Back to Journals » Veterinary Medicine: Research and Reports » Volume 15
Antibiogram of Escherichia coli Isolated from Dairy Cattle and in-Contact Humans in Selected Areas of Central Ethiopia
Authors Tadesse T , Alemayehu H , Medhin G, Akalu A, Eguale T
Received 22 December 2023
Accepted for publication 3 April 2024
Published 10 April 2024 Volume 2024:15 Pages 117—127
DOI https://doi.org/10.2147/VMRR.S456247
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Tekalign Tadesse,1,* Haile Alemayehu,2 Girmay Medhin,2 Aberaw Akalu,3 Tadesse Eguale2,4,*
1Department of Veterinary Science, Mattu University, Mattu, Ethiopia; 2Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia; 3Food, Medicine and Healthcare Administration and Control, Addis Ababa, Ethiopia; 4The Ohio State University, Global One Health LLC, Addis Ababa, Ethiopia
*These authors contributed equally to this work
Correspondence: Tekalign Tadesse, Department of Veterinary Science, Mattu University, P.O Box 318, Mattu, Ethiopia, Email [email protected]
Background: Antimicrobial resistance (AMR) is a global threat to public and animal health. Escherichia coli is considered an indicator organism for monitoring AMR among gram-negative Enterobacteriaceae in humans and animals. The current study aims to assess the antibiogram profile of E. coli isolated from dairy cattle and in-contact humans in central Ethiopia and to identify risk factors associated with multidrug resistance (MDR).
Methods: A cross-sectional study was conducted in which 58 farms were recruited from selected districts of central Ethiopia. E. coli was isolated using standard bacteriological techniques. A total of 200 representative isolates (140 from cattle and 60 from humans in contact) were randomly selected and tested for susceptibility to a panel of 13 antimicrobials using the Kirby-Bauer disc diffusion assay.
Results: The highest rate of resistance was observed for sulfamethoxazole+trimethoprim (58.6%, 82/140) and amoxicillin+clavulanic acid (70.0%, 42/60) among E. coli isolates from cattle and hmans, respectively. In contrast, resistance rates in isolates from in contact humans with the cattle were 30%, 33.3%, and 66.7%, respectively. Resistance to tetracycline (p=0.02), streptomycin (p=0.03), and sulfamethoxazole+trimethoprim (p=0.007) was significantly high in E. coli isolated from cattle on commercial dairy farms than in those isolated from cattle on smallholder farms. There was no significant difference (p> 0.05) in the rate of resistance between E. coli isolated from in contact humans with smallholder and commercial dairy farms. Antimicrobial use for treatment purpose (p=0.04) and non-compliance with the drug withdrawal period (p=0.03) were significantly associated with the farm-level occurrence of MDR.
Conclusion: A high rate of resistance was detected in E. coli isolated from the feces of dairy cattle and in-contact humans. This necessitates an effective intervention through a one-health approach and further molecular studies are required to establish source attribution.
Keywords: antimicrobial resistance, dairy cattle, E. coli, humans
Introduction
In the year 2050, the world population is projected to be 9.8 billion, developing nations like Africa, Asia, and Latin America will account for most of the population growth.1 To meet the population demand for proteins of animal origin, mainly milk and other dairy products, developing countries are expected to supply 61% of the world’s milk production.2 This demand can only be fulfilled if the focus is on improving production efficiency, ensuring health, and tackling the issues of antimicrobial dependency and resistance in farming practices, including dairy production.3
Antimicrobials are used in food animals for prophylaxis, therapeutic, and growth promotion.4 Although these practices are essential for animal health and provide benefits to farmers, they have been a concern regarding the global challenge of AMR.5 The indiscriminate use of antimicrobials in food animals is a potential cause of the emergence of antimicrobial-resistant bacterial strains owing to increasing selection pressure.6,7 According to the WHO-OIE-FAO tripartite report, AMR is among the top three priorities for health risks within the area of zoonotic diseases.8 Zoonotic transmission of resistant bacteria from animals to humans can occur through the food chain, direct contact with animals, and indirect contact with the environment, leading to the emergence of infectious diseases that are difficult to manage9–12 and require collaboration between different disciplines and sectors.13
Even though antimicrobial treatment has revolutionized the management of many infectious diseases, their increasing usage, inaccurate and indiscriminate prescription, incorrect dosage and duration of therapy and the over-the-counter distribution of antimicrobials has supported the increase in resistance to antimicrobial agents.14,15
The trend of an increase in AMR, particularly among gram-negative bacteria, is alarming because of the limited therapeutic options available for these organisms. Escherichia coli is generally considered a useful sentinel for AMR because of its wide host range and its ability to easily receive and donate resistance genes.16 Hence, the investigation of the AMR in E. coli, a representative population of gram-negative bacteria, is a good means of establishing the antimicrobial susceptibility status of major pathogenic and commensal gram-negative organisms circulating in a given community or environment, and can serve as a key indicator of other related bacterial pathogens in the study area.17
Although research on the prevalence of AMR in E. coli from livestock and humans has been conducted in various regions of Ethiopia,18–20 little is known about the overall epidemiology of AMR in E. coli isolates from dairy cattle under different farming scales and their in-contact humans. Therefore, this study aimed to investigate the antibiogram profile of E. coli isolated from dairy farms under smallholder and commercial setting and corresponding in-contact humans in central Ethiopia and to identify risk factors associated with occurrence of multidrug resistance (MDR).
