Back to Journals » Journal of Multidisciplinary Healthcare » Volume 8
An intervention designed to improve sensory impairments in the elderly and indoor lighting in their homes: an exploratory randomized controlled trial
Authors Haanes G, Kirkevold M, Hofoss D, Horgen G, Eilertsen G
Received 24 July 2014
Accepted for publication 26 September 2014
Published 7 January 2015 Volume 2015:8 Pages 11—20
DOI https://doi.org/10.2147/JMDH.S71718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Gro Gade Haanes,1 Marit Kirkevold,2 Dag Hofoss,2 Gunnar Horgen,1 Grethe Eilertsen1
1Department of Health Sciences, Buskerud and Vestfold University College, Drammen, Norway; 2Institute of Health and Society, University of Oslo, Oslo, Norway
Background: Vision and hearing impairments in the elderly (aged over 80 years) and poor indoor lighting conditions in a home-care setting are risk factors for functional decline, reduced social participation, withdrawal, and accidents.
Objective: We aimed to evaluate the changes in vision, hearing, and lighting conditions in the homes of participants aged over 80 years after implementation of a clinical intervention.
Methods: We undertook an exploratory randomized, controlled experimental study of sensory impairments and lighting conditions in the homes of elderly aged over 80 years who received home care. The intervention group (IG) received advice and encouragement to improve their vision, hearing, and indoor lighting conditions in the home, with a 10-week follow-up period. The control group (CG) received their usual care and underwent the same vision and hearing tests but were provided no intervention.
Results: Vision and hearing (self-assessed) and tested by Wilcoxon rank-sum test were significantly better (P=0.025 and P=0.008, respectively) in the IG after the intervention and follow-up. The test between the groups showed a significance of P=0.026 for visual acuity and P=0.098 for pure-tone average. The maximum and minimum lighting levels were significantly improved in the IG after the intervention (P=0.002 and P=0.039, respectively) but were unchanged in the CG.
Conclusion: Several of the IG participants did not follow all of the advice; however, among those who did, vision, hearing, and lighting conditions were all significantly improved. It appears that modest interventions have great potential for improving vision and hearing. Older patients in the home-care setting cannot be expected to take the necessary action to improve their sensory impairments by themselves. They require close monitoring, help from a specialist, and help to improve the indoor lighting conditions in their homes.
Keywords: vision and hearing impairments, home lighting conditions, old people, home care
Corrigendum has been published
Introduction
Multimorbidity, increased risk of diseases in the sensory organs, and age-related changes in the eyes and ears not only lead to reduced vision and hearing but also render the elderly aged over 80 years who are in a home-care setting vulnerable to many disabilities.1–4
Older persons experience hearing loss, which may be a combination of sensory, neural, strial (metabolic), and cochlear conductive presbycusis.5,6 The older person thus experiences considerable problems understanding rapid speech and unfamiliar accents.5,7 Sounds may be difficult to localize, particularly when different hearing aids are used in the left and right ears. Even a mild hearing loss can result in 25%–40% of speech being missed.5,6 It is well documented that impairments in vision and hearing are risk factors for social withdrawal and depression and can have a serious impact on a person’s quality of life.6,8,9 Vision and hearing impairments can affect both general function and the ability 1) to read and access information and 2) to communicate with others.
