. 2017;18(9):533–547. doi:10.1038/nrm.2017.60
8. Estrada-Cuzcano A, Roepman R, Cremers FPM, den Hollander AI, Mans DA. Non-syndromic retinal ciliopathies: translating gene discovery into therapy. Hum Mol Genet. 2012;21(R1):R111–R124. doi:10.1093/hmg/dds298
9. Gupta N, D’Acierno M, Zona E, Capasso G, Zacchia M. Bardet-Biedl syndrome: the pleiotropic role of the chaperonin-like BBS6, 10, and 12 proteins. Am J Med Genet C Semin Med Genet. 2022;190(1):9–19. doi:10.1002/ajmg.c.31970
10. Ishikawa H, Thompson J, Yates JR, Marshall WF. Proteomic analysis of mammalian primary cilia. Curr Biol. 2012;22(5):414–419. doi:10.1016/j.cub.2012.01.031
11. Zacchia M, Marchese E, Trani EM, et al. Proteomics and metabolomics studies exploring the pathophysiology of renal dysfunction in autosomal dominant polycystic kidney disease and other ciliopathies. Nephrol Dial Transplant. 2019:gfz121. doi:10.1093/ndt/gfz121
12. Long H, Huang K. Transport of ciliary membrane proteins. Front Cell Dev Biol. 2019;7:381. doi:10.3389/fcell.2019.00381
13. Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36(6):437–446. doi:10.1136/jmg.36.6.437
14. Forsythe E, Kenny J, Bacchelli C, Beales PL. Managing Bardet-Biedl syndrome-now and in the future. Front Pediatr. 2018;6:23. doi:10.3389/fped.2018.00023
15. Simonini C, Floeck A, Strizek B, Mueller A, Gembruch U, Geipel A. Fetal ciliopathies: a retrospective observational single-center study. Arch Gynecol Obstet. 2022;306(1):71–83. doi:10.1007/s00404-021-06265-7
16. Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013;21(1):8–13. doi:10.1038/ejhg.2012.115
17. Pomeroy J, Krentz AD, Richardson JG, Berg RL, VanWormer JJ, Haws RM. Bardet-Biedl syndrome: weight patterns and genetics in a rare obesity syndrome. Pediatr Obes. 2021;16(2):e12703. doi:10.1111/ijpo.12703
18. Niederlova V, Modrak M, Tsyklauri O, Huranova M, Stepanek O. Meta-analysis of genotype-phenotype associations in Bardet-Biedl syndrome uncovers differences among causative genes. Hum Mutat. 2019;40(11):2068–2087. doi:10.1002/humu.23862
19. Forsyth R, Gunay-Aygun M, Forsyth RL, Gunay-Aygun M. Bardet-Biedl syndrome overview. 2003 Jul 14 [Updated 2020 Jul 23]. In: Adam MP, Everman DB, Mirzaa GM, editors. GeneReviews®. Seattle: University of Washington; 1993. Available from:. http://www.ncbi.nlm.nih.gov/books/NBK1363/.
20. Marshall JD, Maffei P, Collin GB, Naggert JK. Alström syndrome: genetics and clinical overview. Curr Genomics. 2011;12(3):225–235. doi:10.2174/138920211795677912
21. Denniston AK, Beales PL, Tomlins PJ, et al. Evaluation of visual function and needs in adult patients with bardet–biedl syndrome. Retina. 2014;34(11):2282–2289. doi:10.1097/IAE.0000000000000222
22. Weihbrecht K, Goar WA, Pak T, et al. Keeping an eye on Bardet-Biedl syndrome: a comprehensive review of the role of Bardet-Biedl syndrome genes in the eye. Med Res Arch. 2017;5:9. doi:10.18103/mra.v5i9.1526
23. Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12(4):238–249. doi:10.2174/138920211795860107
24. Guo D, Ru J, Xie L, et al. Tmem138 is localized to the connecting cilium essential for rhodopsin localization and outer segment biogenesis. Proc Natl Acad Sci U S A. 2022;119(15):e2109934119. doi:10.1073/pnas.2109934119
25. Patel MM, Tsiokas L. Insights into the regulation of ciliary disassembly. Cells. 2021;10(11):2977. doi:10.3390/cells10112977
26. Focșa I, Budișteanu M, Burloiu C, et al. A case of Bardet-Biedl syndrome caused by a recurrent variant in BBS12: a case report. Biomed Rep. 2021;15(6):103. doi:10.3892/br.2021.1479
27. Lee CH, Kang GM, Kim MS. Mechanisms of weight control by primary cilia. Mol Cells. 2022;45(4):169–176. doi:10.14348/molcells.2022.2046