Materials and Methods
Study Area
The study was conducted at selected sites in central Ethiopia: Akaki-Kaliti sub-city of Addis Ababa, Sululta, and Bishoftu towns in the Oromia Region. The Akaki-Kaliti sub-city is one of the 11 sub-cities in Addis Ababa. It is the city’s southernmost suburb bordering the subcities of Nifas Silk-Lafto and Bole. Bishoftu and Sululta towns are in Oromia Regional State, 47 km southeast and 23 km northwest of Addis Ababa, respectively. These areas are known to have a large number of peri-urban dairy farms and a dense human population, which paves the way for interaction between animals and humans (Figure 1).21
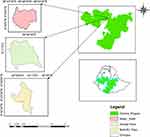 |
Figure 1 Map of the study area. |
Study Design and Study Population
This cross-sectional study was conducted between December 2022 and August 2023. The study population included Zebu, Holstein-Friesian, and crossbred cattle from smallholder and commercial dairy farms. For this study, smallholder dairy farming was defined as subsistence dairy farming systems established on small land whereby 5–20 heads of cattle are kept with the primary aim of home consumption. Commercial dairy farming is market-oriented dairy farming that uses improved dairy stocks and those kept under intensive production. They contain more than 20 cattle and require special feed inputs.22,23 Farm attendants were the subjects of this study.
Sample Size Determination and Sampling Strategies
The number of study animals was determined based on the expected prevalence of E. coli and the desired absolute precision according to a previously reported formula.24 Accordingly, the sample size was 368, as determined from a previous fecal prevalence report of E. coli in the feces of slaughtered cattle at Karalo abattoir (37%).25 The list of dairy farms available in each study area was obtained from the district agricultural office, and representative farms were selected based on accessibility, owner willingness, and representation of farms with different farm scales. A total of 58 farms (27 smallholder dairy farms and 31 commercial dairy farms) were included in this study. At each farm, farm attendants were approached for voluntary participation in interviews and stool sample collection. Therefore, 368 fecal samples from cattle and 90 stool samples from in-contact humans were collected. Dairy cattle from each farm scale were randomly selected for sample collection.
Data Collection, E.coli Isolation and Identification
The questionnaire was developed by adopting the WHO multi-country public awareness survey standard26 and previous related studies.27,28 The questionnaire was then translated into the Afan Oromo and Amharic languages and pre-tested on five dairy farms. Necessary revisions were made before the questionnaire was completed. The final approved assessment tool was established using an Open Data Kit (ODK) on mobile tablet devices. Following verbal consent from farm representatives, information about farm characteristics and the type, purpose, and frequency of antimicrobial use were collected using a structured questionnaire.