The probability of developing cataracts is almost 100% if the individual lives long enough;10 although this condition is curable in Norway and many Western countries, it is nevertheless stressful for the individual.11 Glaucoma, age-related macular degeneration, and diabetic retinopathy are frequent diseases in aging populations.4 Persons with cataract or uncorrected refractive errors have greatly reduced visual acuity (VA) and increased sensitivity to luminary contrast.12 Pupillary dilation in darkness diminishes with age,13 as does scotopic vision.14 The age-related reduction in scotopic visibility is greatest for violet light and light of shorter wavelengths, while the measurable reduction in scotopic, age-related visibility is much smaller for light of longer wavelengths and white light.14
Indoor lighting conditions become increasingly important as people get older and spend more time indoors, and good lighting is fundamental to a person’s ability to cope in his or her everyday life. Indoor lighting should provide a good working light and adequate overall illumination.15 Room lighting should be adapted to the function of particular areas of a room; for visually demanding work, such as reading and needlework, general room lighting is often inadequate.15 Lighting conditions in the home are also important for those with a hearing impairment, enabling them to read faces and lips as this compensates for their hearing loss.4 Adequate lighting in the home may also help to prevent accidents and falls.4,16 Despite these well-known issues, the lighting requirements and adapted conditions that should be imposed to compensate for the loss of eyesight and hearing in the elderly have been discussed infrequently within health-care research. Indeed, we have identified only one study concerning the relationship between indoor home lighting and quality of life in the healthy elderly. Sörensen and Brunnström17 have showed that improved indoor lighting significantly increased the quality of life in healthy elderly people living at home. In an ongoing Norwegian study,18 preliminary results indicate that the indoor lighting levels in the homes of 75-year-old persons are far below the recommendations.19,20
In a previous descriptive study,9 self-assessed vision and hearing were not strongly correlated with the results from standardized tests. The elderly persons themselves and health personnel tend to under-communicate both the existence of this problem and any problems related to sensory impairments.5,21,22 Simple actions, such as the removal of earwax or improving the lighting conditions in the home, may improve the vision and hearing and contribute to greater enjoyment of life among the elderly. A functional house, adapted to meet future challenges in different phases of life, is an official goal for Norwegian health authorities.23
Adaptation and adjustment of the environment, including the home, is also important in order to promote participation of the elderly in social gatherings with family and friends;24 participation in meaningful activities is necessary for the individual’s experience of having a good aging period.25,26 An increased focus on the amounts of daylight and artificial light in a home could help to prevent accidents therein.27,28 The presiding assumptions of the current study were that elderly aged over 80 years living in the home-care situation could be suffering from untreated vision and hearing impairments and that their houses were insufficiently lit, with lighting levels below the recommended Norwegian lux values.19 The aim of this intervention study was thus to evaluate the changes in vision, hearing, and lighting conditions at home following a clinical intervention. It was hypothesized that after a 10-week follow-up, the patients in the intervention group (IG) would have improved vision and hearing and enhanced indoor lighting in the home compared to the control group (CG) participants.
Methods
Design
This study was an exploratory randomized controlled study of sensory impairments and the lighting conditions in the homes of elderly persons aged over 80 years who receive home care.
Setting
Five municipalities (four rural and one borough), with populations ranging from ~9,000 to ~26,500, located in the southeast of Norway participated in the study. For all participants, the hearing center and eye specialists were located within a 1-hour drive (there were opticians in each municipality).
Sample and randomization process
The sampling process is shown in the flow chart in Figure 1.
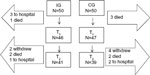  | Figure 1 Flow chart showing the samples at T1 and T2. |
The intervention was planned for two groups of 50 patients, based on a calculation to detect 1) an improvement of 20 lux with a probability of 0.90 from Test time 1 (T1) to Test time 2 (T2) and 2) a maximum risk of 5% of a type 1 error occurring. Inclusion criteria for the participants were ≥80 years old, receiving home care, and able to speak Norwegian. The exclusion criteria were the presence of obvious cognitive impairment, dementia, or palliation as documented in the medical chart or by clinical judgment by the primary nurse. Initially, the 50 participants for the IG were randomly drawn from the home-care patient lists. Then, the 50 patients to be included in the CG were randomly drawn from among the remaining home-care patients.
All of the subjects provided written informed consent to participate. The study was approved by the Norwegian Social Science Data Service and by the Regional Ethics Committee for Medical Research.
Training program
The ten nurses who mapped, surveyed, and conducted the follow-up for the elderly persons in the IG attended a training program, which was developed and performed in order to increase their knowledge about vision, hearing, and the lighting conditions in the homes of the elderly. The participants were trained to apply the metrics for these three parameters. The measures that could be taken to improve the conditions within each of the three parameters were also explored.
Intervention
The intervention consisted of evaluations at T1 and T2 which were executed at the start and the end of the 10-week intervention period, respectively, in both the IG and the CG. The patients in the IG received individual advice regarding improvements to their home lighting conditions at T1. If the lux values were below the recommended values19 or if there were lighting factors that negatively affected the participants’ daily functioning, reading, or communication abilities, the IG participants were advised as to how to improve the lighting conditions in their homes. The IG patients were referred to a specialist if the evaluation at T1 revealed impairments of vision and/or hearing or were advised to have their ears rinsed of earwax. If the hearing test revealed a Pure Tone Average (PTAV) value at T1 of ≥35 dB or if the vision test yielded a VA of ≤0.7, the IG patient was referred to a specialist. They were not referred to a specialist if they already were in a treatment course. The nurses visited the patients in the IG once a week during the 10-week intervention period. These visits were the nurses’ regular visits to these patients, and they were instructed to ask the patients while they were there whether they needed any help to book an appointment with the doctor or if there were other issues related to the project for which they needed help. All the participants in the study were surveyed to identify those who had problems, including those who were unaware of their impairments, and to provide specific and individual advice.