28. Guo DF, Searby C, Zhang Q, Nishimura D, Sheffield V, Rahmouni K. Abstract P459: the BBSome mediate the sorting of the serotonin 5-HT2C receptor to the plasma membrane in POMC neurons. Hypertension. 2017;70(suppl_1). doi:10.1161/hyp.70.suppl_1.p459
29. Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18(7):1323–1331. doi:10.1093/hmg/ddp031
30. Marion V, Stoetzel C, Schlicht D, et al. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci U S A. 2009;106(6):1820–1825. doi:10.1073/pnas.0812518106
31. Starks RD, Beyer AM, Guo DF, et al. Regulation of insulin receptor trafficking by Bardet Biedl syndrome proteins. PLoS Genet. 2015;11(6):e1005311. doi:10.1371/journal.pgen.1005311
32. Priya S, Nampoothiri S, Sen P, Sripriya S. Bardet-Biedl syndrome: genetics, molecular pathophysiology, and disease management. Indian J Ophthalmol. 2016;64(9):620–627. doi:10.4103/0301-4738.194328
33. Schaefer E, Lauer J, Durand M, et al. Mesoaxial polydactyly is a major feature in Bardet-Biedl syndrome patients with LZTFL1 (BBS17) mutations. Clin Genet. 2014;85(5):476–481. doi:10.1111/cge.12198
34. Marchese E, Ruoppolo M, Perna A, Capasso G, Zacchia M. Exploring key challenges of understanding the pathogenesis of kidney disease in Bardet–Biedl syndrome. Kidney Int Rep. 2020;S2468024920313401. doi:10.1016/j.ekir.2020.06.017
35. O’Dea D, Parfrey PS, Harnett JD, Hefferton D, Cramer BC, Green J. The importance of renal impairment in the natural history of Bardet-Biedl syndrome. Am J Kidney Dis. 1996;27(6):776–783. doi:10.1016/S0272-6386(96)90513-2
36. Zacchia M, Blanco FDV, Torella A, et al. Urine concentrating defect as presenting sign of progressive renal failure in Bardet-Biedl syndrome patients. Clin Kidney J. 2021;14(6):1545–1551. doi:10.1093/ckj/sfaa182
37. Zacchia M, Zacchia E, Zona E, et al. Renal phenotype in Bardet-Biedl syndrome: a combined defect of urinary concentration and dilution is associated with defective urinary AQP2 and UMOD excretion. Am J Physiol Renal Physiol. 2016;311(4):F686–F694. doi:10.1152/ajprenal.00224.2016
38. Forsythe E, Sparks K, Best S, et al. Risk factors for severe renal disease in Bardet-Biedl syndrome. J Am Soc Nephrol. 2017;28(3):963–970. doi:10.1681/ASN.2015091029
39. Viggiano D, Zacchia M, Simonelli F, et al. The renal lesions in Bardet-Biedl syndrome: history before and after the discovery of BBS genes. G Ital Nefrol. 2018;35(Suppl 70):95–100.
40. Imhoff O, Marion V, Stoetzel C, et al. Bardet-Biedl syndrome: a study of the renal and cardiovascular phenotypes in a French cohort. Clin J Am Soc Nephrol. 2011;6(1):22–29. doi:10.2215/CJN.03320410
41. Zacchia M, Di Iorio V, Trepiccione F, Caterino M, Capasso G. The kidney in Bardet-Biedl syndrome: possible pathogenesis of urine concentrating defect. Kidney Dis. 2017;3(2):57–65. doi:10.1159/000475500
42. Borrelli P, Zacchia M, Cavaliere C, et al. Diffusion tensor imaging for the study of early renal dysfunction in patients affected by bardet-biedl syndrome. Sci Rep. 2021;11(1):20855. doi:10.1038/s41598-021-00394-4
43. Patnaik SR, Farag A, Brücker L, et al. Tissue-dependent differences in Bardet-Biedl syndrome gene expression. Biol Cell. 2020;112(2):39–52. doi:10.1111/boc.201900077
44. Marchese E, Caterino M, Fedele R, et al. Multi-omics studies unveil extraciliary functions of BBS10 and show metabolic aberrations underlying renal disease in Bardet-Biedl syndrome. Int J Mol Sci. 2022;23(16):9420. doi:10.3390/ijms23169420
45. Zacchia M, Capolongo G, Trepiccione F, Marion V. Impact of local and systemic factors on kidney dysfunction in Bardet-Biedl syndrome. Kidney Blood Press Res. 2017;42(5):784–793. doi:10.1159/000484301
