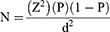Back to Journals » Veterinary Medicine: Research and Reports » Volume 14
Assessment of the Epidemiology of the Gastrointestinal Tract Nematode Parasites in Sheep in Toke Kutaye, West Shoa Zone, Ethiopia
Authors Desalegn C, Berhanu G
Received 20 July 2023
Accepted for publication 27 September 2023
Published 3 October 2023 Volume 2023:14 Pages 177—183
DOI https://doi.org/10.2147/VMRR.S427828
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Chaltu Desalegn,1 Gemechu Berhanu2
1College of Agriculture and Veterinary Sciences, Ambo University, Ambo, Oromia, Ethiopia; 2School of Veterinary Medicine, Dambi Dollo University, Dambi Dollo, Oromia, Ethiopia
Correspondence: Gemechu Berhanu, Email [email protected]
Introduction: Gastrointestinal tract nematodes are considered to be the most significant and underestimated problems that hinder sheep productivity.
Objective: This study aimed to evaluate the epidemiology of gastrointestinal tract nematode infestation of sheep in the Toke Kutaye District of West Shoa Zone, Ethiopia.
Methods: A cross-sectional study was conducted to determine the prevalence and risk factors associated with gastrointestinal tract nematode parasite infestation in sheep. Faecal samples were collected from 384 sheep and subjected to coprological examination, including direct smear, flotation techniques, and Baermann techniques for screening and identifying gastrointestinal nematodes.
Results: The overall prevalence of gastrointestinal tract nematode parasite infestation in sheep in the study area was 284 (73.96%). From the total positive cases, males and females were 97 (82.91%) and 187 (70.04%), respectively. The predominantly detected gastrointestinal tract nematodes of sheep in the study area were Trichostrongylus 111 (28.91%), Oestartagia 55 (14.32%), Haemonchus 42 (10.94%), Oesophagostomum 48 (12.50%), and Trichuris 23 (5.99%). Mixed nematode eggs were noticed in some of the sheep beside the single type of nematode eggs, with a prevalence of 85 (22.14%). The relationship in the occurrence of parasites between sex, age groups, body conditions, and seasons was statistically significant (P = 0.008, P = 0.014, P = 0.001 and P = 0.003), respectively.
Conclusion: The present study is of great importance to add to the existing knowledge of the epidemiology of gastrointestinal tract nematodes of sheep, and the findings are very important to apply the proper control and prevention strategies for gastrointestinal tract nematodes of sheep in the area.
Keywords: coprological examination, nematodes, prevalence, sheep, Toke Kutaye
Introduction
Sheep production contributes to the global livestock industry, rural development, and the provision of essential products such as meat, wool, milk, and manure.1 However, parasitic diseases are leading constraints to sheep production in many countries.2 Sheep in intensive and extensive production systems are extremely vulnerable to the effects of a variety of nematode internal parasites.3 The abundant gastrointestinal parasites that affect sheep are Haemonchus, Cooperia, Ostertagia, Trichostrongylus, Bunostomum, Chabertia, Nematodirus, and Oesophagostomum.4 Gastrointestinal nematodes cause a reduction in appetite, hypoproteinemia, anemia, impaired digestive system, death,5 and reduced feed consumption, and reduced immunity. These lead to low fertility and a reduction in productivity.6
Some studies have been conducted in other different parts of Ethiopia showing the prevalence of 74.7% gastrointestinal nematodiasis in sheep in Borena, Ethiopia;2 98.9% in Southern Ethiopia,7 86.7% in Bishoftu, Ethiopia;8 90.9% in sheep around Gondar, Northern Ethiopia;9 94.1% in sheep of the Mendayo in Bunch, Southeast Ethiopia;10 36.7% in Bako, Ethiopia;11 68.1% in Asella, Ethiopia;12 61.9% in Western Hararghe;13 and 24.7% in Western Oromia.14
However, there is no study regarding the epidemiology of gastrointestinal tract nematode infection in sheep in the study area. Thus, detecting the epidemiology of gastrointestinal nematodes in sheep is very important to apply the proper control and prevention strategies of the parasites in sheep in the area. Hence, this study aimed to evaluate the prevalence of gastrointestinal nematode parasite infestations and associated risk factors in sheep in the study area.
Materials and Methods
Description of the Study Area
Toke Kutaye District is one of the West Shoa Zone districts in Ethiopia. It is geographically located between 8°17 “north to 9°60” north latitude and 37°17 “east to 38°45” east longitude.15 The mean annual temperature of the district is 22–25°C, while its altitude ranges from 1500 to 3200 meters above sea level.16 The total livestock population of West Shoa Zone is 5,522,474, including 2,294,593 cattle, 1,074,939 sheep, 264,931 goats, 263,558 horses, 11,210 mules, 265,736 donkeys, and 1,347,507 poultry.17 Map of the study area is shown in Figure 1.