The intervention was based on the theory of self-care management.29 We assumed that it would be in the best interests of the elderly patients to follow the advice they were given. The nurses were not able to come with the participants to the doctor, but they were able to help them to arrange the visit. The nurses provided information to the IG patients regarding how to increase the lighting levels in their homes, if necessary, and the changes that would be wise to make, such as adding more lamps or installing a lighting control system that would allow the user to operate all of the lamps in one room with a single switch.
The patients in the CG were evaluated in the same manner by two specially trained nursing assistants and the first author (GGH), who obtained the same training as the nurses. In addition, a lighting designer controlled the lux values for 20 patients in the CG and 20 patients in the IG to ensure that the experimental setup was correct. However, the CG patients were not referred to specialists if their vision or hearing was considered to be impaired, and they were not provided with advice as to how to improve their home lighting conditions if found to be inadequate, until after the completion of the study.
Data collection
The data for T1 were collected during October and November 2011, when general outdoor light levels in Norway are low for much of the day. Similarly, the data for T2 were collected during January and Feburary 2012, to match the lighting conditions for T1. The lighting values were measured at approximately the same time of the day both at T1 and T2.
Instruments
The instruments used for the mapping and the variables measured are listed in Table 1.
  | Table 1 Instruments and measured variables |
Logarithm of the minimum angle of resolution chart
The mapping tools used in this study included VA screening with a logarithm of the minimum angle of resolution (LogMAR) distance acuity chart, which measures the minimum angle of resolution on a logarithmic scale.30 The LogMAR chart was originally developed for use in children, but it has been shown to give measurements equivalent to the Snellen chart in adults.31 It has also been used in other surveys in older people.32 It was chosen because it provides the most valid data, and it is easy to learn, easy to use, and easy for the nurses to transport on home visits. VA (LogMAR) values ranging from 0.0 to 1.0 (equivalent to Snellen 20/20 to 20/200) were measured at a distance of 4 or 6 m, depending on the room, under the participants’ usual lighting conditions (ie, habitual lighting). When using a distance of 4 m, the results were recalculated to express comparable values. The VA test was performed in the presence of best correction (ie, spectacles). The reference values for vision were categorized according to the reference values cited by the World Health Organization (WHO)33 as follows: VA ≥0.8, normal vision; VA =0.4–0.8, slightly visually impaired; and VA ≤0.4, visually impaired.
Eardrum examination
The ear was inspected for earwax, and an otoscope (Mini 3000®; HEINE Optotechnik, Herrsching, Germany) was used to examine the eardrum.
Pure-tone audiometry
Pure-tone audiometry was conducted in accordance with the modified Hughson–Westlake ascending technique, as specified in EN ISO 8253-1:2010,34 using a portable manual audiometer (SA201-IV; Entomed Norge AS, Lillestrøm, Norway) equipped with circumaural earphones (Sennheiser HDA200). Audiometric thresholds were established separately for the left and right ears using the M4 recommendation of the WHO, which requires the sensitivity to be measured at frequencies of 500 Hz, 1,000 Hz, 2,000 Hz, and 4,000 Hz to estimate mean hearing loss.35 The PTAV is the average value of these frequencies. The severity of hearing impairment was thus categorized using the PTAV value. Reference values for hearing were also categorized according to those cited by the WHO36 as follows: PTAV ≤25 dB, no impairment; PTAV =25–40 dB, slight impairment (hearing aid may be needed); PTAV =40–60 dB, moderate impairment (hearing aid usually recommended); PTAV =61–80 dB, severe impairment; and PTAV ≥80 dB, profound impairment.36 A hearing loss of >40 dB is considered a disabling hearing impairment.37 Pure-tone audiometric tests are constructed to examine the hearing capacity without hearing aids and standard procedure was used.