46. McIntyre JC, Hege MM, Berbari NF. Trafficking of ciliary G protein-coupled receptors. Methods Cell Biol. 2016;132:35–54. doi:10.1016/bs.mcb.2015.11.009
47. Baker K, Northam GB, Chong WK, Banks T, Beales P, Baldeweg T. Neocortical and hippocampal volume loss in a human ciliopathy: a quantitative MRI study in Bardet-Biedl syndrome. Am J Med Genet. 2011;155(1):1–8. doi:10.1002/ajmg.a.33773
48. Mockel A, Perdomo Y, Stutzmann F, Letsch J, Marion V, Dollfus H. Retinal dystrophy in Bardet–Biedl syndrome and related syndromic ciliopathies. Prog Retin Eye Res. 2011;30(4):258–274. doi:10.1016/j.preteyeres.2011.03.001
49. Mujahid S, Hunt KF, Cheah YS, et al. The endocrine and metabolic characteristics of a large Bardet-Biedl syndrome clinic population. J Clin Endocrinol Metab. 2018;103(5):1834–1841. doi:10.1210/jc.2017-01459
50. Meyer JR, Krentz AD, Berg RL, et al. Kidney failure in
51. Panny A, Glurich I, Haws RM, Acharya A. Oral and craniofacial anomalies of Bardet-Biedl syndrome: dental management in the context of a rare disease. J Dent Res. 2017;96(12):1361–1369. doi:10.1177/0022034517716913
52. Kulaga HM, Leitch CC, Eichers ER, et al. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nat Genet. 2004;36(9):994–998. doi:10.1038/ng1418
53. Braun JJ, Noblet V, Kremer S, et al. Value of MRI olfactory bulb evaluation in the assessment of olfactory dysfunction in Bardet-Biedl syndrome. Clin Genet. 2016;90(1):79–83. doi:10.1111/cge.12697
54. Braun JJ, Noblet V, Durand M, et al. Olfaction evaluation and correlation with brain atrophy in Bardet-Biedl syndrome. Clin Genet. 2014;86(6):521–529. doi:10.1111/cge.12391
55. Elbedour K, Zucker N, Zalzstein E, Barki Y, Carmi R. Cardiac abnormalities in the Bardet-Biedl syndrome: echocardiographic studies of 22 patients. Am J Med Genet. 1994;52(2):164–169. doi:10.1002/ajmg.1320520208
56. Olson AJ, Krentz AD, Finta KM, Okorie UC, Haws RM. Thoraco-abdominal abnormalities in Bardet-Biedl syndrome: situs inversus and heterotaxy. J Pediatr. 2019;204:31–37. doi:10.1016/j.jpeds.2018.08.068
57. Digilio MC, Calcagni G, De Luca A, Guida V, Marino B. Atrioventricular canal defect as partial expression of heterotaxia in patients with Bardet-Biedl syndrome. J Pediatr. 2020;218:263–264. doi:10.1016/j.jpeds.2019.10.050
58. Branfield Day L, Quammie C, Héon E, et al. Liver anomalies as a phenotype parameter of Bardet-Biedl syndrome. Clin Genet. 2016;89(4):507–509. doi:10.1111/cge.12684
59. Kerr EN, Bhan A, Héon E. Exploration of the cognitive, adaptive and behavioral functioning of patients affected with Bardet-Biedl syndrome: exploration of the cognitive, adaptive and behavioral functioning of patients affected with BBS. Clin Genet. 2016;89(4):426–433. doi:10.1111/cge.12614
60. Bennouna-Greene V, Kremer S, Stoetzel C, et al. Hippocampal dysgenesis and variable neuropsychiatric phenotypes in patients with Bardet-Biedl syndrome underline complex CNS impact of primary cilia. Clin Genet. 2011;80(6):523–531. doi:10.1111/j.1399-0004.2011.01688.x
61. Haws RM, McIntee TJ, Green CB. Cutaneous findings in Bardet-Biedl syndrome. Int J Dermatol. 2019;58(10):1160–1164. doi:10.1111/ijd.14412
62. Shoemark A, Dixon M, Beales PL, Hogg CL. Bardet Biedl syndrome: motile ciliary phenotype. Chest. 2015;147(3):764–770. doi:10.1378/chest.13-2913
63. Tsyklauri O, Niederlova V, Forsythe E, et al. Bardet-Biedl Syndrome ciliopathy is linked to altered hematopoiesis and dysregulated self-tolerance. EMBO Rep. 2021;22(2):e50785. doi:10.15252/embr.202050785
64. Peiker G, Böhm W, Carol W. Laurence-Moon-Bardet-Biedl-Syndrome und Endometrionkarcinom [Laurence-Moon-Bardet-Biedl syndrome and endometrial carcinoma]. Zentralbl Gynakol. 1978;100(17):1126–1130. German.