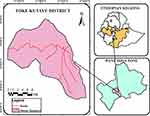 |
Figure 1 Map of the Study Area. |
Study Design
A cross-sectional study was used to determine the prevalence of sheep gastrointestinal nematodes using the coprological study method. The study animals included Menz breed pasture-foraging sheep reared in extensive management systems of different sexes, ages, body conditions, and locations. Those animals with an age of less than one year were considered as young while those greater than or equal to one year were considered adults according to the classification of age groups by Kumsa et al.18 Body condition scoring of sampled animals was carried out according to the method described by Cooper and Thomas19 and categorized into three as poor, medium and good.
The villages in the study area were selected purposively depending on their geographical locations including Goro Sole (relatively highland) when compared to Mutulu (relatively midland) and Guder (relatively lowland). Then, a simple random sampling technique was used to select the sheep from each village for this study. The sample size was calculated according to Thrusfield20 formula,
whereas P = 50% expected prevalence, Z = 1.96 with 5% desired absolute precision (d).
Coprological Examination
A total of 384 sheep faecal samples were collected from the rectum of sheep and taken to the Ambo University Veterinary Laboratory. About 10 g of fresh faecal sample was collected from each sheep directly from the rectum in a screw-capped universal bottle.21 Each sample was labeled with the required information (identification number, sex, age, body condition, location, and season) and transported in an icebox soon to Ambo University Veterinary Laboratory for analysis. Those fecal samples which were not examined on the same day were stored at 4°C and examined the next day.
For the parasitological examination of the faecal samples, direct smear22 and the flotation technique described by Hansen and Perry23 were employed, and the slides prepared were examined under a microscope (x40 magnification). Eggs of the different nematodes were identified on the base of morphological appearance and size of eggs.10 Positive faecal samples from each animal for nematode eggs were cultured to obtain third-stage larvae (L3) and identification of the most important genera of nematode eggs in sheep. Finally, larvae were recovered using the Baermann technique after 14 to 21 days of culture at room temperature (25°C). The recovered larvae were examined, and identification of the genus level was done based on morphological characteristics.24
Data Analysis
The raw data obtained from the study were recorded and coded in Microsoft Office Excel and analyzed using STATA version 11 software. Descriptive statistics in which the proportion of animals with a particular parasite that was investigated at the time was divided by the total number of animals was used to determine the prevalence of the gastrointestinal nematode. Pearson Chi-square statistics (χ2) was used to assess the degree of association between each risk factor (sex, age, body condition, location, and seasons) and the occurrence of gastrointestinal nematodes. A P-value less than 0.05 was considered to have a significant difference at a 95% confidence interval.
Results
Of the 384 sheep examined, 284 (73.96%) were positive for different types of gastrointestinal nematodes. From the total positive cases, males and females were 97 (82.91%) and 187 (70.04%), respectively. The difference in the predominance of the two sexes was statistically significant (P = 0.008). Additionally, age-related prevalence of the parasites was 43 (62.32%) and 241 (76.51%), in young and adults, respectively. The difference in the predominance of parasites between the age groups was significant (P = 0.014). Moreover, the body conditions related prevalence of the parasites was 160 (65.04%), 77 (84.62%), and 47 (100%), in sheep with good, medium, and poor body conditions, respectively. The difference in the prevalence of the parasites between the body conditions was statistically significant (P = 0.001). Furthermore, the geographic location-related prevalence of the parasite in Goro Sole, Mutulu, and Guder was 84 (65.63%), 92 (74.19%), and 108 (81.82%), respectively. The difference between them was not statistically significant. Besides, the season-related prevalence of the parasite in sheep in summer, spring, winter, and autumn was 89 (92.71%), 70 (72.92%), 53 (55.21%) and 72 (75.00%), respectively. The difference in the prevalence of the parasites between the seasons was statistically significant (P = 0.003) (Table 1).
 |
Table 1 Prevalence of Gastrointestinal Nematodes in Sheep by Sex, Age, Body Condition, Location and Seasons |
Among the positive cases, 111 (28.91%) were infested with Trichostrongylus, 55 (14.32%) with Ostertagia, 42 (10.94%) with Haemonchus, 48 (12.50%) with Oesophagostomum, and 23 (5.99%) with Trichuris. The most prevalent gastrointestinal nematodes identified in sheep were Trichostrongylus, followed by Ostertagia and Oesophagostomum, with a general predominance of 28.91%, 14.32%, and 12.50%, respectively (Table 2).
 |
Table 2 Prevalence of Sheep Gastrointestinal Nematodes in the Study Area |
Mixed nematode eggs were noticed in some of the sheep beside the single type of nematode eggs, with a prevalence of 22.14% (85/384).