Assessment of indoor lighting
A light-measuring device (Hagner ScreenMaster; B Hagner AB, Solna, Sweden) was used to measure the lighting levels (lux values) in the patients’ homes. The living room was divided into 1×1 m2 grids and light levels were measured 80 cm above the floor level in each square.15 The minimum and maximum lighting levels in the living room were measured, and the average lux value was calculated, as was that for a reading light if the participant had one. Recommendations do exist for lighting levels in certain environments; eg, a lighting level of 500–700 lux is recommended in a ward office. Different recommendations for elderly or visually impaired people have been suggested, but there does not seem to be any international consensus on lower lux limits. According to a Norwegian guideline, the recommended values for older people are as follows: hall and corridor, 300–500 lux; living room (general lighting), 200 lux; dining table, 500 lux; and reading light, 750–1000 lux.21 An American guideline sets 300 lux as the recommended lowest limit for ambient light in living areas (including the living room), and 750 lux for task lighting.20
Kombinert Alvorlig Sansesvikt: the Combined Serious Sensory Impairment interview guide
The severity of the sensory impairments was assessed by the participants themselves using the Kombinert Alvorlig Sansesvikt (KAS) screen,16 which consists of 110 standardized questions designed to reveal the subject’s assessment of their own sensory impairments. The KAS screen, which is reportedly adequate for detecting vision and hearing impairments in the elderly,16,38 provides patient information about the following nine subscales: 1) background (sociodemographic data), 2) vision and hearing, 3) verbal communication and social life, 4) access to information, 5) orientation and mobility, 6) activities of daily living (ADL)/instrumental ADL, 7) health issues and the need for help, 8) social network and where they live, and 9) financial situation and special circumstances. The questions considered to be of relevance for this study are from the following subscales: background, vision and hearing, verbal communication and social life, access to information, and orientation and mobility. Certain questions that were considered irrelevant were excluded, such as those pertaining to whether they used a white stick or a hand alphabet, because none of the patients used them.
Sociodemographic data
Demographic data were obtained from the KAS screen questionnaire.16
Data analysis
Statistical analysis was performed with the Statistical Package for Social Sciences software for Windows (version 20.0). Descriptive statistics were used to examine the frequencies for lighting conditions, vision, and hearing, as well as for data from the KAS screen questionnaire. Independent-samples t-test, paired-samples statistics, and one-way analysis of variance (ANOVA) were used to calculate the relationships between groups and variations within groups. Linear regression analysis, logistic regression, and χ2 tests across tabulations were used to examine the relationship between variables. The cutoff for statistical significance was set at P<0.05.
Results
Participants
At T1, the IG comprised 38 females and eight males and the CG comprised 34 females and 13 males. These figures at T2 were 34 females and seven males in IG and 29 females and ten males in CG. The sociodemographic data of the participants are presented in Table 2.
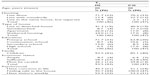  | Table 2 Sociodemographics at T1 |
All of the participants had serious health challenges. Most of them rarely left their houses, and 62% never left home alone. As many as 73% of the participants could not use public transport because of health issues, and 26% needed an escort or help to take a taxi.
Visual status
The results from the vision test are presented in Table 3. There were some slight improvements in the VA in both groups between T1 and T2; these improvements did not differ significantly between the groups (one-way ANOVA between the groups, P=0.57; Table 3).
  | Table 3 Measured lighting levels (lux) at T1 and T2 |
While there was no significant improvement in VA in the IG, there was a significant improvement in the CG from T1 to T2. The result from the vision test analyzed by paired-samples t-test revealed that mean change from T1 to T2 in IG changed from VA =0.49 to 0.51 and from VA =0.40 to 0.43 in the CG (P=0.28 and 0.03, respectively). The statistical change was a minimal improvement (from 0.40 to 0.43). Independent-samples t-test between the groups revealed that there was a significant change (P=0.026). This test showed that the change in the IG was different from the change in the CG.
Following instructions to offer referrals to those in the IG with a VA of <0.7, 38 participants should have received a referral, but a referral was only provided for eleven participants. The remaining 27 participants were already in a treatment course for their vision impairment. However, notes from the open-ended questions in the KAS screen revealed that 15 of the 27 participants did not want any advice or specialist referrals. They stated that they were too old and sick and that it was too much trouble for them to apply the suggested interventions. Furthermore, nine had been to an ophthalmologist or optician during the previous year, and three had regular appointments with an ophthalmologist. The remaining eleven patients were offered a referral to an optician or an ophthalmologist, but only two had been to the specialist by T2, and none of them had bought new spectacles.
Hearing status
The results from the pure-tone audiometry test are presented in Table 4. The changes within the groups revealed small improvements in the IG and no change in the CG between T1 and T2 The mean change between these two time points was −0.94 in the IG and 0.38 in the CG. One-way ANOVA showed that the difference between the groups was not significant (P=0.10). Paired-samples t-test showed that self-assessment of hearing had changed significantly in the IG (P=0.012) but not in the CG by T2.
There were no significant improvements in PTAV either in IG or in CG from T1 to T2. Paired-samples t-test revealed that mean change from T1 to T2 was PTAV =38.69 to PTAV =37.74 in the IG and from PTAV =42.48 to PTAV =42.86 in the CG (P=0.13 and P=0.45, respectively). Independent-samples t-test between the groups revealed that there was no significant change (P=0.098).