65. Schwab G, Kriz K. Zwei Schwestern mit Laurence-Bardet-Biedl-Moon Syndrome und gleichzeitig bestehendem korpuskarzinom [Two sisters with Laurence Bardet-Biedl-Moon syndrome and concomittant carcinoma of the body of the uterus (author’s transl)]. Geburtshilfe Frauenheilkd. 1980;40(3):279–281. German. doi:10.1055/s-2008-1037011
66. Grechukhina O, Gressel GM, Munday W, Wong S, Santin A, Vash-Margita A. Endometrial carcinoma in a 26-year-old patient with Bardet-Biedl syndrome. Case Rep Obstet Gynecol. 2018;2018:1952351. doi:10.1155/2018/1952351
67. Beales PL, Reid HA, Griffiths MH, Maher ER, Flinter FA, Woolf AS. Renal cancer and malformations in relatives of patients with Bardet-Biedl syndrome. Nephrol Dial Transplant. 2000;15(12):1977–1985. doi:10.1093/ndt/15.12.1977
68. Hjortshøj TD, Grønskov K, Rosenberg T, Brøndum-Nielsen K, Olsen JH. Risk for cancer in patients with Bardet-Biedl syndrome and their relatives. Am J Med Genet A. 2007;143A(15):1699–1702. doi:10.1002/ajmg.a.31805
69. Zaghloul NA, Liu Y, Gerdes JM, et al. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome. Proc Natl Acad Sci U S A. 2010;107(23):10602–10607. doi:10.1073/pnas.1000219107
70. Badano JL, Leitch CC, Ansley SJ, et al. Dissection of epistasis in oligogenic Bardet-Biedl syndrome. Nature. 2006;439(7074):326–330. doi:10.1038/nature04370
71. Manara E, Paolacci S, D’Esposito F, et al. Mutation profile of BBS genes in patients with Bardet-Biedl syndrome: an Italian study. Ital J Pediatr. 2019;45(1):72. doi:10.1186/s13052-019-0659-1
72. Abu-Safieh L, Al-Anazi S, Al-Abdi L, et al. In search of triallelism in Bardet-Biedl syndrome. Eur J Hum Genet. 2012;20(4):420–427. doi:10.1038/ejhg.2011.205
73. M’hamdi O, Ouertani I, Chaabouni-Bouhamed H. Update on the genetics of bardet-biedl syndrome. Mol Syndromol. 2014;5(2):51–56. doi:10.1159/000357054
74. Zacchia M, Blanco FDV, Trepiccione F, et al. Nephroplex: a kidney-focused NGS panel highlights the challenges of PKD1 sequencing and identifies a founder BBS4 mutation. J Nephrol. 2021;34(6):1855–1874. doi:10.1007/s40620-021-01048-4
75. Janssen S, Ramaswami G, Davis EE, et al. Mutation analysis in Bardet-Biedl syndrome by DNA pooling and massively parallel resequencing in 105 individuals. Hum Genet. 2011;129(1):79–90. doi:10.1007/s00439-010-0902-8
76. Harville HM, Held S, Diaz-Font A, et al. Identification of 11 novel mutations in eight BBS genes by high-resolution homozygosity mapping. J Med Genet. 2010;47(4):262–267. doi:10.1136/jmg.2009.071365
77. Kim SK, Shindo A, Park TJ, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329(5997):1337–1340. doi:10.1126/science.1191184
78. Sathya Priya C, Sen P, Umashankar V, et al. Mutation spectrum in BBS genes guided by homozygosity mapping in an Indian cohort. Clin Genet. 2015;87(2):161–166. doi:10.1111/cge.12342
79. Abu Safieh L, Aldahmesh MA, Shamseldin H, et al. Clinical and molecular characterisation of Bardet-Biedl syndrome in consanguineous populations: the power of homozygosity mapping. J Med Genet. 2010;47(4):236–241. doi:10.1136/jmg.2009.070755
80. Smaoui N, Chaabouni M, Sergeev YV, et al. Screening of the eight BBS genes in tunisian families: no evidence of triallelism. Invest Ophthalmol Vis Sci. 2006;47(8):3487. doi:10.1167/iovs.05-1334
81. Billingsley G, Deveault C, Héon E. BBS mutational analysis: a strategic approach. Ophthalmic Genet. 2011;32(3):181–187. doi:10.3109/13816810.2011.567319