Sample Collection, Processing and Bacteria Identification
Fecal samples were collected directly from the rectum of dairy cattle by using disposable gloves in sterile zippered plastic bags. Human stool samples were obtained from any in-contact humans willing to participate in a sterile universal bottle prepared for this purpose. The collected samples were labeled and transported in an icebox to the Microbiology Laboratory of the Aklilu Lemma Institute of Pathobiology, Addis Ababa University, for microbiological analysis. Escherichia coli was isolated using techniques recommended by the International Organization for Standardization29 based on colony morphology in different media and biochemical properties. Five grams of feces was diluted in 45 mL of buffered peptone water, BPW incubated at 37°C for 24 h, and then plated on MacConkey agar. Presumptive E. coli isolates with smooth, circular, and pink colonies were selected, streaked on Eosin Methylene Blue (EMB) agar, and grown at 37°C for 24 h. Isolates with a green metallic sheen appearance on the EMB plate were considered E. coli which was further confirmed by biochemical tests such as triple sugar iron (TSI) agar, Citrate and Indole tests.30 Isolates that were positive for the TSI test, negative for citrate utilization, and positive for the indole test were considered E. coli.
Antimicrobial Susceptibility Testing
Two hundred E. coli isolates representing all 58 dairy farms were randomly selected: 140 representative isolates from cattle (68 isolates from smallholder farms and 72 isolates from commercial farms) and 60 E. coli isolates from humans in contact (30 isolates from each farm type) from 45 volunteer dairy farms were subjected to antimicrobial susceptibility testing. The selection of antimicrobials used in this study was based on the history of their local use in the treatment of cattle and human infections. Aminoglycosides, cephalosporins, tetracycline, penicillin, and sulfonamides are common antibiotics used in livestock production in the study area. Antimicrobial susceptibility testing was performed using the agar disc diffusion method, as described in the Clinical and Laboratory Standards Institute Guidelines.31 Briefly, bacterial suspensions were prepared in 0.85% sterile normal saline solution, and the concentration of bacteria was adjusted to 0.5 McFarland using standard McFarland solution.
A sterile cotton swab dipped into the standardized suspension of bacteria was uniformly streaked over the entire surface of the Mueller-Hinton agar. After the plates were dried, 13 antimicrobial disks namely: gentamicin (10 μg), chloramphenicol (30 μg), ciprofloxacin (5 μg), sulfisoxazole (25 μg), streptomycin (10 μg), tetracycline (30 μg), nalidixic acid (30 μg), ampicillin (10 μg), ceftriaxone (30 μg), cephalothin (30μg), amikacin (30μg), sulfamethoxazole+trimethoprim (23.75μg) and amoxacillin+clavulanic acid (30μg) were placed on the inoculated Muller Hinton agar plates using sterile forceps. It was then gently pressed to ensure firm contact with the agar surface and allowed to diffuse for 15 min in the prepared media. All the antimicrobial discs used in this study were Sensi-Discs (Becton, Dickinson and Company, Loveton, USA). The plates were then incubated at 37°C for 24 h. The diameter of the zone of inhibition of each antimicrobial disc was measured using calipers to the nearest millimeter and recorded using interpretative guidelines as resistant, intermediate, or susceptible.31 E. coli isolates exhibiting resistance to more than two antimicrobials belonging to different classes of antimicrobials were regarded as MDR.
Data quality was maintained throughout the laboratory work process by strictly adhering to standard operating procedures (SOPs) and the manufacturer’s instructions. Before using the prepared media for bacterial growth, sterility was tested by incubating randomly selected plates at 37°C for 24 h and analyzing for potential contamination. E. coli ATCC 25922 reference strains were used for quality control in both the culture and antibiotic susceptibility testing.
Statistical Analysis
The data were analyzed using SPSS® version 26.0 (SPSS Inc. Chicago, Il). The occurrence of E. coli in different study areas and the antimicrobial susceptibility status were summarized using frequencies and percentages. Chi-square and Fischer’s exact tests were used to compare the occurrence of antimicrobial-resistant E. coli isolated from cattle and humans in contact with the two dairy farming scale. Chi-square was used to assess the association of the occurrence of MDR with relevant factors, such as dairy farm characteristics and antimicrobial use practices. The fitness of the model was assessed using the Hosmer-Lemeshow test (goodness-of-fit). Statistical significance was set at p<0.05, indicating a significant association with the outcome of interest.
Results
Antimicrobial Usage in Dairy Farms
All farms (100%) in this study used antimicrobials for their dairy cattle, and the primary purpose was to treat sick animals in 39 (67.3%) of the farms. On many farms, veterinarians 31 (53.5%) were responsible for the treatment of cattle and veterinary drug shops and 22 (37.9%) were the main source of antimicrobials. Most of the farms 43 (74.1%) did not comply with the drug withdrawal period. The most commonly used antimicrobials in the farms were oxytetracycline, sulfonamides, streptomycin, and penicillin+streptomycin in 29 (50%), 11 (19%), 8 (13.8%), 8 (13.8%), and 2 (3.5%) of the farms, respectively.