Following instructions to offer referrals to those in the IG with a PTAV of >35 dB and who were not in a current course of treatment for their hearing impairment, 23 participants were assessed as suffering from hearing impairment, and all were referred to a specialist. Two of the respondents (one each in the IG and the CG) were not able to complete the pure-tone audiometry test at T1; the nurses reported that this was either because they could not hear sufficiently well to perform the test or because they failed to adhere to the instructions they were given when performing the test. The analysis in this paper is based on those who completed the test at both T1 and T2.
Notes from the open-ended questions in the KAS screen and the nurses’ logbooks revealed that 23 participants in the IG were offered a referral to a hearing specialist and five were advised to contact their general practitioners to have earwax removed. Half of those who received a referral to a hearing specialist had made an appointment to attend or had already visited a specialist at least once by T2, but they had not yet received a new hearing aid. All five participants who were advised to visit their general practitioners for removal of earwax had done this by T2.
Self-assessment of vision and hearing
Self-assessment of vision and hearing was tested by the nonparametric Wilcoxon rank-sum test. For the IG, the self-assessment was significantly higher at T2. The P-value was 0.025 and 0.008 for vision and hearing, respectively. This means that the IG participants assessed both their vision and their hearing to be better by T2 compared to the same at T1.
Indoor lighting
The lighting levels measured at T1 and T2 are presented in Table 3. It is worth noting that the indoor lighting level increased between T1 and T2 by an average of 70 lux in the IG, while it decreased by 5 lux in the CG (P=0.009). The maximum lighting level in the living room increased by 129 lux in the IG (n=34) and decreased by 26 lux in the CG (n=36). The difference between the two groups was significant (P=0.002). The minimum lighting level in the living room increased by 35 lux in the IG and decreased by 0.37 lux in the CG (P=0.039). The reading light level increased by 94 lux in the IG and decreased by 38 lux in the CG (P=0.20).
Four participants had moved into residential care after T1, rendering it impossible to compare the lighting conditions in their homes between T1 and T2. The lux values were miscalculated in ten homes in one of the municipalities, and so the number of participants for whom the lighting levels in their homes were measured at T1 was 36 in the IG and 37 in the CG; these figures at T2 were 32 and 31, respectively.
The lighting levels measured in the living room were generally low and were also significantly below the recommended levels19,20 at T2. At T1, there was considerable variation across the participants, but no significant sex difference in the average lighting levels. A reading light was used by 31.2% of the men but only by 17.3% of the women. The lux values of these reading lights were also clearly below the recommended value of 750–1000 lux (Table 3).
Notes from the open-ended questions in the KAS screen and the nurses’ logbooks revealed that 30 of the IG participants received advice concerning their indoor lighting conditions. Advice about artificial lighting included adding more lamps or moving lamps, changing to brighter light bulbs, reducing glare, using more of the lamps already in the house, or installing a light control system that connected all of the lamps in the living room to one light switch with a dimmer. Advice about natural lighting comprised removing plants or objects from the window frame and changing the curtains if they were dark. At T2, none of the IG patients had installed a lighting control system or bought new lamps, but some had changed to brighter light bulbs and switched on more of the lamps in their homes. None of them had changed their curtains, but three had removed objects or plants from the window to allow more daylight to enter the room.
KAS screen
The results from the KAS screen questionnaire are presented in Table 4. The KAS screen scores changed significantly from T1 to T2 in the IG with respect to the ability to understand when people talk too fast, too quietly, or not clearly (P=0.03).
Discussion
The baseline data of the first evaluation at T1 uncovered that the vision and hearing of the participants in the study (both in the IG and the CG) were impaired.9 The data demonstrated that the indoor lux values were clearly below the recommended values.19,20
The aim of this intervention study was to identify changes between the IG and the CG following a randomized, controlled, 10-week clinical intervention. Although there were significant changes between T1 and T2 in the lighting levels between the two groups, the lux values in the IG were still clearly below recommended values at T2. The actions taken by the elderly in the IG to improve the lighting conditions in their homes during the follow-up period were not sufficiently in line with the recommendations given by the nurses. This might partly explain why the improvements in visual function at T2 in the IG were not statistically significant. At the same time, an unexpected finding was a statistically significant improvement in VA in the CG at T2, which could be due to a placebo effect.39–41 It is not uncommon that people concentrate more at the follow-up test (T2).10,11,42
Another possible explanation is that only two of the patients consulted a specialist and obtained a recommendation for new custom spectacles, with none of the participants actually obtaining new spectacles or vision or hearing aids during the intervention period. Moreover, there were no detailed notes on how often the elderly maintained or cleaned the spectacles or hearing aids they already had.