82. Khan OA, Majeed R, Saad M, Khan A, Ghassan A. Rarity of Laurence Moon Bardet Biedl syndrome and its poor management in the Pakistani population. Cureus. 2019;11(2):e4114. doi:10.7759/cureus.4114
83. Bouzidi A, Charoute H, Charif M, et al. Clinical and genetic spectrums of 413 North African families with inherited retinal dystrophies and optic neuropathies. Orphanet J Rare Dis. 2022;17(1):197. doi:10.1186/s13023-022-02340-7
84. Suspitsin EN, Imyanitov EN. Bardet-Biedl syndrome. Mol Syndromol. 2016;7(2):62–71. doi:10.1159/000445491
85. Chen J, Smaoui N, Hammer MBH, et al. Molecular analysis of Bardet-Biedl syndrome families: report of 21 novel mutations in 10 genes. Invest Ophthalmol Vis Sci. 2011;52(8):5317–5324. doi:10.1167/iovs.11-7554
86. M’hamdi O, Redin C, Stoetzel C, et al. Clinical and genetic characterization of Bardet-Biedl syndrome in Tunisia: defining a strategy for molecular diagnosis. Clin Genet. 2014;85(2):172–177. doi:10.1111/cge.12129
87. Innes AM, Boycott KM, Puffenberger EG, et al. A founder mutation in BBS2 is responsible for Bardet-Biedl syndrome in the Hutterite population: utility of SNP arrays in genetically heterogeneous disorders. Clin Genet. 2010;78(5):424–431. doi:10.1111/j.1399-0004.2010.01481.x
88. Florea L, Caba L, Gorduza EV. Bardet-Biedl syndrome-multiple kaleidoscope images: insight into mechanisms of genotype-phenotype correlations. Genes. 2021;12(9):1353. doi:10.3390/genes12091353
89. Otto EA, Hurd TW, Airik R, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet. 2010;42(10):840–850. doi:10.1038/ng.662
90. Schaefer E, Zaloszyc A, Lauer J, et al. Mutations in SDCCAG8/NPHP10 cause Bardet-Biedl syndrome and are associated with penetrant renal disease and absent polydactyly. Mol Syndromol. 2011;1(6):273–281. doi:10.1159/000331268
91. Billingsley G, Vincent A, Deveault C, Héon E. Mutational analysis of SDCCAG8 in Bardet-Biedl syndrome patients with renal involvement and absent polydactyly. Ophthalmic Genet. 2012;33(3):150–154. doi:10.3109/13816810.2012.689411
92. Jin H, White SR, Shida T, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141(7):1208–1219. doi:10.1016/j.cell.2010.05.015
93. Gascue C, Tan PL, Cardenas-Rodriguez M, et al. Direct role of Bardet–Biedl syndrome proteins in transcriptional regulation. J Cell Sci. 2012;125(2):362–375. doi:10.1242/jcs.089375
94. Anosov M, Birk R. Bardet-Biedl syndrome obesity: BBS4 regulates cellular ER stress in early adipogenesis. Mol Genet Metab. 2019;126(4):495–503. doi:10.1016/j.ymgme.2019.03.006
95. Klink BU, Gatsogiannis C, Hofnagel O, Wittinghofer A, Raunser S. Structure of the human BBSome core complex. Elife. 2020;9:e53910. doi:10.7554/eLife.53910
96. Blaess S, Wachten D. The BBSome: a nexus controlling energy metabolism in the brain. J Clin Invest. 2021;131(8):148903. doi:10.1172/JCI148903
97. Seo S, Baye LM, Schulz NP, et al. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci U S A. 2010;107(4):1488–1493. doi:10.1073/pnas.0910268107
98. Álvarez-Satta M, Castro-Sánchez S, Bardet-Biedl VD. Syndrome as a chaperonopathy: dissecting the major role of chaperonin-like BBS proteins (BBS6-BBS10-BBS12). Front Mol Biosci. 2017;4:55. doi:10.3389/fmolb.2017.00055
99. Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi:10.1016/j.cell.2007.03.053
100. Sodini SM, Kemper KE, Wray NR, Trzaskowski M. Comparison of genotypic and phenotypic correlations: cheverud’s conjecture in humans. Genetics. 2018;209(3):941–948. doi:10.1534/genetics.117.300630