Occurrence of E.coli in Feces of Dairy Cattle and in-Contact Humans
Of the 368 fecal samples and 90 stool samples collected from cattle and humans in contact, 280 (76%) and 60 (67%), respectively, were positive for E. coli. Although the isolation of E. coli was relatively high in the feces of dairy cattle from Sululta town 97 (79%), there was no significant difference (p>0.05) in the proportion of E. coli isolated from the feces of dairy cattle from different study areas. E. coli was isolated from at least one animal in all 58 dairy farms included in the study (Table 1).
 |
Table 1 Occurrence of E. coli in Feces of Cattle and in-Contact Humans from Different Study Areas |
Antimicrobial Susceptibility Profile of E.coli Isolates
Variable degrees of susceptibility to the tested antimicrobials were also recorded. Among the E. coli isolates tested from cattle, 82 (58.6%) were resistant to sulfamethoxazole+trimethoprim, 78 (55.7%) isolates were resistant to sulfisoxazole, 76 (54.3%) were resistant to cephalothin and 64 (45.7%) of the isolates were resistant to tetracycline (Figure 2A). E. coli isolates from humans in contact with dairy cattle showed a high proportion of resistance to amoxicillin+clavulanic acid 84 (70.0%) and ampicillin 80 (66.7%). Resistance to sulfamethoxazole+trimethoprim, streptomycin, tetracycline, and ciprofloxacin was observed in 76 (63.3%), 60 (50.0%), 52 (43.3%), and 40 (33.3%) of in-contact humans, respectively (Figure 2B). Complete resistance to ciprofloxacin, nalidixic acid, and ceftriaxone was not detected in isolates from cattle, whereas 30%, 33.3%, and 66.7% resistance was recorded among in-contact humans.
Resistance to tetracycline (p=0.02), streptomycin (p=0.03), and sulfamethoxazole+trimethoprim (p=0.007) was significantly higher in E. coli isolated from cattle in commercial dairy farms compared to those isolated from cattle in smallholder farms whereas resistance to cephalothin was higher in cattle from small scale farms (p=0.03) compared to those from commercial farms (Figure 3A). There was no significant difference (p>0.05) in the rate of resistance to all antimicrobials among E. coli isolated from in-contact humans in smallholder farms and commercial dairy farms (Figure 3B).
Antimicrobial Resistance Pattern of E.coli Isolates
Multi-drug resistance (MDR) in E. coli isolated from cattle was observed in 126 isolates (90%). Among the total isolates from cattle, 40 (28%) were resistant to three antimicrobials. A slightly higher prevalence of MDR was recorded in E. coli isolates from dairy farms in Sululta 40 (34.5%) and commercial dairy farms 54 (46.6%). MDR to as many as eight antimicrobials was recorded in two isolates obtained from cattle on a dairy farm in Bishoftu Town. The most commonly observed resistance patterns were CF-SXT, AMP-CF, TET-AMC-SXT, SLF-AMC-SXT, AMP-CF-SXT, TET-S-SLF-CF-AMC-SXT, and TET-SLF-CHL-AMC-SXT (Table 2). Among E. coli isolates from humans tested for antimicrobial susceptibility, 56 (93%) exhibited resistance to three or more antimicrobials. The highest occurrence of MDR was recorded for resistance to ten antimicrobials in the two isolates (Table 3).
 |
Table 2 Antimicrobial Resistance Pattern of E. coli Isolated from Cattle |
 |
Table 3 Antimicrobial Resistance Pattern of E. coli Isolated from in-Contact Humans |
Factors Associated with the Occurrence of Multidrug Resistant E. coli in Cattle
The occurrence of MDR was slightly higher in farms that used antimicrobials for the treatment of sick animals than in those that used antimicrobials for the prevention of various diseases and dry cow therapy for the prevention of mastitis. However, this difference was not statistically significant (p>0.05). Most of the farms 41 (95.3%) did not comply with the drug withdrawal period, and this was significantly associated with the occurrence of MDR (p=0.03). Occurrence of MDR was not significantly associated with the study areas, source of the drug and, antimicrobial administration (p>0.05) (Table 4).