Of the 46 participants in the IG at T1, only 17% had normal VA values, while at the same time, 57% assessed their vision as being good. In addition, Wilcoxon rank-sum test revealed a significant improvement in the self-assessment of vision in the IG by T2. There was also a significant change in lux values from T1 to T2 for this group. Thus, although there was no significant improvement in the VA for the IG participants, they may have experienced a difference at T2. They may have found it somewhat easier to read or to manage their daily life by T2 when they had switched on more lights. Another explanation could be the Hawthorne effect,43–46 whereby the participants felt that they were expected to say that they had experienced an improvement after the intervention.
The Wilcoxon rank-sum test for the self-assessment of vision and hearing revealed a significant improvement in the IG by T2 (P=0.025 and P=0.008, respectively).
One of the assumptions for this intervention study was that because self-care management would be in the elderly’s own best interests, they would follow the advice given to them. Although several of them did not follow the advice they were given, those who did follow at least some of the advice experienced improved hearing, vision, and home lighting conditions. The presence of some changes despite the relatively modest nature of the interventions suggests that even relatively small efforts have a great potential for improving sensory impairments.
As a consequence of the normal aging process, the changes in vision and hearing often are gradual; so, the person may not notice it and perhaps neither see nor consider the need for an improvement. It may also be difficult for the elderly to admit the loss of either vision or hearing or to accept that situation.47–49 Furthermore, the everyday life of a nurse is hectic and involves many tasks; the nurses who delivered the intervention and follow-up had attended only a 4-day course in preparation. Focusing on the senses involves new tasks and, in practice, probably requires a little more time to develop the necessary skills to both measure the impairments and to implement the improvements.
When asked about their finances, more than 50% of the participants stated that they must be careful with money, and so economic factors could also have influenced why they did not buy new lamps or new spectacles. In addition, the participants were on average 89 years old and thus constituted a group of elderly people who might have experienced economic hardship during their childhood and upbringing because they grew up during the recession in the 1930s and during World War II. According to Hauge et al,50 those born from the beginning of the 20th century to the 1930s are often called the “strugglers” as many grew up with fathers who were unemployed. Similarly, those having less financial concerns might be characterized by a cautious attitude toward spending money. If purchasing lamps or spectacles required them to save money, or would adversely affect their budget, they may not prioritize it.51
Furthermore, taking a taxi to a specialist was costly in terms of both money and effort for these participants; if they were not guaranteed a positive result, they might not consider the effort to be worthwhile. It is possible that when they realized they had to implement the improvements themselves, they considered the costs – both financial and with respect to the effort required – too high.9
We suggest that future recommendations could be closely and carefully drafted to assist the elderly to achieve optimal indoor lighting in their homes and to optimize their vision and hearing functions. The home-care nurse could play an important role in this context. Simple periodic evaluations could reveal impairments or restrictions at an early stage. It is important that nurses who work with the elderly have knowledge of 1) age-related changes and diseases related to vision and hearing and 2) the consequences of sensory loss on factors of daily life like ADL/instrumental ADL, falls, loneliness, and the quality of life.10,11,16,17 Furthermore, we recommend increased knowledge and awareness about what can be improved with regard to sensory impairments and lighting conditions in the home.
The intervention implemented in this study was not sufficient for this patient group, perhaps because of reduced motivation to make any changes. We assume it could be related to their age and to their health condition. The sum total of high age, frailty in combination with expenses, and strenuous movement could be an explanation. This indicates that the organization of health services related to vision and hearing may be brought home to the patients instead of the elderly having to seek the service in a hospital outside of their home. In addition, the competence in these areas needs to be increased among nurses in community health care.
The study showed that the participants were too old and too fragile to fully comply with the advice and that they needed a lot more help and supervision than was envisaged in the design of this study. However, the intervention may be adequate for younger seniors.
Limitations
A possible limitation is that it was not possible to blind the nurses as to the patients’ group identity because the evaluations and follow-up sessions were conducted by the same nurses. In addition, some nurses may have been more motivated than others, perhaps making a greater effort to persuade their patients to follow their advice. To have more control with the placebo effect, which is common in intervention studies,38–40 it would probably have been an advantage to have had a control group with another intervention and a reference group.