101. Esposito G, Testa F, Zacchia M, et al. Genetic characterization of Italian patients with Bardet-Biedl syndrome and correlation to ocular, renal and audio-vestibular phenotype: identification of eleven novel pathogenic sequence variants. BMC Med Genet. 2017;18(1):10. doi:10.1186/s12881-017-0372-0
102. Daniels AB, Sandberg MA, Chen J, Weigel-DiFranco C, Fielding Hejtmancic J, Berson EL. Genotype-phenotype correlations in Bardet-Biedl syndrome. Arch Ophthalmol. 2012;130(7):901–907. doi:10.1001/archophthalmol.2012.89
103. Estrada-Cuzcano A, Koenekoop RK, Senechal A, et al. BBS1 mutations in a wide spectrum of phenotypes ranging from nonsyndromic retinitis pigmentosa to Bardet-Biedl syndrome. Arch Ophthalmol. 2012;130(11):1425–1432. doi:10.1001/archophthalmol.2012.2434
104. Riise R, Tornqvist K, Wright AF, Mykytyn K, Sheffield VC. The phenotype in Norwegian patients with Bardet-Biedl syndrome with mutations in the BBS4 gene. Arch Ophthalmol. 2002;120(10):1364–1367. doi:10.1001/archopht.120.10.1364
105. Hjortshøj TD, Grønskov K, Philp AR, et al. Bardet-Biedl syndrome in Denmark--report of 13 novel sequence variations in six genes. Hum Mutat. 2010;31(4):429–436. doi:10.1002/humu.21204
106. Pawlik B, Mir A, Iqbal H, et al. A novel familial BBS12 mutation associated with a mild phenotype: implications for clinical and molecular diagnostic strategies. Mol Syndromol. 2010;1(1):27–34. doi:10.1159/000276763
107. Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Hum Mol Genet. 2012;21(9):1945–1953. doi:10.1093/hmg/dds004
108. Héon E, Westall C, Carmi R, et al. Ocular phenotypes of three genetic variants of Bardet-Biedl syndrome. Am J Med Genet A. 2005;132A(3):283–287. doi:10.1002/ajmg.a.30466
109. Mourão A, Nager AR, Nachury MV, Lorentzen E. Structural basis for membrane targeting of the BBSome by ARL6. Nat Struct Mol Biol. 2014;21(12):1035–1041. doi:10.1038/nsmb.2920
110. Zhang Q, Yu D, Seo S, Stone EM, Sheffield VC. Intrinsic protein-protein interaction-mediated and chaperonin-assisted sequential assembly of stable Bardet-Biedl syndrome protein complex, the BBSome. J Biol Chem. 2012;287(24):20625–20635. doi:10.1074/jbc.M112.341487
111. Katoh Y, Nozaki S, Hartanto D, Miyano R, Nakayama K. Architectures of multisubunit complexes revealed by a visible immunoprecipitation assay using fluorescent fusion proteins. J Cell Sci. 2015;128(12):2351–2362. doi:10.1242/jcs.168740
112. Klink BU, Zent E, Juneja P, Kuhlee A, Raunser S, Wittinghofer A. A recombinant BBSome core complex and how it interacts with ciliary cargo. Elife. 2017;6:e27434. doi:10.7554/eLife.27434
113. Woodsmith J, Apelt L, Casado-Medrano V, Özkan Z, Timmermann B, Stelzl U. Protein interaction perturbation profiling at amino-acid resolution. Nat Methods. 2017;14(12):1213–1221. doi:10.1038/nmeth.4464
114. Riise R. The cause of death in Laurence-Moon-Bardet-Biedl syndrome. Acta Ophthalmol Scand Suppl. 1996;219:45–47. doi:10.1111/j.1600-0420.1996.tb00385.x
115. Gourdol O, David L, Colon S, et al. L’atteinte rénale dans le syndrome de Laurence-Moon-Bardet-Biedl. A propos de trois observations [Renal involvement in the Laurence-Moon-Bardet-Biedl syndrome. Apropos of 3 cases]. Pediatrie. 1984;39(3):175–181. French.
116. Churchill DN, McManamon P, Hurley RM. Renal disease-a sixth cardinal feature of the Laurence-Moon-Biedl syndrome. Clin Nephrol. 1981;16(3):151–154.
117. Bardet-Biedl Syndrome Guideline Development Group. Management of Bardet-Biedl Syndrome. A clinical guideline; 2014. Available from: https://www.esvision.es/wp-content/uploads/2018/10/bardet_bield_guia_seguimiento_clinico.pdf.