 |
Table 4 Factors Associated with Occurrence of Multidrug Resistance E. coli Isolates at Farm Level |
Discussion
Although the detection of a high number of fecal E. coli isolates was not unexpected, the isolates were resistant to several antimicrobials commonly used in cattle and humans. Even if most of these isolates are commensal and do not cause disease in the host animals or humans, they serve as carriers of drug-resistant genes that are transmitted to other microorganisms, including pathogenic ones. These resistant bacterial isolates may directly cause infections, making treatment difficult in animals and increasing the risk of human infection through the food chain or direct contact.32
In the current study, the overall occurrence of E. coli in fecal and stool samples was 76% in cattle and 67% in in-contact humans. This agrees with a previous 70% report33 from Bangladesh and 69% report34 from Tanzania. However, the current figure is lower than 100% reported in a previous study in Algeria.35 The current finding was relatively higher than that reported from Kenya,36 who reported 33.7% E. coli from human stool. Such differences could be due to variation in the composition of the fecal microbiota of cattle and humans in different geographic areas.
In the current study E. coli isolates from cattle showed a higher proportion of resistance to sulfamethoxazole+trimethoprim, sulfisoxazole, cephalothin, tetracycline, and streptomycin, which is consistent with previous findings in Jordan37 and Ethiopia.25 The high number of isolates resistant to tetracycline, sulfamethoxazole, trimethoprim, and streptomycin may be linked to their wide use in most farms to treat bacterial infections in the study area.38 Resistance to cephalothin in cattle was reported in this study, despite the lack of reports on its use in cattle. This might be due to the resistance determinants from the use of other antimicrobials belonging to the beta-lactam class, such as penicillin and ampicillin. Resistance to ciprofloxacin, nalidixic acid, and ceftriaxone was not detected among E. coli isolates from dairy cattle, whereas a significantly high rate of resistance was detected among E. coli isolates from in-contact humans. This might be related to the limited use and misuse of these antimicrobials in dairy cattle and in-contact humans, respectively.
The observed high resistance rate in E. coli isolates from in-contact humans with sulfamethoxazole+trimethoprim and tetracycline agrees with the resistance rate in E. coli from cattle attendants in Tanzania against sulfamethoxazole+trimethoprim (49.0%) and tetracycline (40.8%).34 For the reported high resistance, factors such as the non-adherence to rational antimicrobial use in livestock production,39 poor farming practices and waste management systems as well as a close contact of humans with animals might have favored the increased rate of resistant E. coli and transmission from animals.16
Overall, MDR in E. coli was 90% in cattle and 93% in human isolates in the current study. The high proportion of MDR E. coli recorded in the present study might be a result of the administration of antimicrobials by relying only on tentative diagnosis and the common use of broad-spectrum antimicrobials in the study area.38–41 Human isolates of E. coli appear to have a higher occurrence of MDR than isolates from cattle. Given the consumption of raw meat and milk, frequent contact with cattle, and inadequate sanitary management, there is a considerable risk of human exposure to MDR pathogens. Even though MDR is high in both cattle and humans, the study was not able to prove zoonotic transmission of MDR strains to humans as we did not perform molecular characterization of the isolates.
The high rate of AMR to tetracycline in isolates from cattle on commercial farms compared to isolates from smallholder dairy farms is consistent with previous reports from Jordan.37 Similarly, the high rates of resistance to streptomycin, sulfamethoxazole+trimethoprim, and gentamicin in commercial farms compared to smallholder farms observed in the current study are comparable to those of previous reports.42 The high rate of resistance to these antimicrobials could be due to the common long-term and extensive use of these antimicrobials in the study area.38 The observed dissimilarity in the rate of resistance in E. coli isolates from cattle between the two farm types might be due to some driving factors of AMR, such as the intensive use of antimicrobials, availability of antimicrobials, and difference in the use of antimicrobials in cattle in a commercial farm setting.43
The proportion of resistance to selected antimicrobials, such as tetracycline, sulfisoxazole, cephalothin, sulfamethoxazole+trimethoprim, was similar in isolates from cattle and in-contact humans, which could probably be related to the possible co-selection of these antimicrobials or because most of these antimicrobials are commonly used in animals and humans in the study area. Resistance to tetracycline and sulfamethoxazole+trimethoprim was positively correlated with the history of use of these antimicrobials in dairy farms. A higher rate of resistance was recorded in cattle reared in the intensive production system. This finding is consistent with the previous reports.44 The intensification of livestock production has been associated with keeping animals in a high density in close proximity to each other, which favors the easy circulation of microorganisms within cattle on the farm.45 Moreover, animals maintained in intensive farming systems tend to have reduced variability in their normal flora and similar vulnerability to organisms.