Conclusion
Several of the elderly in the study did not follow all of the advice offered by the study nurses; however, those who did follow at least some of the advice experienced improvements in vision, hearing, and home lighting. There is thus reason to believe that even modest measures have great potential for improving vision and hearing. Elderly home-care patients cannot be expected to take the necessary actions to meet their sensory impairments themselves; they need close assistance and help to see a specialist and to improve the indoor lighting conditions in their homes. Even small steps such as changing to brighter light bulbs and switching on more lamps in the house can have an encouraging effect. It is recommended that such interventions be applied to the elderly at an earlier age than those evaluated in this study, who had a mean age of 89 years.
Disclosure
The authors report no conflicts of interest in this work.
References
Aarhus L, Kvestad E, Tambs K, Engdahl B. Aldersrelatert hørselstap: En kort oppsummering av resultater fra Hørselsundersøkelsen i Nord-Trøndelag [Age-related hearing loss: a summary of results from the hearing survey in Nord Trøndelag]. Nor Epidemiol. 2012;22(2):175–176. Norwegian. | |
Abdelhafiz AH, Austin CA. Visual factors should be assessed in older people presenting with falls or hip fracture. Age Ageing. 2003;32(1):26–30. | |
Arlinger S. Negative consequences of uncorrected hearing loss-a review. Int J Audiol. 2003;42(2s):17–20. | |
Grue EV. Vision and hearing impairment in old age, Thesis, University of Oslo, Oslo, 2010. | |
Solheim J. Hearing loss in the elderly: consequences of hearing loss and considerations for audiological rehabilitation, Thesis, University of Oslo, Oslo, 2011. | |
Bergman B, Rosenhall U. Vision and hearing in old age. Scand Audiol. 2001;30(4):255–263. | |
Gates G, Mills J. Presbycusis. Lancet. 2005;366(9491):1111–1120. | |
Rees T, Duckert L, Carey J. Auditory and vestibular dysfunction. In Hazzard WR, Blass JP, Ettinger WH, Halter JB, Oustlander JG, editors. Principles of Geriatric Medicine and Gerontology. New York: McGraw-Hill; 1999:617–631. | |
Haanes GG, Kirkevold M, Horgen G, Hofoss D, Eilertsen G. Sensory impairments in community health care: a descriptive study of hearing and vision among elderly norwegians living at home. J Multidiscip Healthc. 2014;7(42):217–225. | |
Burmedi D, Becker S, Heyl V, Wahl H, Himmelsbach I. Emotional and social consequences of age-related low vision. Vis Impair Res. 2002;4(1):47–71. | |
Brody BL, Gamst AC, Williams RA, et al. Depression, visual acuity, comorbidity, and disability associated with age-related macular degeneration. Ophthalmology. 2001;108(10):1893–1900. | |
Seim T, Valberg A. Image diffusion in cataracts affects chromatic and achromatic contrast perception differently. In: Drum B, editor. Colour Vision Deficiencies XI. Vol 56. Netherlands: Springer; 1993:153–161. | |
Kadlecova V, Peleska M. Dependence on age of the diameter of the pupil in the dark. Nature. 1958;182(4648):1520–1521. | |
Gunkel R, Gouras P. Changes in scotopic visibility thresholds with age. Arch Ophthalmol. 1963;69(1):4. | |
Bjørset H-H. Lys og belysning i arbeidsmiljøet [Light and Lighting Issues in Working Environment]. Trondheim: Tapir; 1994. Norwegian. | |
Lyng K, Svingen EM. Kartlegging av alvorlig, kombinert sansetap hos eldre [Screening of Serious, Combined Impairment in Elderly]. Oslo: NOVA; 2001. Norwegian. | |
Sörensen S, Brunnström G. Quality of light and quality of life: an intervention study among older people. Lighting Res Technol. 1995;27(2):113–118. | |
Egilsdottir O, Falkenberg H, Horgen G, Eilertsen G. Happy living in darkness! Lighting in old age is related to thriving and healthy ageing in 75 year old Norwegians. In: The 20th IAGG Congress of Gerontology and Geriatrics; June 23–27, 2013; Seoul, Korea. | |
Lyskultur. Belysning for eldre og svaksynte [Lighting Conditions for Elderly and Visually Impaired People]. Vol. 11/97. Sandvika: Lyskultur; 1997. Norwegian. | |
America IESoN. Recommended Practice for Lighting and the Visual Environment for Senior Living. New York: ANSI/IESNA; 2007. Product ID: RP-28-07. | |
Wallhagen M, Pettengill E. Hearing impairment: Significant but underassessed in primary care settings. J Gerontol Nurs. 2008;34(2):36–42. | |