Discussion
Gastrointestinal nematodes are an important cause of productivity loss in sheep production in Ethiopia.25 The overall prevalence of gastrointestinal nematode parasites, 73.96%, in this study was in agreement with the study conducted by Handiso et al2 who reported an overall prevalence of 74.7% gastrointestinal nematodiasis in sheep in Borena, Ethiopia. However, this result was less than that of previous studies on sheep from different parts of Ethiopia, including 98.9% in Southern Ethiopia,7 86.7% in Bishoftu,8 90.9% in sheep around Gondar, Northern Ethiopia,9 and 94.1% in sheep of the Mendayo in Bunch, Southeast Ethiopia.10 In addition, this finding was higher than the previous results, including 36.7% in Bako, Ethiopia,11 68.1% in Asella, Ethiopia,12 61.9% in Western Hararghe,13 and 24.7% in Western Oromia.14 The reason for this difference may be due to a combination of different factors, including climate and weather conditions, vegetation and grazing practices, animal movement, host genetics and immunity, parasite species, and drug resistance.26
Moreover, the finding related to the sex group agrees with a report by Getachew et al13 that gastrointestinal helminths influence both sexes. However, the current study contradicts a report by Temesgen et al2 who reported that the predominance of gastrointestinal nematodes was higher in females than in males. Male and female sheep have a higher chance of contamination if they are exposed to the same contaminated communal grazing pasture. Additionally, the male sheep (rams) infection rate might be related to their behavioral characteristics (aggressiveness), hormonal fluctuations during the breeding season, stress, and poor management.27,28
Additionally, the prevalence of nematodiasis in this study related to age agrees with the report by Yimer et al29 who reported a significant association between age categories. The reasons for the higher prevalence of GIT nematode infections in young sheep may be due to the immaturity of their immune system,30 increased exposure to infective larvae,31 the presence of maternal immunity that decreases over time, and limited acquired immunity.32 This finding suggests that age plays a crucial role in determining the vulnerability of sheep to gastrointestinal tract nematode parasites.
Besides, gastrointestinal nematode infection observed in body condition showed that shedding of nematode eggs increased with poor body condition (100%), compared to medium and good body condition, 65.04% and 84.62%, respectively. This agrees with a report by Getachew et al13 who reported that a good body condition score was associated with a lower prevalence rate of nematodiasis. This could be because sheep with poor body conditions are more susceptible to parasitic contamination due to weakened immune systems, stress, compromised mobility, gastrointestinal health, and potential lapses in husbandry practices.
Additionally, this study showed that there was a higher prevalence of gastrointestinal nematode parasites in Guder (relatively lowland) when compared to Mutulu (mid-land) and Goro Sole (highland). This may be due to the reason that nematode parasites thrive in the warm and humid environments of lowland areas.33
Furthermore, a higher prevalence of the sheep gastrointestinal nematode parasites in this study was observed during the summer season, and this finding agrees with the results discovered in Southeastern Ethiopia.10 The higher prevalence of sheep gastrointestinal nematodes in the rainy season (summer) can be attributed to favorable environmental conditions for parasite survival and development, increased pasture contamination, and increased grazing activity.32
Additionally, this study revealed that Trichostrongylus (28.91%) was the most prevalent among those positive for gastrointestinal nematode parasites. This result was higher than the report of Yimer et al29 which was 6.8% in North East Ethiopia but lower than the report of Shankute et al8 that was 74.37% in Central Ethiopia. The reason for the higher prevalence of Trichostrongylus in the study area than other gastrointestinal nematodes might be due to the complex life cycle and rapid development of the parasites, favorable environmental conditions for their survival and transmission, broad host range, host factors such as stress and immunocompromised states, as well as certain agricultural practices and management strategies.
Likewise, the prevalence of mixed nematode eggs observed in this study, 22.14%, is lower than the report of Tibebu et al11 who reported 36.7% in Horro District, Ethiopia, but higher than the report of Dawit et al31 who reported 12.5% mixed type of gastrointestinal tract nematode parasites in sheep in Hawassa Town, Southern Ethiopia. The presence of mixed gastrointestinal tract nematode infections in sheep in the study area might be due to the geographical and climatic conditions, complex life cycles of the parasites, host-parasite interactions, practices of grazing, and pasture management.