Irrespective of economic benefits for owners and consumers and promotion of livestock well-being, frequent antimicrobial use for treatment without prior confirmatory diagnosis supplemented with irrational use of antimicrobials could give rise to the emergence and dissemination of resistant organisms.5
Conclusion
This study revealed high rates of multidrug resistance E. coli in both humans and animals, which could be attributed to the irrational use of antimicrobials in animals and humans in the study area. A high rate of antimicrobial resistance was detected in dairy cattle at the commercial farms than at small-holder dairy farms. The design of this study did not allow us to draw solid conclusions regarding the zoonotic transmission of resistant E. coli. Hence, further molecular studies are recommended to establish the genetic relatedness between E. coli isolates from dairy cattle and humans in different production systems. A follow up is recommended with a more advanced technique in antibiogram to detect the minimal inhibitory and cidal concentration of antimicrobial agents. Educating dairy farmers on the rational use of antimicrobials and their consequences is vital for reducing the emergence and spread of AMR.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki guidelines. Ethical approval for this study was obtained from the Institutional Review Board (IRB) of Aklilu Lemma Institutes of pathobiology, Addis Ababa University (ALIPB-IRB/45/2013/21). An official Letter was written to District Livestock and Fisheries Resource Office from the Aklilu Lemma Institute of Pathobiology. IRB committee approved the protocol and recommended to obtain verbal consent instead of written consent since majority of study participants were illiterate and the study does not involve invasive procedure. All information obtained from them was kept confidential. During the collection of fecal samples, the cattle were handled and treated with the utmost veterinary care.
Acknowledgments
The authors would like to express their gratitude to the farm owners and study participants for their cooperation during the collection of data and samples. Additionally, they extend their thanks to Miss Azeb Teklu for her technical assistance in the laboratory.
Author Contributions
All authors made a significant contribution to conception, study design, execution, acquisition of data, analysis and interpretation; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was financially supported by Addis Ababa University thematic research project.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. UN. Department of economic and social affairs, population division. world population prospects. Working Paper No. ESA/P/WP/248; 2018:283.
2. Food and Agriculture Organization. The future of food and agriculture: trends and challenges. In: The Future of Food and Agriculture: Trends and Challenges; 2017. Available from: www.fao.org/3/i6583e/i6583e.pdf.
3. Jayarao B, Almeida R, Oliver SP. Antimicrobial resistance on dairy farms. Foodborne Pathog Dis. 2019;16(1):1–4. doi:10.1089/fpd.2019.29011
4. Economou V, Gousia P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist. 2015;8:49–61. doi:10.2147/IDR.S55778
5. Van T, Yidana Z, Smooker PM, Coloe PJ. Antibiotic use in food animals worldwide, with a focus on Africa: pluses and minuses. J Glob Antimicrob Resist. 2020;20:170–177. doi:10.1016/j.jgar.2019.07.031
6. Aarestrup F, Wegener HC, Collignon P. Resistance in bacteria of the food chain: epidemiology and control strategies. Expert Rev Anti Infect Ther. 2008;6(5):733–750. doi:10.1586/14787210.6.5.733
7. Van Boeckel T, Brower C, Gilbert M, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci. 2015;112(18):5649–5654.
8. World Health Organization (WHO), Food and Agriculture Organisation (FAO), World Organisation for Animal Health (OIE). A Tripartite Guide to Addressing Zoonotic Diseases in Countries.; 2019. Available from: https://apps.who.int/iris/handle/10665/325620.