118. Dervisoglu E, Isgoren S, Kasgari D, Demir H, Yilmaz A. Obesity control and low protein diet preserve or even improve renal functions in Bardet-Biedl syndrome: a report of two cases. Med Sci Monit. 2011;17(1):CS12–14. doi:10.12659/msm.881320
119. Haws RM, Joshi A, Shah SA, Alkandari O, Turman MA. Renal transplantation in Bardet–Biedl syndrome. Pediatr Nephrol. 2016;31(11):2153–2161. doi:10.1007/s00467-016-3415-4
120. Mujahid S, Huda MSB, Beales P, Carroll PV, McGowan BM. Adjustable gastric banding and sleeve gastrectomy in Bardet-Biedl syndrome. Obes Surg. 2014;24(10):1746–1748. doi:10.1007/s11695-014-1379-7
121. Boscolo M, Féry F, Cnop M. Beneficial outcomes of sleeve gastrectomy in a morbidly obese patient with Bardet-Biedl syndrome. J Endocr Soc. 2017;1(4):317–322. doi:10.1210/js.2017-00071
122. Gantz MG, Driscoll DJ, Miller JL, et al. Critical review of bariatric surgical outcomes in patients with Prader-Willi syndrome and other hyperphagic disorders. Obesity. 2022;30(5):973–981. doi:10.1002/oby.23385
123. Hamlington B, Ivey LE, Brenna E, Biesecker LG, Biesecker BB, Sapp JC. Characterization of courtesy stigma perceived by parents of overweight children with Bardet-Biedl syndrome. PLoS One. 2015;10(10):e0140705. doi:10.1371/journal.pone.0140705
124. Jeziorny K, Zmyslowska-Polakowska E, Wyka K, et al. Identification of bone metabolism disorders in patients with Alström and Bardet-Biedl syndromes based on markers of bone turnover and mandibular atrophy. Bone Rep. 2022;17:101600. doi:10.1016/j.bonr.2022.101600
125. Yaman F, Çekmen N. Anesthetic management of a patient with Bardet-Biedl syndrome undergoing renal transplantation: a case report. Medicine. 2020;99(38):e22300. doi:10.1097/MD.0000000000022300
126. Genuis SJ, Lobo RA. Potential amelioration of morbidity in patients with chromosomal anomalies: relevance to Bardet-Biedl syndrome. Clin Genet. 2011;79(5):482–488. doi:10.1111/j.1399-0004.2010.01475.x
127. Kenny J, Forsythe E, Beales P, Bacchelli C. Toward personalized medicine in Bardet–Biedl syndrome. Per Med. 2017;14(5):447–456. doi:10.2217/pme-2017-0019
128. Seo S, Mullins RF, Dumitrescu AV, et al. Subretinal gene therapy of mice with Bardet-Biedl syndrome type 1. Invest Ophthalmol Vis Sci. 2013;54(9):6118–6132. doi:10.1167/iovs.13-11673
129. Simons DL, Boye SL, Hauswirth WW, Wu SM. Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci U S A. 2011;108(15):6276–6281. doi:10.1073/pnas.1019222108
130. Food and Drug Administration. FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss; 2017. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-novel-gene-therapy-treat-patients-rare-form-inherited-vision-loss#:~:text=The%20U.S.%20Food%20and%20Drug%20Administration%20today%20approved,of%20vision%20loss%20that%20may%20result%20in%20blindness.
131. Rubin JD, Barry MA. Improving molecular therapy in the kidney. Mol Diagn Ther. 2020;24(4):375–396. doi:10.1007/s40291-020-00467-6
132. Khan A, Barber DL, Huang J, et al. Lentivirus-mediated gene therapy for Fabry disease. Nat Commun. 2021;12(1):1178. doi:10.1038/s41467-021-21371-5
133. Bukowy-Bieryllo Z, Dabrowski M, Witt M, Zietkiewicz E. Aminoglycoside-stimulated readthrough of premature termination codons in selected genes involved in primary ciliary dyskinesia. RNA Biol. 2016;13(10):1041–1050. doi:10.1080/15476286.2016.1219832
134. Goldmann T, Overlack N, Wolfrum U, Nagel-Wolfrum K. PTC124-mediated translational readthrough of a nonsense mutation causing Usher syndrome type 1C. Hum Gene Ther. 2011;22(5):537–547. doi:10.1089/hum.2010.067
135. Schwarz N, Carr AJ, Lane A, et al. Translational read-through of the RP2 Arg120stop mutation in patient iPSC-derived retinal pigment epithelium cells. Hum Mol Genet. 2015;24(4):972–986. doi:10.1093/hmg/ddu509
136. Eintracht J, Forsythe E, May-Simera H, Moosajee M. Translational readthrough of ciliopathy genes BBS2 and ALMS1 restores protein, ciliogenesis and function in patient fibroblasts. EBioMedicine. 2021;70:103515. doi:10.1016/j.ebiom.2021.103515
137. Schmid F, Glaus E, Barthelmes D, et al. U1 snRNA-mediated gene therapeutic correction of splice defects caused by an exceptionally mild BBS mutation. Hum Mutat. 2011;32(7):815–824. doi:10.1002/humu.21509
138. Pomeroy J, VanWormer JJ, Meilahn JR, Maki T, Murali HR, Haws RM. Sleep and physical activity patterns in adults and children with Bardet-Biedl syndrome. Orphanet J Rare Dis. 2021;16(1):276. doi:10.1186/s13023-021-01911-4
139. Haws R, Brady S, Davis E, et al. Effect of setmelanotide, a melanocortin‐4 receptor agonist, on obesity in
140. Haws RM, Gordon G, Han JC, Yanovski JA, Yuan G, Stewart MW. The efficacy and safety of setmelanotide in individuals with Bardet-Biedl syndrome or Alström syndrome: Phase 3 trial design. Contemp Clin Trials Commun. 2021;22:100780. doi:10.1016/j.conctc.2021.100780
141. Ganawa S, Santhosh SH, Parry L, Syed AA. Weight loss with glucagon-like peptide-1 receptor agonists in
142. Tobin JL, Beales PL. Restoration of renal function in zebrafish models of ciliopathies. Pediatr Nephrol. 2008;23(11):2095–2099. doi:10.1007/s00467-008-0898-7
143. Husson H, Bukanov NO, Moreno S, et al. Correction of cilia structure and function alleviates multi-organ pathology in Bardet-Biedl syndrome mice. Hum Mol Genet. 2020;29(15):2508–2522. doi:10.1093/hmg/ddaa138