Wallhagen M, Strawbridge W, Shema S, Kurata J, Kaplan G. Comparative impact of hearing and vision impairment on subsequent functioning. J Am Geriatr Soc. 2001;49(8):1086–1092. | |
Norwegian Ministry of Health and Services. Samhandlingsreformen: rett behandling – på rett sted – til rett tid [The Coordination Reform]. Oslo: Norwegian Ministry of Health and Services; 2009:149. Norwegian. | |
Vik K. Older adults’ participation in occupation in the context of home-based rehabilitation, Thesis, Karolinska Institutet, Division of Occupational Therapy, Huddinge, 2008. | |
Carlson M, Clark F, Young B. Practical contributions of occupational science to the art of successful ageing: how to sculpt a meaningful life in older adulthood. J Occup Sci. 1998;5(3):107–118. | |
Gabriel Z, Bowling A. Quality of life from the perspectives of older people. Ageing Soc. 2004;24(5):675–691. | |
Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence: unifying the approach to geriatric syndromes. JAMA. 1995;273(17):1348–1353. | |
Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Health. 2004;94(5):823. | |
Orem DE, Taylor SG, Renpennig KM. Nursing: Concepts of Practice/Dorothea E Orem; with a Contributed Chapter by Susan G Taylor and Kathie McLaughlin Renpenning. 6th ed. St Louis, MO: Mosby; 2001. | |
McGraw PV, Winn B. Glasgow acuity cards: a new test for the measurement of letter acuity in children. Ophthalmic Physiol Opt. 1993;13(4):400–404. | |
McGraw PV, Winn B, Gray LS, Elliott DB. Improving the reliability of visual acuity measures in young children. Ophthalmic Physiol Opt. 2000;20(3):173–184. | |
van der Pols JC, Bates CJ, McGraw PV, et al. Visual acuity measurements in a national sample of British elderly people. Br J Ophthalmol. 2000;84(2):165–170. | |
WHO. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease. Geneva: ERIC Clearinghouse; 1994. | |
International Organization for Standardization. Acoustics–Audiometric test methods–Part 1: Pure-tone air and bone conduction audiometry. ISO 8253-1:2010. Geneva: International Organization for Standardization; 2010. | |
WHO. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision: Introduction; List of Three-Character Categories; Tabular List of Inclusions and Four-Character Subcategories; Morphology of Neoplasms; Special Tabulation Lists for Mortality and Morbidity; Definitions; Regulations. Geneva: World Health Organization; 1992. | |
WHO. Grades of Hearing Impairment; 2014. Available from: http://www.who.int/pbd/deafness/hearing_impairment_grades/en/. Accessed February 10, 2014. | |
Mathers C, Smith A, Concha M. Global burden of hearing loss in the year 2000. Global Burden of Disease 2000; Available from: http://www.who.int/healthinfo/statistics/bod_hearingloss.pdf. Accessed February 11, 2014. | |
Grue EV, Kirkevold M, Mowinchel P, Ranhoff AH. Sensory impairment in hip-fracture patients 65 years or older and effects of hearing/vision interventions on fall frequency. J Multidiscip Healthc. 2009;2:1–11. | |
Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159(17):1602–1606. | |
Moerman DE. Meaning, Medicine, and the “Placebo Effect”. Vol. 28. Cambridge: Cambridge University Press; 2002. | |
Moerman DE, Jonas WB. Deconstructing the placebo effect and finding the meaning response. Ann Intern Med. 2002;136(6):471–476. | |
Young L, George J. Do guidelines improve the process and outcomes of care in delirium? Age Ageing. 2003;32(5):525–528. | |
McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7(1):30. | |
Adair JG. The Hawthorne effect: a reconsideration of the methodological artifact. J Appl Psychol. 1984;69(2):334. | |
Parsons HM. What happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974;183(4128):922–932. | |
Jones SR. Was there a Hawthorne effect? Am J Sociol. 1992;98:451–468. | |
Brennan M, Bally SJ. Psychosocial adaptations to dual sensory loss in middle and late adulthood. Trends Amplif. 2007;11(4):281–300. | |
Tolson D, Swan I, Knussen C. Hearing disability: a source of distress for older people and carers. Br J Nurs. 2002;11(15):1021–1025. | |
Heine C, Browning C. Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disabil Rehabil. 2002;24(15):763–773. | |
Hauge S, Jacobsen FF, Alvsvåg H. Hjem: eldre og hjemlighet [Housing for the Elderly]. Oslo: Cappelen akademisk forl; 2008. Norwegian. | |
Ytrehus S. Interpretation of housing needs? A critical discussion. Hous Theory Soc. 2000;17(4):166–174. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