9. Timofte D, Maciuc I, Evans N, et al. Detection and molecular characterization of Escherichia coli CTX-M-15 and Klebsiella pneumoniae SHV-12 β-lactamases from bovine mastitis isolates in the United Kingdom. Antimicrob Agents Chemother. 2014;58(2):789–794. doi:10.1128/AAC.00752-13
10. Oliver S, Murinda S, Jayarao B. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: a comprehensive review. Foodborne Pathog Dis. 2011;8(3):337–355. doi:10.1089/fpd.2010.0730
11. Landers T, Cohen B, Wittum T, Larson E. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127(1):4–22. doi:10.1177/003335491212700103
12. Shankar P. Book review: tackling drug-resistant infections globally. Arch Pharm Pract. 2016;7(3):110. doi:10.4103/2045-080x.186181
13. World Health Organization. WHO member states adopt global action plan on antimicrobial resistance. Euro Surveill. 2015;20(21):2009. doi:10.2807/ese.20.21.21137-en
14. Jwair N, Al-Ouqaili M, Al-Marzooq F. Inverse association between the existence of crispr/cas systems with antibiotic resistance, extended spectrum β-lactamase and carbapenemase production in multidrug, extensive drug and pandrug-resistant Klebsiella pneumoniae. Antibiotics. 2023;12(6):980. doi:10.3390/antibiotics12060980
15. Al-Ouqaili M. Synthesize of pluronic-based nanovesicular formulation loaded with Pistacia atlantica extract for improved antimicrobial efficiency. Arab Journ of Chem. 2023;16:6. doi:10.1016/j.arabjc.2023.104704
16. Alonso C, Zarazaga M, Ben Sallem R, Jouini A, Ben Slama K, Torres C. Antibiotic resistance in Escherichia coli in husbandry animals: the African perspective. Lett Appl Microbiol. 2017;64(5):318–334.
17. Nyirabahizi E, Tyson G, Dessai U, et al. Evaluation of Escherichia coli as an indicator for antimicrobial resistance in Salmonella recovered from the same food or animal ceca samples. Food Control. 2020;115:107280.
18. Disassa N, Sibhat B, Mengistu S, Muktar Y, Belina D. Prevalence and antimicrobial susceptibility pattern of E. coli O157: H7 isolated from traditionally marketed raw cow milk in and around Asosa town, western Ethiopia. Vet Med Int. 2017. doi:10.1155/2017/7581531
19. Bedasa S, Shiferaw D, Abraha A, Moges T. Occurrence and antimicrobial susceptibility profile of Escherichia coli O157: H7 from food of animal origin in Bishoftu town, Central Ethiopia. Int J Food Contam. 2018;5(1):1–8. doi:10.1186/s40550-018-0064-3
20. Tuem K, Gebre A, Atey T, Bitew H, Yimer E, Berhe D. Drug resistance patterns of Escherichia coli in Ethiopia: a Meta-Analysis. Biomed Res Int. 2018;2018. doi:10.1155/2018/4536905
21. Eguale T, Engidawork E, Gebreyes WA, et al. Fecal prevalence, serotype distribution and antimicrobial resistance of Salmonellae in dairy cattle in central Ethiopia. BMC Microbiol. 2016:1–11. doi:10.1186/s12866-016-0638-222
22. CSA (Central Statistics Agency). Agricultural Sample Survey. Volume II Report on Livestock and Livestock Characteristics (Private Peasant Holdings), Central Statistical Agency (CSA). Addis Ababa, Ethiopia; 2015.
23. Gizaw S, Abera M, Muluye M, Hoekstra D, Gebremedhin B, Tegegne A. Smallholder dairy farming systems in the highlands of Ethiopia: system-specific constraints and intervention options. Int Livest Res Inst. 2016;2:24.
24. Thrusfield M. Veterinary Epidemiology.
25. Tilahun G Antimicrobial susceptibility of Escherichia coli isolates from feaces of slaughtered cattle, beef carcass and abattoir environment at Karalo abattoir and surrounding butcher shops, Addis Ababa, Ethiopia; 2020:2416. Available from: http://etd.aau.edu.et/handle/123456789/23198.
26. World Health Organization. Antibiotic Resistance: Multi-Country Public Awareness Survey. Geneva, Switzerland: World Health Organization; 2015.
27. Sadiq M, Syed-Hussain S, Ramanoon S, et al. Knowledge, attitude and perception regarding antimicrobial resistance and usage among ruminant farmers in Selangor, Malaysia. Prev Vet Med. 2018;156:76–83.