Conclusion
Gastrointestinal nematode parasites are the major constraints in sheep production and contribute to loss in productivity and economy. In this study, the overall prevalence of gastrointestinal nematodes was 73.96% in sheep which was based on Coproscopical examination for detection of the nematode eggs. The predominant gastrointestinal nematode parasites identified were Trichostrongylus, Ostertagia, Haemonchus, Oesophagostomum, and Trichuris. There was a significant relationship between the occurrence of parasites and sex, age groups, body conditions, and seasons. The presence of gastrointestinal tract nematode infections in sheep in the study area might be due to the geographical and climatic conditions, complex life cycles of the parasites, host-parasite interactions, practices of grazing, and pasture management. The present study is of great importance to add to the existing knowledge of the epidemiology of gastrointestinal tract nematodes of sheep. The high prevalence rate emphasizes the need for effective control and prevention strategies to minimize the impact of these parasites on sheep health and productivity.
Ethical Clearance
This study was approved, and ethical clearance was given by the Dambi Dollo University Ethical Review Committee, Dambi Dollo, Oromia, Ethiopia, with a reference number of Ref. No. RCS034/2020 regarding the use of animals for research purposes and all protocols or procedures in this study. All methods in this study were carried out by the research guidelines of the European Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Informed written consent was also obtained from the sheep owners. The objectives of the study were explained, and informed written consent to participate in the study was taken from the sheep owners.
Acknowledgment
The authors would like to thank Ambo University for its financial support during data collection, transportation, and laboratory tests during this study.
Funding
All materials and reagents used in this study were funded by Ambo University, but they didn’t participate in the study design, data collection, analysis, and writing of the report.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
1. Degen AA. Sheep and goat milk in pastoral societies. Small Rumin Res. 2007;68(2):7–19. doi:10.1016/j.smallrumres.2006.09.020
2. Handiso T, Yohannes B, Alemu B. Prevalence of gastrointestinal nematodes in. Dairy Vet Sci J. 2019;11(3):1–5. doi:10.19080/JDVS.2019.11.555813
3. Yimer A, Birhan E. Prevalence and identification of gastrointestinal nematodes of small ruminants in northern Ethiopia. Middle East J Sci Res. 2016;24(8):2602–2608. doi:10.5829/idosi.mejsr.2016.24.08.23834
4. Seyoum Z, Getnet K, Chanie M, Derso S, Fentahun S. Morbidity parameters associated with gastrointestinal tract nematodes in sheep in Dabat District, Northwest Ethiopia. Biomed Res Int. 2018;9247439:1–7. doi:10.1155/2018/9247439
5. Demewez G, Birhan M, Awoke T. Prevalence and risk factors of gastrointestinal nematode parasites of shoat in Andabet District, North West Ethiopia. Online J Anim Feed Res. 2017;7(6):134–137.
6. Perry BD. Investing in Animal Health Research to Alleviate Poverty. ILRI (aka ILCA and ILRAD); 2002.
7. Tolke AA. Epidemiology of gastrointestinal tract nematodosis of small ruminants in three different agro-ecological zones of Southern Ethiopia; 2005.
8. Shankute G, Basaznew Bogale AM. An abattoir survey on gastrointestinal nematodes in sheep and goats in hemex-export abattoir, Debre Ziet, Central Ethiopia. J Adv Vet Res. 2013;3:60–63.
9. Dagnachew S, Amamute A, Temesgen W. Epidemiology of gastrointestinal helminthiasis of small ruminants in selected sites of North Gondar zone, Northwest Ethiopia. Ethiop Vet J. 2011;15(2):57–68. doi:10.4314/evj.v15i2.67694
10. Zeryehun T. Helminthosis of sheep and goats in and around Haramaya, Southeastern Ethiopia. J Vet Med Anim Heal. 2012;4(3):48–55. doi:10.5897/JVMAH12.0014
11. Tibebu A, Tamiru Y, Abdeta D. Prevalence of major gastrointestinal nematode and degree of parasite infestation in sheep of bako agricultural research center community based breeding program project small holder farms at horro district. Dairy Vet Sci J. 2018;8(3):1–12. doi:10.19080/JDVS.2018.08.555740
12. Lemma D, Abera B. Prevalence of ovine gastrointestinal nematodes in and around Asella, South Eastern Ethiopia. J Vet Med Anim Heal. 2013;5(8):222–228. doi:10.5897/JVMAH12.022
13. Getachew M, Tesfaye R, Sisay E. Prevalence and risk factors of gastrointestinal nematodes infections in small ruminants in Tullo District, Western Harerghe, Ethiopia. J Vet Sci Technol. 2017;8(2):8–11. doi:10.4172/2157-7579.1000428
14. Aga TS, Hailu Y, Terefe G. Epidemiology of gastrointestinal nematodes of Horro sheep in Western Oromiya, Ethiopia. J Vet Med Anim Heal. 2013;5(10):297–304. doi:10.5897/JVMAH2013.0234
15. Abebaye H, Mengistu A, Tamir B, Assefa G, Feyissa F. Feed resources availability and feeding practices of smallholder farmers in selected districts of West Shewa Zone, Ethiopia. World J Agric Sci. 2019;15(1):21–30. doi:10.5829/idosi.wjas.2019.21.30
16. Etefa Y, Dibaba K. Physical and Socio Economic Profile of West Shewa Zone and Districts’. BFED-Regional Data and Information Core Process; 2011.