144. The human protein atlas. Available from: https://www.proteinatlas.org.
145. Zhou, Z, Qui, H, Castro-Araya, RF. Impaired cooperation between IFT74/BBS22-IFT81 and IFT25-IFT27/BBS19 causes Bardet-Biedl syndrome. Human Mol Genet. 2022;31(10):1681–1693. doi:10.1093/hmg/ddab354.
146. Wingfield JL, Lechtreck K-F, Lorentzen E, Wakefield JG, Moores CA. Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery. Essays Biochem. 2018;62(6):753–763. doi:10.1042/EBC20180030
147. Senatore E, Iannucci R, Chiuso F, Delle Donne R, Rinaldi L, Feliciello A. Pathophysiology of primary cilia: signaling and proteostasis regulation. Front Cell Dev Biol. 2022;10:833086. doi:10.3389/fcell.2022.833086
148. Bateman A, Martin M-J, Orchard S, et al.; The UniProt Consortium. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 2023;51(D1):D523–D531. doi:10.1093/nar/gkac1052
149. Johnson CA, Collis SJ. Ciliogenesis and the DNA damage response: a stressful relationship. Cilia. 2016;5(1):19. doi:10.1186/s13630-016-0040-6
150. Cui C, Chatterjee B, Lozito TP, et al. Wdpcp, a PCP protein required for ciliogenesis, regulates directional cell migration and cell polarity by direct modulation of the actin cytoskeleton. PLoS Biol. 2013;11(11):e1001720. doi:10.1371/journal.pbio.1001720
151. Novas R, Cardenas-Rodriguez M, Irigoín F, Badano JL. Bardet-Biedl syndrome: is it only cilia dysfunction? FEBS Lett. 2015;589(22):3479–3491. doi:10.1016/j.febslet.2015.07.031
152. Pruski M, Hu L, Yang C, et al. Roles for IFT172 and primary cilia in cell migration, cell division, and neocortex development. Front Cell Dev Biol. 2019;7:287. doi:10.3389/fcell.2019.00287
153. CFAP418 cilia and flagella associated protein 418 [Homo sapiens (human)] Gene ID: 157657; 2022. Available from: https://www.ncbi.nlm.nih.gov/gene/157657.
154. Kanie T, Abbott KL, Mooney NA, Plowey ED, Demeter J, Jackson PK. The CEP19-RABL2 GTPase complex binds IFT-B to initiate intraflagellar transport at the ciliary base. Dev Cell. 2017;42(1):22–36.e12. doi:10.1016/j.devcel.2017.05.016
155. Mannella V, Quilici G, Nigro EA, et al. The N-terminal domain of NPHP1 folds into a monomeric left-handed antiparallel three-stranded coiled coil with anti-apoptotic function. ACS Chem Biol. 2019;14(8):1845–1854. doi:10.1021/acschembio.9b00582
156. Wormser O, Gradstein L, Yogev Y, et al. SCAPER localizes to primary cilia and its mutation affects cilia length, causing Bardet-Biedl syndrome. Eur J Hum Genet. 2019;27(6):928–940. doi:10.1038/s41431-019-0347-z
157. Lee H, Moon KH, Song J, Je S, Bok J, Ko HW. Tissue-specific requirement of sodium channel and clathrin linker 1 (Sclt1) for ciliogenesis during limb development. Front Cell Dev Biol. 2022;10:1058895. doi:10.3389/fcell.2022.1058895
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.