28. Azabo R, Mshana S, Matee M, Kimera SI. Antimicrobial usage in cattle and poultry production in Dar es Salaam, Tanzania: pattern and quantity. BMC Vet Res. 2022;18(1):1–12. doi:10.1186/s12917-021-03056-929
29. ISO. Microbiology of food and animal feeding stuffs — horizontal method for the detection of Escherichia coli and Salmonella in animal faeces and in environmental samples from the primary production stage. Geneva. 2007;6579:2017.
30. Quinn P, Markey B, Leonard C, Hartigan P, Fanning S. A Text Book of Veterinary Microbiology and Microbial Disease.
31. CLIS. Clinical and laboratory standards institute: performance standards for antimicrobial susceptibility testing supplement M100S.; 2017.
32. Founou L, Founou R, Essack S. Antibiotic resistance in the food chain: a developing country-perspective. Front Microbiol. 2016;7:1–19. doi:10.3389/fmicb.2016.01881
33. Gupta M, Sen A, Sarker MS, Das A. Prevalence and antibiotic susceptibility pattern of Escherichia coli in cattle on Bathan and intensive rearing system. Microbes Heal. 2017;6(1):1–4. doi:10.3329/mh.v6i1.34062
34. Madoshi B, Kudirkiene E, Mtambo A, Muhairwa A, Lupindu A, Olsen JE. Characterisation of commensal Escherichia coli isolated from apparently healthy cattle and their attendants in Tanzania. PLoS One. 2016;11(12):1–19. doi:10.1371/journal.pone.0168160
35. Barour D, Berghiche A, Boulebda N. Antimicrobial resistance of Escherichia coli isolates from cattle in Eastern Algeria. Vet World. 2019;12:1195–1203. doi:10.14202/vetworld.2019.1195-1203
36. Muloi D, Kiiru J, Ward MJ, et al. sympatric humans and livestock in a rapidly urbanizing city. Int J Antimicrob Agents. 2019;8:15–21. doi:10.1016/j.ijantimicag.2019.08.014
37. Obaidat MM, Bani Salman AE, Davis MA, Roess AA. Major diseases, extensive misuse, and high antimicrobial resistance of Escherichia coli in large- and small-scale dairy cattle farms in Jordan. J Dairy Sci. 2018;101(3):2324–2334. doi:10.3168/jds.2017-13665
38. Beyene T, Assefa S. Assessment of rational veterinary drugs use in livestock at adama district veterinary clinic, Central Ethiopia. J Vet Sci Technol. 2015;07(03):1–7. doi:10.4172/2157-7579.1000319
39. Kifle T, Tadesse G. Antimicrobial prescription practices in the veterinary clinics of Addis Ababa, Ethiopia. Ethiop Vet J. 2014;18(2):65–70.
40. Al-Ouqaili M, Jal’oot A, Badawy A. Identification of an OprD and bla(IMP) gene-mediated carbapenem resistance in Acinetobacter baumannii and pseudomonas aeruginosa among patients with wound infections in Iraq. Asian J Pharma. 2019;12(3):S959–S965.
41. Al-Ouqaili M, Al-Kubaisy S, Al-Ani N. Biofilm antimicrobial susceptibility pattern for selected antimicrobial agents against planktonic and sessile cells of clinical isolates of staphylococci using MICs, BICs and MBECs. Asian J Pharma. 2018;12(4):S1375–S1383.
42. Lunha K, Leangapichart T, Jiwakanon J, et al. Antimicrobial resistance in fecal Escherichia coli from humans and pigs at farms at different levels of intensification. Antibiotics. 2020;9(10):1–11. doi:10.3390/antibiotics9100662
43. Ström G, Halje M, Karlsson D, et al. Antimicrobial use and antimicrobial susceptibility in Escherichia coli on small- and medium-scale pig farms in north-eastern Thailand. Antimicrob Resist Infect Control. 2017;6(1):1–9. doi:10.1186/s13756-017-0233-9
44. Salyers A, McManus P. Possible Impact on Antibiotic Resistance in Human Pathogens Due to Agricultural Use of Antibiotics. New York, NY: Antibiot Dev Resist Taylor Fr Inc; 2001.
45. Schokker D, Zhang J, Zhang L, et al. Early-life environmental variation affects intestinal microbiota and immune development in new-born piglets. PLoS One. 2014;9(6). doi:10.1371/journal.pone.0100040
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