17. CSA, Central Statistical Agency. Report on livestock and livestock characteristics (Private Peasant Holdings). Agricultural Sample Survey 2021, Federal Democratic Republic of Ethiopia. Addis Ababa, Ethiopia; 2021.
18. Kumsa B, Tadesse T, Sari T, Duguma R, Hussen B. Helminths of sheep and goats in central Oromia (Ethiopia) during the dry season. J Anim Vet Adv. 2011;10(14):1845–1849. doi:10.3923/javaa.2011.1845.1849
19. Cooper JO, Thomas RE. A body condition scoring system for sheep. J Agric Sci. 1985;57(1):135–144.
20. Thrusfield M. Veterinary Epidemiology. John Wiley & Sons; 2007.
21. Hendrix CM, Robinson E. Nematodes that infect domestic animals. Diagnostic parasitology for veterinary technicians. Mosby Inc. 1998;63146:136–139.
22. Taylor MA. Parasitological examinations in sheep health management. Small Rumin Res. 2010;92(1–3):120–125. doi:10.1016/j.smallrumres.2010.04.012
23. Hansen J, Perry B. The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants. ILRAD; 1994:1–110.
24. Van Wyk JA, Cabaret J, Michael LM. Morphological identification of nematode larvae of small ruminants and cattle simplified. Vet Parasitol. 2004;119(4):277–306. doi:10.1016/j.vetpar.2003.11.012
25. Biffa D, Jobre Y, Chakka H. Ovine helminthosis, a major health constraint to productivity of sheep in Ethiopia. Anim Heal Res Rev. 2007;7(2):107–118. doi:10.1017/S1466252307001132
26. Gemeda BA, Amenu K, Magnusson U, et al. Antimicrobial use in extensive smallholder livestock farming systems in Ethiopia: knowledge, attitudes, and practices of livestock keepers. Front Vet Sci. 2020;7:1–15. doi:10.3389/fvets.2020.00055
27. Maquivar MG, Smith SM, Busboom JR. Reproductive management of rams and ram lambs during the pre-breeding season in us sheep farms. Animals. 2021;11(9):1–12. doi:10.3390/ani11092503
28. Binns SH, Cox IJ, Rizvi S, Green LE. Risk factors for lamb mortality on UK sheep farms. Prev Vet Med. 2002;52(3–4):287–303. doi:10.1016/S0167-5877(01)00255-0
29. Yimer A, Sissay D, Nazir S. Prevalence and associated risk factors of gastrointestinal nematodiasis in small ruminants in North East Ethiopia. J Anim Res. 2016;6(2):165–170. doi:10.5958/2277-940X.2015.00188.6
30. Cardia DFF, Rocha-Oliveira RA, Tsunemi MH, Amarante AFT. Immune response and performance of growing Santa Ines lambs to artificial Trichostrongylus colubriformis infections. Vet Parasitol. 2011;182(2–4):248–258. doi:10.1016/j.vetpar.2011.05.017
31. Dawit I, Weldegebriel W, Dejene D, Israel I. Prevalence of gastrointestinal tract nematodes parasites in sheep in Hawasa Town, Southern Ethiopia. Biomed J Sci Tech Res. 2022;41(5):33046–33052. doi:10.26717/bjstr.2022.41.006661
32. Singh E, Kaur P, Singla LD, Bal MS. Prevalence of gastrointestinal parasitism in small ruminants in western zone of Punjab, India. Vet World. 2017;10(1):61–66. doi:10.14202/vetworld.2017.61-66
33. Torres-Acosta JFJ, Sandoval-Castro CA, Hoste H, Aguilar-Caballero AJ, Cámara-Sarmiento R, Alonso-Díaz MA. Nutritional manipulation of sheep and goats for the control of gastrointestinal nematodes under hot humid and subhumid tropical conditions. Small Rumin Res. 2012;103(1):28–40. doi:10.1016/j.smallrumres.2011.10.016
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.